The prevalence of low serum vitamin D levels in cancer patients with fatigue or poor appetite and their association with symptom burden and other correctable endocrine abnormalities were investigated.
Keywords: Vitamin D, Testosterone, Cancer, Symptoms
Abstract
Background.
Vitamin D deficiency in noncancer patients is associated with symptoms of fatigue, muscle weakness, and depression. These symptoms are common among advanced cancer patients. We investigated the prevalence of low serum vitamin D levels in cancer patients with fatigue or poor appetite and their association with symptom burden and other correctable endocrine abnormalities.
Methods.
This was a retrospective review of 100 consecutive cancer patients with appetite or fatigue scores of ≥4 of 10 referred to a supportive care clinic. We investigated serum levels of 25(OH) vitamin D, cortisol, thyroid-stimulating hormone, and bioavailable testosterone. Symptoms were measured by the Edmonton Symptom Assessment Scale. Serum 25(OH) vitamin D <20 ng/mL was considered deficient; ≥20 ng/mL and <30 ng/mL were considered insufficient.
Results.
Patients were predominantly male (68%) and white (66%), with a median age of 60 years (range, 27–91 years). Gastrointestinal (30%) and lung (22%) cancers were predominant. Forty-seven patients (47%) were vitamin D deficient and 70 (70%) were insufficient. Thirteen of 70 patients (19%) with vitamin D insufficiency were on supplementation. Vitamin D deficiency was more common among nonwhites (82% versus 36%) and females. No significant association was found between vitamin D and symptoms. Hypogonadic males had a significantly lower mean 25(OH) vitamin D level than eugonadic males.
Conclusions.
Low vitamin D levels were highly prevalent among advanced cancer patients with cachexia or fatigue. Vitamin D deficiency was more frequent among nonwhite and female patients. Vitamin D levels were also significantly lower in male patients with hypogonadism.
Introduction
Among noncancer patients, vitamin D deficiency is associated with joint pain, muscle weakness [1, 2], cognitive changes, and depression [3]. Although these symptoms are frequently found among advanced cancer patients, there are limited data on the association between vitamin D deficiency and other endocrine abnormalities among these patients.
Fatigue and anorexia/cachexia often occur together in patients with cancer [4]. Because these symptoms may share common underlying mechanisms, an effective therapy might alleviate more than one symptom simultaneously. Additionally, patients with fatigue and poor appetite may be at greater risk for vitamin D deficiency/insufficiency because of lower exposure to sunlight and/or low oral intake and a reduced ability to absorb dietary vitamin D. Consequently, we investigated the association of vitamin D deficiency and symptoms as well as other potentially correctable endocrine and laboratory abnormalities among ambulatory advanced cancer patients with moderate to severe symptoms of poor appetite and fatigue.
Methods
We performed a retrospective chart review, which was approved by the MD Anderson Institutional Review Board. In total, 100 consecutive cancer patients referred by their primary oncologists with moderate to severe symptoms including fatigue or poor appetite were evaluated in the Supportive Care Clinic at the University of Texas MD Anderson Cancer Center during January 2009 to December 2010. As a part of a standardized evaluation of all patients referred to our clinic, symptom intensity was measured by the Edmonton Symptom Assessment Scale (ESAS). The ESAS is a validated assessment tool quantifying a patient's response to 10 common symptoms in the past 24 hours, including pain, fatigue, nausea, depression, anxiety, sedation, shortness of breath, appetite, sleep, and sense of well-being [5]. Symptoms are scored for intensity at 0 (best) to 10 (worst). All patients had an ESAS score ≥4 for appetite or fatigue prior to their laboratory tests.
The standardized evaluation of these patients with fatigue or appetite scores ≥4 also included the following laboratory tests—serum 25(OH) vitamin D, bioavailable testosterone (males only), thyroid-stimulating hormone (TSH), and a.m. cortisol. Serum 25(OH) D was measured by chemiluminescent immunoassay (DiaSorin Liaison 25 OH Vitamin D TOTAL Assay, DiaSorin Corporation, Stillwater, MN). Although there is controversy regarding the optimal serum level of vitamin D, we used common cutpoints for vitamin D deficiency and insufficiency: <20 ng/mL and <30 ng/mL, respectively [6]. A consensus regarding the cutpoint of serum testosterone that defines testosterone deficiency for adult males is not established [7]. However, age-associated testosterone deficiency is characterized by symptoms and a deficiency in serum testosterone levels below the young healthy adult male reference range [8]. Because the reference range for bioavailable testosterone (BT) using mass spectrometry and the ammonium precipitation method (Mayo Clinic, Rochester, MN) for men aged 30–39 years is 72–235 ng/dL, we used a cutoff of 70 ng/dL BT as our definition for testosterone deficiency.
Demographic information was collected on age, gender, race, primary cancer diagnosis, Zubrod performance scale, and medications (opioids, megestrol acetate, corticosteroids, and chemotherapy within 3 months of laboratory assessments).
Data were summarized using descriptive statistics and 95% confidence intervals. Spearman's correlation was used to determine associations between laboratory abnormalities and symptom burden. Two-sample t-tests were used when the data were approximately normally distributed, the Wilcoxon two-sample test was used if the data were skewed, and χ2 tests were used for dichotomous variables. A two-sided p-value <.05 was considered statistically significant.
Results
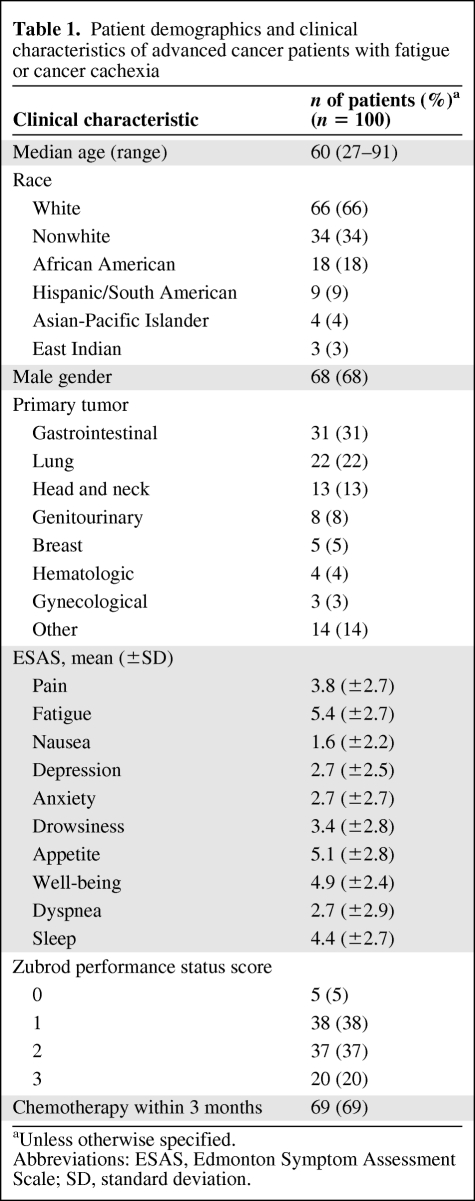
Baseline characteristics of the patients are summarized in Table 1. The median age was 60 years (range, 27–91 years). The majority of patients were male (n = 68, 68%) and white (n = 66, 66%). The most common cancer types were gastrointestinal (n = 31, 31%) and lung (n = 22, 22%).
Table 1.
Patient demographics and clinical characteristics of advanced cancer patients with fatigue or cancer cachexia
aUnless otherwise specified.
Abbreviations: ESAS, Edmonton Symptom Assessment Scale; SD, standard deviation.
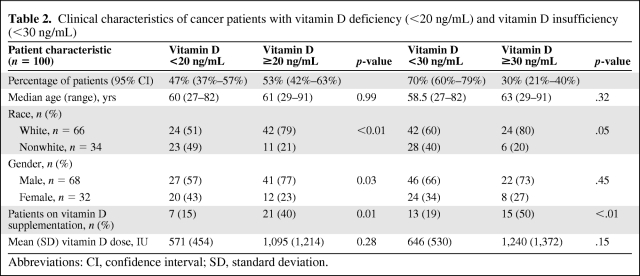
Forty-seven patients (47%) had 25(OH) vitamin D levels <20 ng/mL and 70 patients (70%) had levels <30 ng/mL (Table 2). Compared with whites, among whom 36% (24 of 66) were vitamin D insufficient (<30 ng/mL), vitamin D insufficiency was significantly more common among nonwhites (28 of 34, 82%)—African Americans, 16 of 18 (84%); Hispanics, nine of nine (100%); East Indian or Middle Eastern patients, two of three (67%); and Pacific Islanders, one of three (33%) (p = .02). Only 13 of 70 patients (19%) with vitamin D insufficiency were currently on vitamin D supplementation (Table 2). Vitamin D deficiency was statistically less common in males than in females (p = .03); however, vitamin D insufficiency had no correlation with gender (Table 2).
Table 2.
Clinical characteristics of cancer patients with vitamin D deficiency (<20 ng/mL) and vitamin D insufficiency (<30 ng/mL)
Abbreviations: CI, confidence interval; SD, standard deviation.
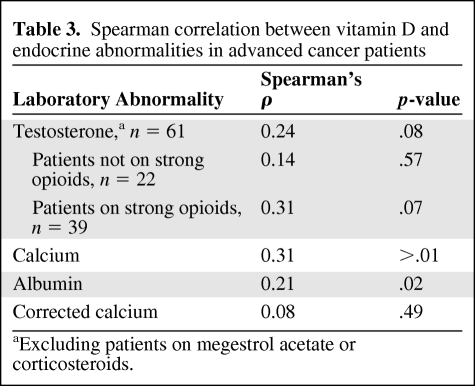
Vitamin D levels were correlated with total serum calcium (Spearman's ρ = 0.31; p < .01) and serum albumin (Spearman's ρ = 0.23; p = .02) (Table 3). The correlation between serum vitamin D and serum calcium was no longer significant when calcium was corrected for low albumin (Spearman's ρ = 0.08; p = .49) (Table 3). No significant correlation was noted between vitamin D and symptoms as measured by the ESAS, the Zubrod performance scale, or chemotherapy. Thirteen of 99 patients (13%) had biochemical hypothyroidism (TSH >5.5 mU/mL), and of the patients not receiving megestrol acetate or corticosteroids, none were noted to have a suppressed a.m. cortisol level (<4 μg/dL) diagnostic for hypoadrenalism.
Table 3.
Spearman correlation between vitamin D and endocrine abnormalities in advanced cancer patients
aExcluding patients on megestrol acetate or corticosteroids.
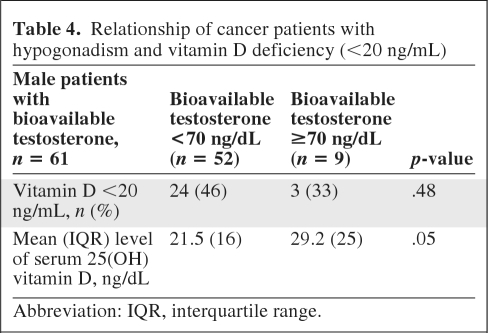
Among 39 male patients taking strong opioids (morphine equivalent daily dose, >0), vitamin D levels were positively associated with bioavailable serum testosterone levels (Spearman's ρ = 0.31; p = .07) (Table 3). Fifty-two of 61 male patients (85%) were hypogonadic (bioavailable testosterone <70 ng/dL). Hypogonadic males had a median 25(OH) vitamin D level of 21.5 ng/mL (interquartile range [IQR], 16 ng/mL) versus 29.2 ng/mL (IQR, 25 ng/mL) for males with testosterone levels ≥70 ng/dL (p = .05) (Table 4).
Table 4.
Relationship of cancer patients with hypogonadism and vitamin D deficiency (<20 ng/mL)
Abbreviation: IQR, interquartile range.
Discussion
Advanced cancer patients referred to our supportive care clinic for symptoms of fatigue or poor appetite have a high prevalence of vitamin D deficiency (47%) and insufficiency (70%). Vitamin D deficiency was significantly more common in nonwhites patients, females, and hypogonadal men.
Other authors have reported vitamin D deficiency among cancer patients, although the prevalence in ambulatory patients with advanced cancer is not well documented [9, 10]. A small study of 41 inpatient palliative care patients [11] reported that 88% had low vitamin D levels. In a large, heterogeneous population of new or previously treated cancer patients, vitamin D insufficiency was found in 75% of patients [12]. We found similar rates of vitamin D insufficiency (70% of patients with levels <30 ng/mL), and although 28% of patients were on some form of supplementation, inadequate levels of vitamin D were found among 46%. Not surprisingly, those patients on higher replacement doses of vitamin D had higher serum vitamin D levels. Whether patients were not prescribed vitamin D or were not compliant with recommendations for replacement or the dose of vitamin D supplementation prescribed was inadequate is unclear [12]. The type of cancer may play a role, because, in a recent study, prostate and lung cancer patients were more likely to respond to oral vitamin D supplementation after 8 weeks (levels >32 ng/mL) than were patients with colorectal and pancreatic cancers. Those authors suggested that the gastrointestinal toxicity (stomatitis and diarrhea) associated with chemotherapy for colorectal cancer may result in poor absorption of vitamin D [9].
Nonwhites in the U.S. are at greater risk for vitamin D deficiency for a variety of reasons, including skin pigmentation and dietary differences [13]. UVB light at wavelengths of 290–315 nm converts 7-dehydrocholesterol to previtamin D3 in the skin and then immediately to vitamin D3 in a heat-dependent process. Because few foods (except for oily fish) contain significant amounts of vitamin D, and supplemented foods (e.g., milk, margarine, orange juice) have very modest levels, patients may be unable to maintain adequate levels of vitamin D through diet alone [6]. Consistent with many other studies, we found a higher prevalence of vitamin D deficiency among females. Although this gender difference is often interpreted to reflect the greater outdoor exposure of males, this explanation seems unlikely in the setting of patients with advanced disease. We speculate that this gender difference may be related to the positive correlation observed between serum 25(OH) vitamin D and serum testosterone. Conversely, this may be a chance finding because the number of female patients (n = 32) was small.
Interventions such as increasing exposure to natural light or oral vitamin D supplementation could maintain adequate vitamin D levels; however, prospective studies are needed to determine their efficacy in cancer patients. A recent study with geriatric nursing home residents reported that weekly exposure to UVB lamps after showering resulted in a significant increase in serum levels of 25(OH) vitamin D [14]. Although sunlight exposure is an inexpensive and effective therapy [15, 16], a cluster randomized controlled trial in the elderly reported poor adherence to the intervention [17]. Many [18–20], but not all [21], observational studies show associations between higher serum 25(OH) vitamin D levels and better survival outcomes in cancer patients, but a survival benefit from vitamin D replacement has yet to be observed in intervention studies.
We found that low vitamin D levels were moderately associated with lower bioavailable testosterone levels in patients on potent opioids (Table 3). Low testosterone levels could be a potential mechanism underlying the association of vitamin D deficiency with fatigue and poor muscle strength. A recent study of men referred for coronary angiography noted that vitamin D levels had a positive association with testosterone levels [22]. Notably, in a placebo-controlled trial among overweight, otherwise healthy men, vitamin D replacement over a period of 1 year significantly increased testosterone levels [23]. In patients with cancer, chronic use of opioid analgesics can result in low testosterone [24], and in combination with low vitamin D levels this could predispose patients to loss of muscle mass and increase the insulin resistance associated with cancer cachexia [25] or obesity. Both vitamin D [26] and testosterone [27] supplementation have been reported to increase insulin sensitivity in selected noncancer populations.
Our study noted an association between vitamin D and albumin, which could be related to poor nutritional status or the metabolic derangements associated with advanced cancer. A lower albumin level has frequently been reported in association with a poor prognosis in cancer patients [28], and whether or not vitamin D has additional prognostic value needs to be examined.
There were no significant correlations between vitamin D levels and symptoms as measured by the ESAS, the Zubrod performance scale, or chemotherapy, which may be attributed to the highly selected nature of our patient cohort. All our clinic patients who underwent laboratory testing for vitamin D deficiency had appetite or fatigue scores of moderate to severe intensity (≥4 on the ESAS). Because of the strong bias in this select group of highly symptomatic patients, future studies should compare groups by including advanced cancer patients with a lower symptom burden. If, however, hypovitaminosis D is truly not associated with a symptom burden, this may explain why health care providers fail to identify patients with vitamin D deficiency.
Limitations of our study include the retrospective nature of the data collection and the lack of measurements of function or muscle strength. A small pilot study of 21 inpatient hospice patients showed an association between vitamin D deficiency and greater functional impairment [29], and a randomized trial of vitamin D supplementation in elderly women showed improved lower limb muscle strength and mobility [30]. Many studies in the elderly [31–33], but not all [34], demonstrate a beneficial effect of vitamin D supplementation in reducing the incidence of falls. One study reported that higher physiological testosterone levels in older men and women may protect against falls, and that the benefit may be additive in those taking vitamin D supplementation [35]. In critically ill patients, a single oral ultrahigh dose of 540,000 IU cholecalciferol corrected vitamin D deficiency within 2 days without causing hypercalcemia [36]. Because vitamin D and testosterone replacement are relatively inexpensive and could improve symptoms, function, and quality of life, prospective intervention studies in patients with cancer are warranted.
Conclusions
Vitamin D deficiency was highly prevalent and largely untreated in advanced cancer patients with cachexia or fatigue. Low levels of vitamin D were more frequent among nonwhites and male patients with hypogonadism. No association was noted between a low vitamin D level and symptom burden. Further studies examining the potential benefits of vitamin D supplementation on functional status, including testosterone levels, among patients with advanced cancer are warranted.
Acknowledgments
Rony Dev and Egidio Del Fabbro share first authorship.
Eduardo Bruera is supported in part by National Institutes of Health grant numbers RO1NR010162–01A1, RO1CA1222292.01, and RO1CA124481–01. Egidio Del Fabbro is supported in part by American Cancer Society grant number PEP-08–299-01-PC1.
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Rony Dev, Egidio Del Fabbro, Gary G. Schwartz, Noah Gutierrez, Eduardo Bruera
Provision of study material or patients: Rony Dev, Egidio Del Fabbro, David Hui, Eduardo Bruera
Collection and/or assembly of data: Rony Dev, Egidio Del Fabbro, David Hui, Shana L. Palla, Noah Gutierrez
Data analysis and interpretation: Rony Dev, Egidio Del Fabbro, Gary G. Schwartz, David Hui, Shana L. Palla, Eduardo Bruera
Manuscript writing: Rony Dev, Egidio Del Fabbro, Gary G. Schwartz, David Hui, Eduardo Bruera
Final approval of manuscript: Rony Dev, Egidio Del Fabbro, Gary G. Schwartz, David Hui, Shana L. Palla, Noah Gutierrez, Eduardo Bruera
References
- 1.Heidari B, Shirvani JS, Firouzjahi A, et al. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis. 2010;13:340–346. doi: 10.1111/j.1756-185X.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 2.Montero-Odasso M, Duque G. Vitamin D in the aging musculoskeletal system: An authentic strength preserving hormone. Mol Aspects Med. 2005;26:203–219. doi: 10.1016/j.mam.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Barnard K, Colón-Emeric C. Extraskeletal effects of vitamin D in older adults: Cardiovascular disease, mortality, mood, and cognition. Am J Geriatr Pharmacother. 2010;8:4–33. doi: 10.1016/j.amjopharm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Walsh D, Rybicki L. Symptom clustering in advanced cancer. Support Care Cancer. 2006;14:831–836. doi: 10.1007/s00520-005-0899-z. [DOI] [PubMed] [Google Scholar]
- 5.Chang VT, Hwang SS, Feruerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 7.Rosner W, Auchus RJ, Azziz R, et al. Position statement: Utility, limitations, and pitfalls in measuring testosterone: An Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Nieschlag E, Swerdloff R, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159:507–514. doi: 10.1530/EJE-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fakih MG, Trump DL, Johnson CS, et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219–224. doi: 10.1007/s00384-008-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuhouser ML, Sorensen B, Hollis BW, et al. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88:133–139. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone CA, Kenny RA, Healy M, et al. Vitamin D depletion: Of clinical significance in advanced cancer? Support Care Cancer. 2011;19:865–867. doi: 10.1007/s00520-011-1117-9. [DOI] [PubMed] [Google Scholar]
- 12.Vashi PG, Trukova K, Lammersfeld CA, et al. Impact of oral vitamin D supplementation on serum 25-hydroxyvitamin D levels in oncology. Nutr J. 2010;9:60. doi: 10.1186/1475-2891-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Chel VG, Ooms ME, Pavel S, et al. Prevention and treatment of vitamin D deficiency in Dutch psychogeratric nursing home residents by weekly half-body UVB exposure after showering: A pilot study. Age Ageing. 2011;40:211–214. doi: 10.1093/ageing/afq159. [DOI] [PubMed] [Google Scholar]
- 15.Chel VG, Ooms ME, Popp-Snijders C, et al. Ultraviolet irradiation corrects vitamin D deficiency and suppresses secondary hyperparathyroidism in the elderly. J Bone Miner Res. 1998;13:1238–1242. doi: 10.1359/jbmr.1998.13.8.1238. [DOI] [PubMed] [Google Scholar]
- 16.Corless D, Gupta SP, Switala S, et al. Response of plasma-25-hydroxyvitamin D to ultraviolet irradiation in long-stay geriatric patients. Lancet. 1978;2:649–651. doi: 10.1016/s0140-6736(78)92760-5. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook PN, Cameron ID, Chen JS, et al. Does increased sunlight exposure work as a strategy to improve vitamin D status in the elderly: A cluster randomised controlled trial. Osteoporos Int. 2011 Mar 3; doi: 10.1007/s00198-011-1590-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Heist RS, Liu G, et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small cell lung cancer patients. J Clin Oncol. 2007;25:479–485. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 20.Tretli S, Hernes E, Berg JP, et al. Association between serum 25(OH)D and death from prostate cancer. Br J Cancer. 2009;100:450–454. doi: 10.1038/sj.bjc.6604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heist RS, Zhou W, Wang Z, et al. Circulating 25-hydroxyvitamin D, VDR polymorphisms, and survival in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5596–5602. doi: 10.1200/JCO.2008.18.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehr E, Pilz S, Boehm BO, et al. Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf) 2010;73:243–248. doi: 10.1111/j.1365-2265.2009.03777.x. [DOI] [PubMed] [Google Scholar]
- 23.Pilz S, Frisch S, Koertke H, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43:223–225. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 24.Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, et al. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;15:100:851–858. doi: 10.1002/cncr.20028. [DOI] [PubMed] [Google Scholar]
- 25.Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol Behav. 2010;100:478–489. doi: 10.1016/j.physbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. Br J Nutr. 2010;103:549–555. doi: 10.1017/S0007114509992017. [DOI] [PubMed] [Google Scholar]
- 27.Malkin CJ, Jones TH, Channer KS. The effect of testosterone on insulin sensitivity in men with heart failure. Eur J Heart Fail. 2007;9:44–50. doi: 10.1016/j.ejheart.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12:223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- 29.To T. Vitamin D deficiency in an Australian inpatient hospice population. J Pain Symptom Manage. 2010 Nov 1; doi: 10.1016/j.jpainsymman.2010.09.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Zhu K, Austin N, Devine A, et al. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr Soc. 2010;58:2063–2068. doi: 10.1111/j.1532-5415.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- 31.Broe KE, Chen TC, Weinberg J, et al. A higher dose of vitamin D reduces the risk of falls in nursing home residents: A randomized, multiple-dose study. J Am Geriatr Soc. 2007;55:234–239. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 32.Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: A 3-year randomized controlled trial. Arch Intern Med. 2006;166:424–430. doi: 10.1001/archinte.166.4.424. [DOI] [PubMed] [Google Scholar]
- 33.Flicker L, MacInnis RJ, Stein MS, et al. Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J Am Geriatr Soc. 2005;53:1881–1888. doi: 10.1111/j.1532-5415.2005.00468.x. [DOI] [PubMed] [Google Scholar]
- 34.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 35.Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Additive benefit of higher testosterone levels and vitamin D plus calcium supplementation in regard to fall risk reduction among older men and women. Osteoporos Int. 2008;19:1307–1314. doi: 10.1007/s00198-008-0573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amrein K, Sourij H, Wagner G, et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: A randomized, double-blind, placebo-controlled pilot study. Crit Care. 2011;15:R104. doi: 10.1186/cc10120. [DOI] [PMC free article] [PubMed] [Google Scholar]