Abstract
Lymphocyte responsiveness in rats was found to depend on serum prolactin levels. Blocking pituitary prolactin release with bromocriptine severely reduces lymphocyte reactivity in vitro (mixed lymphocyte reaction) as well as in vivo (graft-versus-host reaction). In addition, evidence for a prolactin/growth hormone-related mRNA species produced in mitogen- and antigen-stimulated lymphocytes has been obtained. Prolactin was shown to compete in a dose-dependent fashion with the immunosuppressant cyclosporine (cyclosporin A) for a common binding site on the surface of T lymphocytes. Further, stimulation of prolactin secretion reversed the immunosuppression induced by cyclosporine. We conclude that prolactin is involved in the maintenance of T-cell immunocompetence and that the immunosuppressive effects of cyclosporine may be mediated by the displacement of prolactin from binding sites on lymphocytes.
Full text
PDF
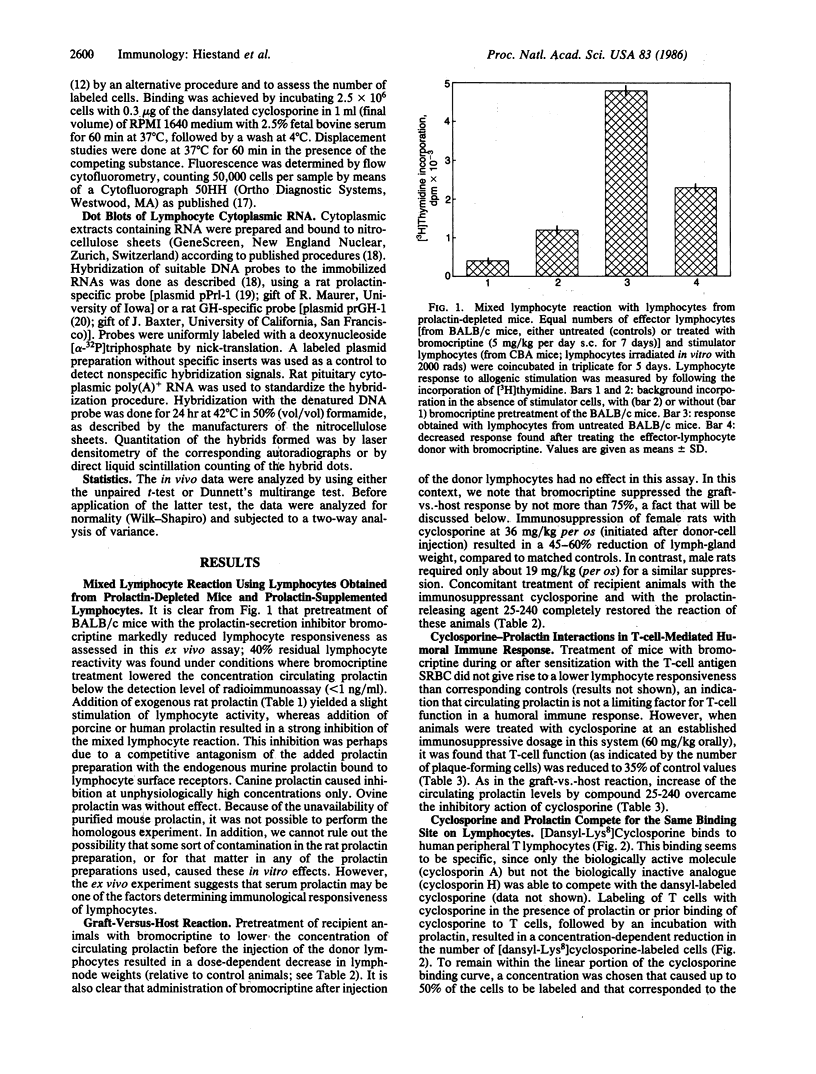
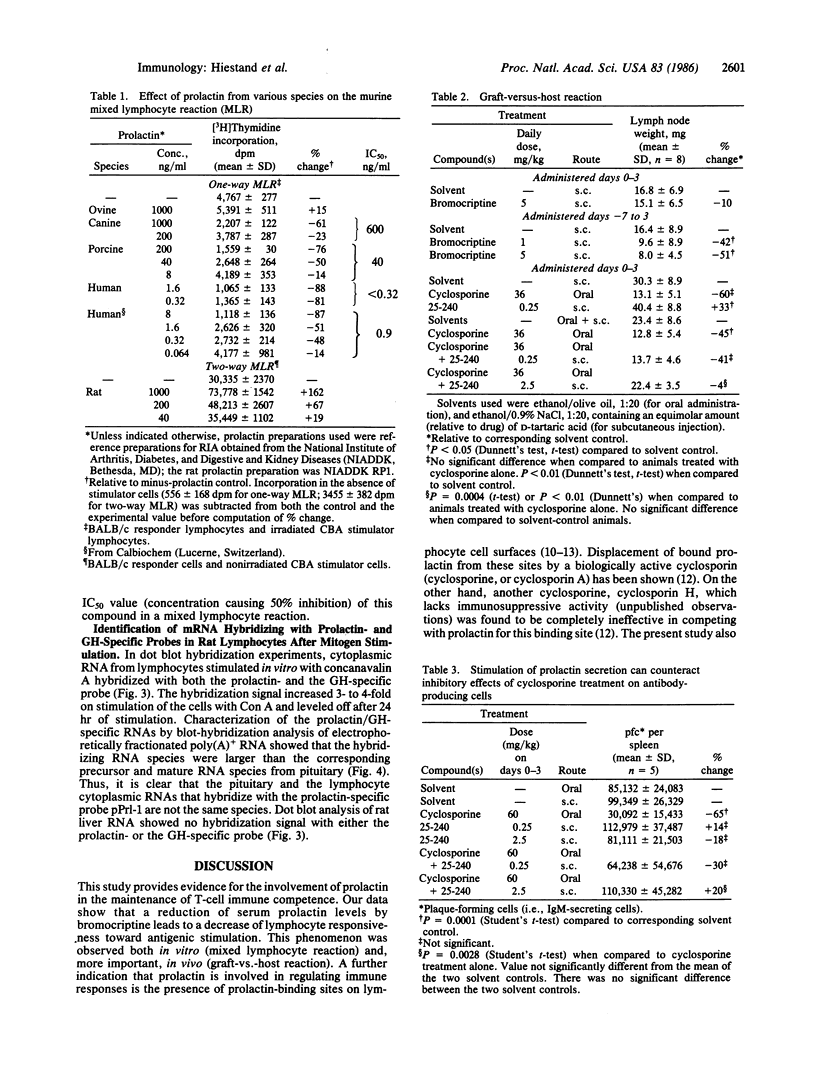
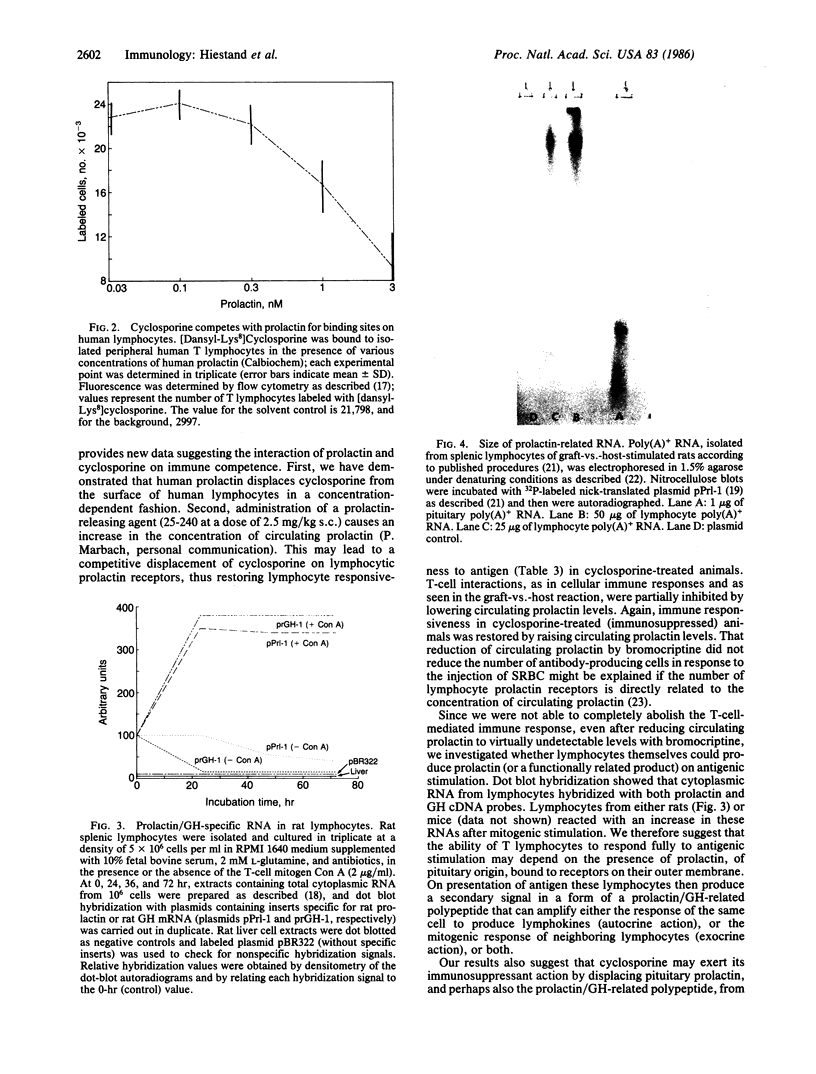

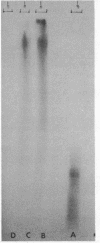
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berczi I., Nagy E. A possible role of prolactin in adjuvant arthritis. Arthritis Rheum. 1982 May;25(5):591–594. doi: 10.1002/art.1780250517. [DOI] [PubMed] [Google Scholar]
- Ford W. L., Burr W., Simonsen M. A lymph node weight assay for the graft-versus-host activity of rat lymphoid cells. Transplantation. 1970 Sep;10(3):258–266. doi: 10.1097/00007890-197009000-00007. [DOI] [PubMed] [Google Scholar]
- Golander A., Hurley T., Barrett J., Hizi A., Handwerger S. Prolactin synthesis by human chorion-decidual tissue: a possible source of prolactin in the amniotic fluid. Science. 1978 Oct 20;202(4365):311–313. doi: 10.1126/science.694535. [DOI] [PubMed] [Google Scholar]
- Gubbins E. J., Maurer R. A., Hartley J. L., Donelson J. E. Construction and analysis of recombinant DNAs containing a structural gene for rat prolactin. Nucleic Acids Res. 1979 Mar;6(3):915–930. doi: 10.1093/nar/6.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J. A., Mode A., Norstedt G., Eneroth P., Hökfelt T. Central control of prolactin and estrogen receptors in rat liver--expression of a novel endocrine system, the hypothalamo-pituitary-liver axis. Annu Rev Pharmacol Toxicol. 1983;23:259–278. doi: 10.1146/annurev.pa.23.040183.001355. [DOI] [PubMed] [Google Scholar]
- Jerne N. K., Nordin A. A. Plaque Formation in Agar by Single Antibody-Producing Cells. Science. 1963 Apr 26;140(3565):405–405. doi: 10.1126/science.140.3565.405. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M., Tobey R. A., Van Dilla M. A. Flow microfluorometric studies of lectin binding to mammalian cells. I. General features. J Cell Physiol. 1973 Jun;81(3):305–314. doi: 10.1002/jcp.1040810303. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Nucleotide sequence of a growth-related mRNA encoding a member of the prolactin-growth hormone family. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4255–4259. doi: 10.1073/pnas.81.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E., Berczi I., Friesen H. G. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol (Copenh) 1983 Mar;102(3):351–357. doi: 10.1530/acta.0.1020351. [DOI] [PubMed] [Google Scholar]
- Nagy E., Berczi I., Wren G. E., Asa S. L., Kovacs K. Immunomodulation by bromocriptine. Immunopharmacology. 1983 Oct;6(3):231–243. doi: 10.1016/0162-3109(83)90023-1. [DOI] [PubMed] [Google Scholar]
- Rich S. S., Rich R. R. Regulatory mechanisms in cell-mediated immune responses. I. Regulation of mixed lymphocyte reactions by alloantigen-activated thymus-derived lymphocytes. J Exp Med. 1974 Dec 1;140(6):1588–1603. doi: 10.1084/jem.140.6.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick D. H., Luciano A. A., Kusmik W. F., Maslar I. A. De novo synthesis of prolactin by human decidua. Life Sci. 1978 Nov 9;23(19):1913–1921. doi: 10.1016/0024-3205(78)90557-x. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Kibler R., Matrisian L., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985 May;134(5):3027–3031. [PubMed] [Google Scholar]
- Russell D. H., Matrisian L., Kibler R., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human lymphocytes and their modulation by cyclosporine. Biochem Biophys Res Commun. 1984 Jun 29;121(3):899–906. doi: 10.1016/0006-291x(84)90762-9. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Baxter J. D., Goodman H. M. Nucleotide sequence and amplification in bacteria of structural gene for rat growth hormone. Nature. 1977 Dec 8;270(5637):486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]