Abstract
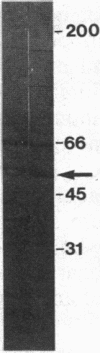
A genomic DNA fragment that encodes a Plasmodium falciparum antigen has been isolated by using human antibodies eluted from the membrane of infected erythrocytes. The antigen has a very unusual primary structure; it is exceptionally rich in asparagine residues, many of which are distributed in clusters (2-15 residues) along the polypeptide chain. Unlike many P. falciparum antigens, this protein lacks tandemly repeated sequences. The antigen is distinct from Pf 155, a merozoite-derived antigen deposited in the membrane of infected erythrocytes, but contains epitopes that crossreact with anti-Pf 155 antibodies. Antisera prepared in mice against the asparagine-rich protein react with late-stage parasites in indirect immunofluorescence. In an in vitro merozoite reinvasion assay, the IgG fraction of a mouse polyclonal antiserum, as well as a mouse monoclonal antibody, gave significant inhibition. Three polypeptides (Mr 36,000, 30,000, and 15,000) were recognized by these antibodies on immunoblots of P. falciparum extracts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berzins K., Perlmann H., Wåhlin B., Carlsson J., Wahlgren M., Udomsangpetch R., Björkman A., Patarroyo M. E., Perlmann P. Rabbit and human antibodies to a repeated amino acid sequence of a Plasmodium falciparum antigen, Pf 155, react with the native protein and inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1065–1069. doi: 10.1073/pnas.83.4.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins K., Wahlgren M., Perlmann P. Studies on the specificity of anti-erythrocyte antibodies in the serum of patients with malaria. Clin Exp Immunol. 1983 Nov;54(2):313–318. [PMC free article] [PubMed] [Google Scholar]
- Björkman A., Hedman P., Brohult J., Willcox M., Diamant I., Pehrsson P. O., Rombo L., Bengtsson E. Different malaria control activities in an area of Liberia--effects on malariometric parameters. Ann Trop Med Parasitol. 1985 Jun;79(3):239–246. doi: 10.1080/00034983.1985.11811914. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Coppel R. L., Saint R. B., Favaloro J., Crewther P. E., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F., Kemp D. J. The ring-infected erythrocyte surface antigen (RESA) polypeptide of Plasmodium falciparum contains two separate blocks of tandem repeats encoding antigenic epitopes that are naturally immunogenic in man. Mol Biol Med. 1984 Jun;2(3):207–221. [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- David P. H., Hadley T. J., Aikawa M., Miller L. H. Processing of a major parasite surface glycoprotein during the ultimate stages of differentiation in Plasmodium knowlesi. Mol Biochem Parasitol. 1984 Apr;11:267–282. doi: 10.1016/0166-6851(84)90071-9. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Biosynthesis and processing of a Plasmodium falciparum schizont antigen recognized by immune serum and a monoclonal antibody. J Exp Med. 1982 Nov 1;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth M. E., Ainley W. M., Shumard D. S., Butow R. A., Grossman L. I. Location and structure of the var1 gene on yeast mitochondrial DNA: nucleotide sequence of the 40.0 allele. Cell. 1982 Sep;30(2):617–626. doi: 10.1016/0092-8674(82)90258-6. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lundgren K., Wahlgren M., Troye-Blomberg M., Berzins K., Perlmann H., Perlmann P. Monoclonal anti-parasite and anti-RBC antibodies produced by stable EBV-transformed B cell lines from malaria patients. J Immunol. 1983 Oct;131(4):2000–2003. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan T. F., Hansen J. L., Dame J. B., Mullins J. A. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. doi: 10.1126/science.6330899. [DOI] [PubMed] [Google Scholar]
- Miller L. H., David P. H., Hadley T. J. Perspectives for malaria vaccination. Philos Trans R Soc Lond B Biol Sci. 1984 Nov 13;307(1131):99–115. doi: 10.1098/rstb.1984.0112. [DOI] [PubMed] [Google Scholar]
- Perlmann H., Berzins K., Wahlgren M., Carlsson J., Björkman A., Patarroyo M. E., Perlmann P. Antibodies in malarial sera to parasite antigens in the membrane of erythrocytes infected with early asexual stages of Plasmodium falciparum. J Exp Med. 1984 Jun 1;159(6):1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Feder R., Pavlovec A., Blobel G. Primary structure and genomic organization of the histidine-rich protein of the malaria parasite Plasmodium lophurae. Nature. 1984 Dec 13;312(5995):616–620. doi: 10.1038/312616a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Troye-Blomberg M., Romero P., Patarroyo M. E., Björkman A., Perlmann P. Regulation of the immune response in Plasmodium falciparum malaria. III. Proliferative response to antigen in vitro and subset composition of T cells from patients with acute infection or from immune donors. Clin Exp Immunol. 1984 Nov;58(2):380–387. [PMC free article] [PubMed] [Google Scholar]
- Udomsangpetch R., Lundgren K., Berzins K., Wåhlin B., Perlmann H., Troye-Blomberg M., Carlsson J., Wahlgren M., Perlmann P., Björkman A. Human monoclonal antibodies to Pf 155, a major antigen of malaria parasite Plasmodium falciparum. Science. 1986 Jan 3;231(4733):57–59. doi: 10.1126/science.3510452. [DOI] [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Björkman A. Characterization of the humoral immune response in Plasmodium falciparum malaria. I. Estimation of antibodies to P. falciparum or human erythrocytes by means of microELISA. Clin Exp Immunol. 1983 Oct;54(1):127–134. [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Björkman A., Perlmann H., Berzins K., Perlmann P. Anti-Plasmodium falciparum antibodies acquired by residents in a holoendemic area of Liberia during development of clinical immunity. Am J Trop Med Hyg. 1986 Jan;35(1):22–29. doi: 10.4269/ajtmh.1986.35.22. [DOI] [PubMed] [Google Scholar]
- Wåhlin B., Wahlgren M., Perlmann H., Berzins K., Björkman A., Patarroyo M. E., Perlmann P. Human antibodies to a Mr 155,000 Plasmodium falciparum antigen efficiently inhibit merozoite invasion. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7912–7916. doi: 10.1073/pnas.81.24.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]