Abstract
Cessation of transcriptional activity is a hallmark of cell division. Many biochemical pathways have been shown and proposed over the past few decades to explain the silence of this phase. In particular, many individual transcription factors have been shown to be inactivated by phosphorylation. In this report, we show the simultaneous phosphorylation and mitotic redistribution of a whole class of modified transcription factors. C2H2 zinc finger proteins (ZFPs) represent the largest group of gene expression regulators in the human genome. Despite their diversity, C2H2 ZFPs display striking conservation of small linker peptides joining their adjacent zinc finger modules. These linkers are critical for DNA binding activity. It has been proposed that conserved phosphorylation of these linker peptides could be a common mechanism for the inactivation of the DNA binding activity of C2H2 ZFPs, during mitosis. Using a novel antibody, raised against the phosphorylated form of the most conserved linker peptide sequence, we are able to visualize the massive and simultaneous mitotic phosphorylation of hundreds of these proteins. We show that this wave of phosphorylation is tightly synchronized, starting in mid-prophase right after DNA condensation and before the breakdown of the nuclear envelope. This global phosphorylation is completely reversed in telophase. In addition, the exclusion of the phospholinker signal from condensed DNA clearly demonstrates a common mechanism for the mitotic inactivation of C2H2 ZFPs.
Key words: mitosis, phosphorylation, C2H2, zinc finger, transcription factor, YY1, biomarker
Introduction
Mitosis is the culminating step of the mammalian cell cycle. After duplicating its DNA content and satisfying growth conditions and checkpoints, one cell divides into two. Convergence of an intricate network of signaling pathways orchestrates the mitotic physical and biochemical processes. Accurate coordination of all these pathways is vital for the execution of mitosis and proper distribution of the genetic material into two new daughter cells.
Cessation of active transcription is a main hallmark of cell division and has long been known.1,2 Although compaction of DNA into condensed chromosomes results in a restrictive barrier, it is not solely responsible for the inhibition of transcription.3,4 Entry into mitosis is accompanied by waves of phosphorylation events regulating the large morphological changes like DNA condensation and nuclear envelope disassembly.5 Phosphorylation has also been shown to be a key player in turning off transcriptional activity, both through general and specific mechanisms.3 The general mechanisms usually involve the inactivation of various components of the basic transcriptional machinery, like phosphorylation of RNA Polymerase II and TFIIH.6–8 In addition, various context-specific phosphorylation events have been shown to differentially inactivate sequence-specific transcription factors, like Myc and Myb.9 The simultaneous inactivation of a whole class of sequence-specific transcription factors by a common mechanism has never been shown. However, two such mechanisms have been proposed in the literature. The first is for the POU homeodomain containing transcription factors, like Oct-1 and GHF-1;10,11 the second is for C2H2 zinc finger proteins (ZFPs).12
C2H2 ZFPs represent the largest class of DNA binding transcription factors comprising hundreds of members in the human genome.13 C2H2 ZFPs are involved in a very wide spectrum of functional diversity, regulating biological processes like cellular growth, proliferation and differentiation.14,15 Each ZFP usually comprises several zinc finger modules which dictate its sequence-specific DNA binding activity. However, optimal binding activity of a ZFP is achieved through cooperative binding of adjacent zinc fingers wrapping around the DNA in locking position.14,16 Small five amino acid linker peptides join adjacent zinc fingers and are critical for this locking position regardless of the sequence specificity of the bound DNA. These linkers are highly conserved among the different ZFPs with TGEKP being the consensus, and most prevalent, sequence.14,16–18
The DNA binding efficiency and specificity of the clusters of zinc finger domains has led to a significant amount of research aimed at designing artificial ZFPs. These have been used to perform a variety of engineered functions at specific targets in the genome, such as controlled gene expression and nuclease activity.19–22 The naturally occurring and very common, TGEKP sequence has also been used as the linker peptide in most of these designed ZFP.20 Although several of the amino acid residues in linker peptides can affect the efficient binding to DNA,17,23,24 the conserved threonine (or serine) residue has a particularly important role, especially through its hydroxyl group.25 This same hydroxyl group can be modified by phosphorylation, resulting in significant reduction of binding affinity.26
In 2002, Dovat et al. showed that two C2H2 ZFPs, Ikaros and Sp1, were phosphorylated at their linker peptides, causing them to lose DNA binding activity in mitotic cells.12 Because of the remarkably high conservation of these sequences, the authors proposed that this could be a common pathway for the inactivation of all C2H2 ZFPs during cell division. In our study of the ZFP YY1, we found that its loss of DNA binding activity and exclusion from chromatin during mitosis were also due to phosphorylation of its linker peptides.27
Here, we provide evidence of the global phosphorylation of C2H2 ZFPs linker peptide in mitosis. We characterize and visualize the temporal and spatial distribution of this massive phosphorylation event during cell division.
Results
Abundance of C2H2 linker phosphorylation in mitotic cells.
The C2H2 ZFP family consists of hundreds of members each containing several linker peptides, in some cases even dozens.13,14 If a common mechanism for the inactivation of C2H2 ZFPs through phosphorylation of their linker peptides does exist, these conserved phosphosites are expected to be found in high abundance. To investigate this, we searched high-throughput databases from published large-scale mass spectrometry analyses.28–32 Indeed, we found that hundreds of phospho-linker peptides have been mapped. However, these reported phosphosites were not particularly categorized under ZFPs or associated with any specific functional relevance. To illustrate the results of this search, we chose 50 ZFPs containing the consensus linker sequence TGEKP. Table S1 lists these 50 proteins, their size (in amino acid numbers), accession numbers, the amino acid position of the TGEKP linker and reported phosphorylation sites.
To enable the experimental investigation of this global mitotic event, we wanted to develop an antibody against the phosphorylated form of the consensus linker sequence (pTGEKP). However, to increase the immunogenicity and specificity of the epitope, we included a histidine residue N-terminally to this sequence. The histidine is part of the preceding zinc finger and is conserved in all C2H2 ZFPs, forming the phospho-epitope HpTGEKP.
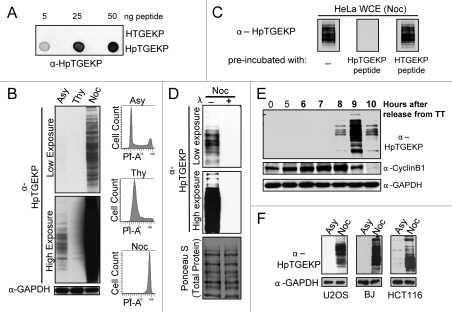
First, to test the phospho-specificity of the new antibody (hereafter referred to as α-HpTGEKP), we spotted synthetic nonphosphorylated or phosphorylated HTGEKP peptides onto a nitrocellulose membrane. As shown in Figure 1A, α-HpTGEKP efficiently recognized only the phosphorylated form of the linker peptide.
Figure 1M.
itotic specificity of the HpTGEKP phosphorylation. (A) Dot-blot assay of non-phosphorylated (HTGEKP) and phosphorylated (HpTGEKP) synthetic peptides probed with α-HpTGEKP. (B) Protein gel blot of WCEs from HeLa cells, growing asynchronously (Asy), arrested with thymidine (Thy) or with nocodazole (Noc). The blot was probed with α-HpTGEKP, then stripped and reprobed with α-GAPDH as a loading control. Fractions of the collected cells were fixed, stained with propidium iodide and analyzed using FACS to show cell cycle distribution based on DNA content (PI-A). (C) Protein gel blot of WCEs from nocodazole-arrested HeLa cells probed with α-HpTGEKP pre-incubated with HTGEKP or HpTGEKP synthetic peptides, as indicated. (D) Protein gel blot of WCEs from nocodazole-arrested HeLa cells incubated with or without Lambda phosphatase. The blot was probed with α-HpTGEKP, and then stained with Ponceau S to show equal loading and the total distribution of protein bands in both lanes. (E) Protein gel blot of WCEs from HeLa cells synchronized at early S phase by double-thymidine block and released for the indicated time points. The blot was probed with α-HpTGEKP, then stripped and reprobed with α-GAPDH as a loading control and α-cyclin B1 to monitor cell cycle progression. (F) Protein gel blots of WCEs from U2OS, BJ and HCT116 asynchronously (Asy) growing or arrested with nocodazole (Noc). The blot was probed with α-HpTGEKP, and then stripped with α-GAPDH as a loading control.
Next, we prepared whole cell extracts (WCE) from HeLa cells growing asynchronously, arrested with thymidine (S phase), or with nocodazole (pro-metaphase of mitosis). Proteins were separated on SDS-PAGE gel, transferred to a nitrocellulose membrane. When the blot was probed with α-HpTGEKP antibody, a large number of bands were detected only in the lane containing proteins extracted from nocodazole-arrested cells. This shows that the phospho-epitope HpTGEKP is found on many proteins in mitotic cells. High exposure of the blot revealed fainter bands in the asynchronous lane but not the thymidine lane. This is reasonable since an asynchronous population typically contains a small percentage of mitotic cells. Equal loading of proteins in the three lanes was controlled for by probing with the GAPDH antibody, and synchrony of the cells confirmed by propidium iodide staining and flow cytometry analyses (Fig. 1B).
To test the specificity of the detected bands, WCEs from nocodazole-arrested HeLa cells were probed with α-HpTGEKP pre-incubated with or without the synthetic peptides HTGEKP or HpTGEKP. As shown in Figure 1C, pre-incubation of the antibody with the non-phosphorylated linker peptide (HTGEKP) did not affect the signal. However, pre-incubation with the phosphorylated peptide (HpTGEKP) completely abolished the signal. Furthermore, pre-treatment of the protein extracts from nocodazole-arrested cells with phosphatase caused the complete disappearance of all bands, even at high exposure, showing the high phospho-specifcity of the α-HpTGEKP antibody (Fig. 1D).
Next, we wanted to ensure that the observed bands are not an artifact of the nocodazole arrest and to determine the timing of this phosphorylation in the cell cycle. For this, HeLa cells were synchronized by double-thymdine block, released and cells were collected for WCE preparation at 5–10 h after release. The progression from S phase, through G2, and through mitosis was monitored by cyclin B1 levels.33 Probing with α-HpTGEKP antibody generated bands peaking at 9 h, when the majority of the cells are in mitosis. The bands rapidly disappeared as cells started exiting mitosis at the 10 h time point (Fig. 1E). In addition, probing WCE from HeLa cells that were released from a nocodazole block, showed that the HpTGEKP bands disappeared as cells progressed toward the end of mitosis (Fig. S1).
To exclude the possibility that this massive mitotic phosphorylation event is specific to HeLa cells, WCEs from asynchronnous or nocodazole arrested U2OS, BJ and HCT116 cells were probed with the α-HpTGEKP antibody. The results were similar to those obtained using HeLa cells (Fig. 1F).
Specificity of α-HpTGEKP to individual C2H2 ZFPs.
Next, we wanted to test the specificity of the new antibody to mitotic phosphorylation of individual ZFPs. The high number of bands detected in WCEs makes it particularly difficult to evaluate individual proteins based on their size distribution. Therefore, conclusive results cannot be obtained by stripping and reprobing the blot with individual ZFP antibodies. Instead, our approach was to immunoprecipitate individual ZFPs using protein-specific antibodies from the WCE and probe for HpTGEKP phosphorylation.
YY1 is a multifunctional C2H2 ZFP involved in the regulation of a wide array of genes controlling cellular growth, proliferation, differentiation, apoptosis,34,35 and tumorigenesis.36,37 YY1 contains four zinc fingers, all of which are needed for its optimal DNA binding activity.38,39 We have previously shown that YY1 gets phosphorylated at two of its linker peptides during mitosis, linker 2 (TGEKP) and linker 3 (TGDRP). We have also shown that mitotic phosphorylation of YY1 at these linkers results in loss of DNA binding activity and exclusion from the condensed DNA.27
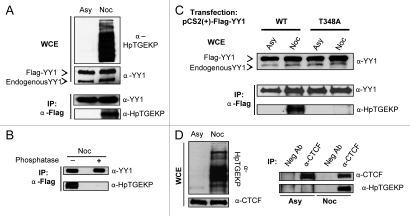
To test if α-HpTGEKP could detect mitotic YY1, Flag-YY1 was immunoprecipitated from HeLa-Flag-YY1 stable transfectant cells, either growing asynchronously or nocodazolearrested. The resulting protein gel blot with α-YY1 antibody showed equal Flag-YY1 protein levels in the immunoprecipitates from asynchronous or nocodazole protein extracts. However, α-HpTGEKP antibody only detected Flag-YY1 in the α-Flag IP from nocodazole extracts (Fig. 2A). In addition, we show that this signal is phospho-specific, since phosphatase treatment of the immunoprecipitated Flag-YY1 from nocodazole extracts abolished the signal (Fig. 2B). YY1 contains only one HTGEKP sequence (threonine 348 of YY1). To examine the specificity of the antibody to its target sequence within YY1, Flag-YY1 wild type (WT) or a threonine to alanine mutant (T348A) were transiently overexpressed in HeLa cells. Each transfection was then divided into two, one growing asynchronously and the other was blocked with nocodazole. Flag-YY1 (WT) and (T348A) were then immunoprecipitated and probed with α-YY1 antibody, showing equal Flag-YY1 protein levels in all of the IPs. While α-HpTGEKP recognized mitotic Flag-YY1 (WT), it did not detect Flag-YY1 (T348A) (Fig. 2C). Since both linker 2 (TGEKP) and linker 3 (TGDRP) are phosphorylated on mitotic YY1,27 this result clearly demonstrates the specificity of α-HpTGEKP for its target phospho-epitope.
Figure 2.
Detecting HpTGEKP phosphorylation on specific ZFPs. (A) Protein gel blot of WCEs of HeLa-Flag-YY1 cells growing asynchronously (Asy) or arrested with nocodazole (Noc), probed with α-HpTGEKP, and then α-YY1 (upper part). Protein gel blot of α-Flag IP from HeLa-Flag-YY1 extracts (Asy or Noc), probed with α-YY1, then with α-HpTGEKP (lower part). (B) Protein gel blot of α-Flag IP from HeLa-Flag-YY1 extracts (Noc) pre-treated with or without Lambda phosphatase. The blot was probed with α-YY1, then with α-HpTGEKP. (C) Protein gel blot of WCEs of HeLa cells transiently overexpressing Flag-YY1 (WT) or (T348A) (Asy or Noc). The blot was probed with α-YY1 (upper part). Protein gel blot of α-Flag IP, probed with α-YY1, then with α-HpTGEKP (lower part). (D) Protein gel blot of WCEs of HeLa cells (Asy or Noc), probed with α-HpTGEKP, and then stripped and reprobed with α-CTCF (left part). Protein gel blot of α-CTCF IP, probed with α-CTCF, then with α-HpTGEKP (right part). Anti-GFP was used as the negative control antibody.
CTCF is another multifunctional ZFP involved in gene regulation and multiple levels of chromatin organization.40,41 The CTCF protein contains several linker peptides, but only one contains the exact TGEKP sequence (threonine 518). Probing immunoprecipitated CTCF from asynchronous or nocodazole arrested HeLa cells with α-HpTGEKP shows that it is phosphorylated at this site in mitosis (Fig. 2D). To our knowledge, this particular CTCF site has not been previously reported to be phosphorylated.
Dynamic distribution of C2H2 linker phosphorylation during mitosis.
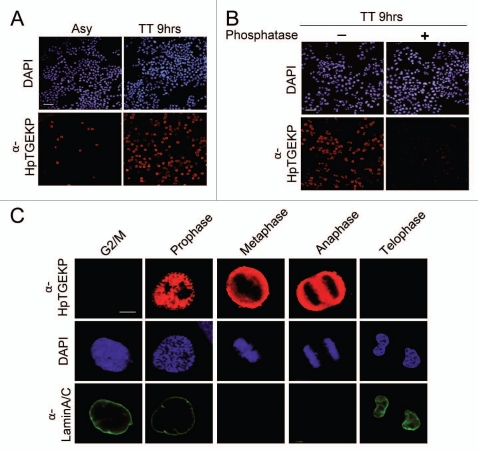
The results presented in the two previous figures, showing protein gel blot analyses, clearly demonstrate the high abundance of linker phosphorylation in mitotic cells. However, the best approach to delineate the exact timing of linker peptide phosphorylation during mitosis is to observe its occurrence in individual cells. Therefore, to study the temporal and spatial distribution of linker peptide phosphorylation during mitosis we immunostained HeLa cells with α-HpTGEKP and examined them using confocal microscopy. Cell nuclei (DNA) were visualized with DAPI stain. First, we looked at cells at low magnification. Figure 3A shows that few cells were positive for HpTGEKP phosphorylation in an asynchronous HeLa population. However, all of the positive cells had condensed chromatin (not shown), suggesting the exclusivity of the phosphorylation to mitotic cells. These positive cells were highly enriched when cells were synchronized with double-thymidine block and released for 9 h (TT9 hrs). This conforms to the protein gel blot results in Figure 1E. The immunostaining was confirmed to be phosphospecific, since treatment of HeLa (TT9 hrs) cells with Lambda phosphatase abolished the signal (Fig. 3B).
Figure 3.
Timing and distribution of linker phosphorylation during mitosis. HeLa cells were grown on coverslips, fixed, permeabilized and stained. Images were captured on a fluorescent confocal microscope. Specific mitotic stage labeling was based on chromatin morphology. (A) HeLa cells, asynchronously growing (Asy) or synchronized with double-thymidine block and released for 9 h (TT 9 hrs). Cells were stained with DAP I (blue) and α-HpTGEKP (red). (Bar, 100 µM). (B) HeLa cells (TT9 hrs) treated with or without Lambda phosphatase prior to immunostaining. Cells were stained with DAPI (blue) and α-HpTGEKP (red). (Bar, 100 µM). (C) HeLa cells synchronized with double-thymidine block and collected 8–10 h after release. Cells were stained with DAP I (blue), α-HpTGEKP (red), and α-lamin A/C (green, to visualize the nuclear envelope). (Bar, 20 µM).
To investigate the sub-mitotic dynamics of HpTGEKP phosphorylation, we examined individual mitotic HeLa cells at high magnification. Cells were double-immunostained with α-HpTGEKP and with α-Lamin A/C to visualize the nuclear envelope. DNA was stained with DAPI and the chromatin morphology was used to determine the mitotic stages. Figure 3C shows that HpTGEKP phosphorylation occurs in prophase prior to the nuclear envelope disassembly. HpTGEKP phosphorylation persists through metaphase, anaphase, and is dephosphorylated by the end of telophase. Throughout these stages, the HpTGEKP signal is excluded from the condensed DNA, as is clearly shown in the metaphase and anaphase parts of Figure 3C and in the prophase part in Figure S2. These observations support the hypothesis that mitotic phosphorylation of linker peptides abolishes the DNA binding activity of ZFPs and contributes to their dispersal away from condensed chromosomes during mitosis. Similar immunostaining patterns were observed for BJ and HCT116 cells (Fig. S3).
To further dissect the initial timing of the HpTGEKP phosphorylation, we investigated HeLa cells at several stages of prophase by monitoring the chromosome condensation morphologies. We found that HpTGEKP phosphorylation occurs in midprophase (Fig. S4). Interestingly, the signal is initially nuclear and distributes to the cytoplasm only at late-prophase, a stage that is associated with the breakdown of the nuclear envelope. Therefore, the presence of this phosphorylation does not appear to be sufficient to translocate the modified ZFPs to the cytoplasm. We have previously shown that phospho-mimetic mutations in the linker peptides of the ZFP YY1 inactivate its DNA binding activity but do not affect its nuclear localization.27 We have also shown that YY1 distribution to the cytoplasm occurs at late prophase.27 This indicates that linker phosphorylation excludes ZFPs from the condensing DNA, but their cytoplasmic distribution is probably due to a different mechanism associated with the disruption of the nuclear envelope barrier. On the other hand, dephosphorylation of HpTGEKP occurs gradually during telophase and cytokinesis, and is complete at the end of mitosis (Fig. S5).
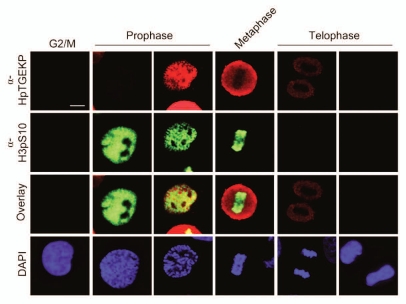
Histone H3 serine 10 phosphorylation (pH3S10) is a widely used mitotic marker. This phosphorylation can occur in specific contexts in association with gene expression. However, its prominent and global distribution is specific to mitosis and is correlated with DNA condensation.42–44 To further characterize the timing of α-HpTGEKP phosphorylation, double-immunostaining of mitotic HeLa cells was performed using α-HpTGEKP and α-pH3S10 antibodies. Figures 4 and S6 show a large overlap in the timing of both phosphorylation events. However, HpTGEKP appears slightly later than pH3S10 and persists longer in telophase. In addition, overlay images show a clear exclusion of the two signals.
Figure 4.
Temporal correlation between phosphorylation of HpTGEKP and pH3S10. HeLa cells grown on coverslips were synchronized by double-thymidine block, released and collected 8–10 h after release. Cells were stained with DAP I (blue) and immunostained with α-HpTGEKP (red) and α-pH3S10 (green). Specific mitotic stage labeling was based on chromatin morphology. (Bar, 20 µM).
The HpTGEKP phospho-epitope is an efficient mitotic marker.
Whether in the context of understanding natural human growth and development or related pathologies, like cancer, the study of the cell cycle and proliferation is largely dependent on efficient and precise biomarkers. Our results clearly demonstrate that the α-HpTGEKP antibody has great potential as a useful proliferation and mitotic marker. Due to the high abundance of the HpTGEKP phospho-epitope in mitotic cells, it is easily detectable in protein gel blot analyses (Figs. 1 and 2). In immunostaining and low magnification fluorescent microscopy, the signal generated from probing with α-HpTGEKP epitope is an indication of the proliferative state of the cellular population (Fig. 3A). At high magnification, linker phosphorylation marks mid-prophase, immediately subsequent to the condensation of DNA (Figs. 3C and S4). The spatial distribution of HpTGEKP phosphorylation offers a clearly visible indicator of progression through prophase. The breakdown of the nuclear envelope is a major morphological event of late prophase.5 However, the exact timing of the breakdown of the nuclear envelope barrier is difficult to assess based on the visible morphological changes of the envelope itself. As mentioned earlier, the linker phosphorylation excludes the ZFPs from mitotic chromatin but is not sufficient for cytoplasmic export; which happens at later mitotic stages in the absence of the nuclear envelope.27 Therefore, the spatial distribution of HpTGEKP signal from the nuclear to the cytoplasmic space in late prophase can be used to mark the exact breaking point of the nuclear envelope barrier (Fig. S4).
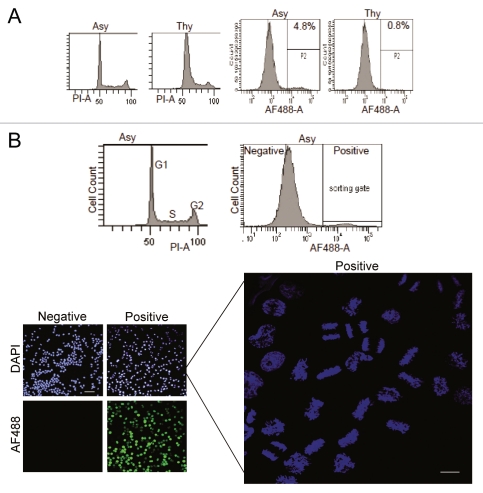
The efficiency of the HpTGEKP immunostaining makes it a good candidate for the quantitative calculation of the mitotic index and enrichment of mitotic cells using flow cytometry analyses. To assess this, asynchronously growing or thymidine-arrested HeLa cells were trypsinized and fixed, and then each population was aliquoted into two fractions. One fraction was immunostained with α-HpTGEKP and the other stained with propidium iodide as a control for cell cycle distribution. As shown in Figure 5A (two right parts), the signal generated from fluorescent immunostaining was very strong (about 100-fold higher than the auto and background fluorescence), allowing gating and calculation of positive cells. According to this result, 4.8% of the asynchronously growing HeLa cells were mitotic compared with only 0.8% in the thymidine arrested cells. This indicates that the thymidine block was not entirely complete and some mitotic cells are present in the population. Conforming to this result, the propidium iodide staining also showed a small peak of 4N DNA content (Fig. 5A, two left parts). pH3S10 immunostaining is the most commonly used marker to calculate mitotic index. To compare mitotic index calculations from these two markers, asynchronous HeLa cells were immunostained with α-HpTGEKP or α-pH3S10, and a third fraction of the cells was stained with propidium iodide to control for normal asynchronous cell cycle distribution (not shown). When calculated using either antibody, the mitotic index was very similar (Results from a triplicate sample: 4.7% ± 0.36 for HpTGEKP and 4.63% ± 0.32 for pH3S10).
Figure 5.
Mitotic index (MI) calculation and enrichment of mitotic cells using α-HpTGEKP immunostaining. (A) Mitotic index calculation for asynchronous (Asy) and thymidine-arrested (Thy) HeLa cells, based on α-HpTGEKP staining (right parts). A fraction of the cells were stained with propidium iodide for cell cycle analysis based on DNA content (left parts). (B) Asynchronous HeLa cells were trypsinized and collected. A fraction of the cells was stained with propidium iodide for cell cycle analysis based on DNA content (B, upper left part). Another fraction was immunostained with α-HpTGEKP, the secondary antibody was coupled to Alexa Fluor 488 fluorescent dye. There was about 100-fold difference in the AF488 intensities between background and positive HpTGEKP staining, allowing gating and sorting of cells (B, upper right part). Samples from the sorted (negative and positive) fractions were stained with DAPI and observed using a fluorescent microscope (Bar, 100 µM) (B, lower left images). The right part shows higher magnification examination of the DNA content of the positive cells showing various mitotic phases (Bar, 20 µM).
In addition, we explored the potential capacity of the new marker for enrichment and purification of mitotic cells. For this purpose, an asynchronous population of HeLa cells was immunostained with α-HpTGEKP. Negatively and positively stained cells were sorted using flow cell cytometry. Samples from each fraction were stained with DAPI and examined under a fluorescent microscope. Figure 5B shows the sorting efficiency; all the positively sorted cells displayed AF488 (HpTGEKP) staining, but none of the negatively sorted cells. When examined at higher magnification, all of the positively sorted cells displayed mitotic condensed chromosomes, indicating high sorting efficiency of mitotic cells (Fig. 5B). Also, all the mitotic stages were represented in the positive fraction. To allow flexibility in subsequent biochemical analyses alternative fixation methods (such as ethanol fixation) were tested, and yielded comparable results in all assays (data not shown).
Discussion
The existence of a common mechanism for the inactivation of all C2H2 ZFPs during mitosis was proposed about nine years ago.12 It remains unproven and unexplored in the scientific literature. Here, we provide evidence for the existence and global nature of this mechanism. It remains unproven and unexplored in the scientific literature. This is of fundamental importance, since the family of transcription factors regulated by this pathway is the largest in the human genome encompassing hundreds of members.13
The study of the simultaneous modification of hundreds of proteins is challenging. To enable the characterization of this biological phenomenon, we exploited the high conservation of the linker peptides, which is the basis of the common phosphorylation pathway. ZFP linker peptides display high sequence similarities with some degree of divergence that would be tolerated as a phosphorylation consensus site, but not for immunogenic specificity. Interestingly, more than half of these ZFP linkers are composed of the same five-amino acid sequence (TGEKP).14 In addition, the linker of C2H2 ZFPs is always preceded by the second histidine residue of the preceding zinc finger. We hypothesized that an HpTGEKP phospho-epitope would have enough immunogenicity and specificity to allow the accurate and global detection of this modification on ZFPs. Therefore, we generated a polyclonal antibody against this epitope (α-HpTGEKP).
The specificity of the new antibody to its phosphorylated target sequence was proven using different approaches, with synthetic peptides or individual ZFPs (YY1 and CTCF), as shown in the results section. We used this antibody in protein gel blots to show the massive and global nature of this phosphorylation event on a large number of proteins. Using immunostaining techniques and fluorescent microscopy, we showed the tight synchrony of this massive event and its mitotic exclusivity. Taken together, these temporal and spatial observations fit perfectly with the proposed biological function of this modification, which is the inactivation of DNA binding activity of C2H2 ZFPs and their exclusion from condensed chromatin during mitosis.12
Although inactivation of the basic transcriptional machinery would probably be sufficient to turn off the transcriptional activity of the cell,3,7 multiple pathways also exist for the inactivation of arrays of specific transcription factors in mitosis.3 Redundancy in biological processes is not uncommon, especially for critical events. On the other hand, multifaceted effects of biological processes are not uncommon either. The implications of mitotic division extend beyond the numeric increase of cellular populations. Interestingly, many of the inhibitory pathways of transcription factors during mitosis are associated with reduced DNA binding affinity which results in exclusion of these factors from condensed DNA. This massive reshuffling and redistribution of proteins has been proposed to be a window for the reestablishment of gene regulation and expression programs.45 Further investigation of the global inactivation of the C2H2 ZFPs could also provide significant insights into the understanding of cellular differentiation and developmental transitions.
Zinc finger modules have also been shown to mediate protein-protein interactions.46 Although this aspect of the zinc finger function is significantly less studied than DNA binding activity, it is well established for many proteins.46–48 In addition, these types of interactions have been shown to be regulated by phosphorylation in several contexts.47,48 The role of ZFP linker peptides in protein-protein interactions is not clear. An interesting possibility to consider is that linker phosphorylation could also affect protein-protein interactions and mediate mitosis-specific protein complex formation or impact the equal distribution (or segregation) of ZFPs into the two new daughter cells. For example, mitotic ZFP Sp1 was shown to biochemically associate with F-actin.49 In addition, Sp1 foci were shown to colocalize with microfilaments during mitosis.49 Further research is needed to investigate the effect of linker phosphorylation on protein-protein interactions and cellular distribution during mitosis.
The high abundance and mitotic exclusivity of the HpTGEKP phosphoepitope make it an excellent candidate for use as a mitotic and proliferation marker. Proliferation biomarkers are of indispensable value in cell cycle research. More importantly, many of these markers have been translated into valuable cancer prognostic and diagnostic tools,50 particularly those used to assess the mitotic index of a cellular mixture.51–54 In this study we have shown the capacity of the α-HpTGEKP antibody for the calculation of the mitotic index, whether with the microscope or in a more automated approach, when coupled with flow cytometry. Although the mitotic occurrence and exclusivity of this event was observed in all of the cell types that we tested, differences were observed in the intensity and the banding patterns of the HpTGEKP containing proteins among different cell types. An intriguing possibility is that this could be due to tissue specific expression of some ZFPs, tissue specific differences in the regulation of this phosphorylation pathway or both. Also, these differences are observed in cells derived from different cancers types. Future work in our laboratory will explore the regulation of this pathway in different tumor cells and the possible diagnostic and prognostic value of the new antibody.
Finally, the identification of the kinase(s) and the signaling pathways governing this global mitotic event is of primary importance. Elucidation of these pathways will have significant implications on our understanding of cellular division and differentiation, possibly presenting novel therapeutic targets for proliferative diseases, like cancer. However, the identification of this kinase has been challenging since the conserved linker elements do not conform to known phosphorylation consensus sites. Previous attempts to identify the linker kinase were not successful.12 Screening of several known mitotic kinases in our laboratory did not yield positive results. Current work in our laboratory is focused on identifying the kinase(s) responsible for the global phosphorylation of C2H2 ZFPs during mitosis.
Materials and Methods
Cell culture and synchronization.
HeLa, HeLa-Flag-YY1 and U2OS cells were grown at 37°C in 5% CO2 in DMEM (Cellgro, Cat# 10-013-CV) with 2 mM L-glutamine. For BJ cells, the growth medium was supplemented with 1% nonessential amino acids (Sigma, Cat# M7145). HCT116 cells were grown in McCoy's 5A medium (Cellgro, Cat# 10-050-CV). All growth media were supplemented with 10% fetal bovine serum (FBS; Sigma, Cat# F0926) and 1% penicillin-streptomycin (Cellgro, Cat# 30-002-CI). HeLa-Flag-YY1 is a stable cell line overexpressing Flag-YY1, constructed as previously described in reference 27. Blocking cells at pro-metaphase of mitosis was performed by adding nocodazole (Sigma, Cat# M1404) (50 ng/ml) for 18 h. To block cells in S phase, thymidine (Sigma, Cat# T1895) was added to a final concentration of 2 mM for 18 h. For double-thymidine synchronization at early S phase, cells were blocked for 18 h, released for 9 h and blocked again for 17 h.
Plasmids and transfections.
pCS2(+)-Flag-YY1 wild type (WT) or (T348A) mutant were constructed as previously described in reference 27. For transient overexpression, cells were transfected with Lipofectamine 2000 transfection reagent (Invitrogen, Cat# 11668-019), according to the manufacturer's instructions.
Antibodies.
α-YY1 (Cat# sc-7341), α-cyclin B1 (Cat# sc-245), α-GAPDH (Cat# sc-25778), α-Plk1 (Cat# sc-5585), α-CTCF (Cat# sc-271474), α-lamin A/C (Cat# sc-7292), α-pH3S10 (Cat# sc-8656-R) and α-GFP (Cat# sc-9996) antibodies were purchased from Santa Cruz Biotechnology. α-Flag (Resin M2) antibody was purchased from Sigma (Cat# A2220). The rabbit polyclonal antibody α-HpTGEKP was raised against the phospho-antigen: Ac-C(Ahx)HpTGEKP-amide and was prepared for our laboratory at New England Peptide company.
WCE, immunoprecipitation (IP) and protein gel blotting.
WCE, immunoprecipitation (IP) and protein gel blotting were performed as previously described in reference 27. Briefly, for WCE preparation, cells were washed with ice-cold PBS and lysed in freshly prepared lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 0.5% Triton-X 100, 1 mM EDTA, 2.5 mM EGTA, 10 mM NaF and 5 mM β-glycerophosphate), on ice. For IP, cell lysates were pre-cleared with protein A/G agarose beads (Santa Cruz, Cat# sc-2003) for 2 h at 4°C. For CTCF IP, cell lysates were incubated with α-CTCF for 3 h at 4°C followed by addition of protein A/G beads for an additional hour. For α-Flag IP, resin-coupled antibody (M2) was added for 4 h. For phosphatase treatment, WCEs or IPs were incubated at 30°C for 1 h with or without Lambda phosphatase (New England Biolabs, Cat# P0753), in the presence of 2 mM MnCl2. For protein gel blotting of WCE and IPs, proteins were separated on SDS-PAGE gels and transferred to a nitrocellulose membrane. After transfer, membranes were blocked in TBSTM (Tris-buffered saline, 0.5% Tween20, 5% Milk) for 30 min. Probing with the indicated primary antibodies was for 2 h at room temperature (RT), or overnight at 4°C. Horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit secondary antibodies (GE Healthcare, Cat# NA934V) were added for 1 h at RT. Visualization of bands was by exposure to X-ray films after incubating the membrane with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Cat# 34078). For the dot-blot experiment, synthetic peptides HTGEKP (non-phosphorylated) and HpTGEKP (phosphorylated) were custom synthesized for our laboratory at New England Peptide. Indicated amount of peptides were spotted on a nitrocellulose membrane and allowed to dry for 1 h. Then, the membrane was wetted in blocking buffer and probed as indicated above. For the antibody pre-blocking experiment, α-HpTGEKP was incubated overnight at 4°C with 1 µg/ml of HTGEKP or HpTGEKP peptides in blocking solution, before addition to the WCE blot.
Indirect immunofluorescence.
Cells seeded on coverslips were grown and synchronized as mentioned above and indicated in the figures. For immunostaining, cells were fixed with 3.7% formaldehyde for 10 min at RT, followed by permeabilization for 10 min with 0.2% Triton-X 100. Cells were blocked in TBST (with 3% IgG-free BSA) for an hour, then incubated with the indicated primary antibodies for 1 h at RT. Anti-mouse and anti-rabbit secondary antibodies, conjugated to Alexa-Fluor 546 (Cat# A10040) and 647 dyes (Cat# 31571), were purchased from Molecular Probes. DNA was stained with DAPI (2 µg/ml). For phosphatase treatment, fixed cells on coverslips were incubated at 30°C for 1 h with or without Lambda phosphatase, in the presence of 2 mM MnCl2. Images were captured using a confocal fluorescent microscope (Leica micro-systems).
Flow cytometry.
For flow cytometry analysis, cells were collected by trypsinization. For cell cycle analysis based on DNA content, cells were fixed with 70% ethanol, washed and resuspended in propidium iodide (PI) solution (50 µg/ml PI, 200 µg/ml RNase A, 0.1% Triton-X 100 in PBS) and incubated for 30 min at 30°C. For counting and enrichment of mitotic cells based on HpTGEKP staining, indirect immunostaining was performed as detailed above. For this procedure, cells were immunostained in suspension using α-HpTGEKP or α-pH3S10 as primary antibodies, then with Alexa-Fluor 488 conjugated anti-rabbit secondary antibody (Molecular Probes, Cat# A-11017). Cells were analyzed on a fluorescence-activated cell sorter (FACS; FACS Canto; Becton Dickinson), and images were generated using BD FACS Diva software. For counting and enrichment of mitotic cells gating was adjusted based on the positive HpTGEKP signal (as shown in Fig. 5). Enrichment of mitotic cells was performed on a FACSAria instrument.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Ruth Didier, the director of the FSU COM confocal microscopy and flow cytometry facilities, for her excellent technical assistance. This work was supported by the Florida State University College of Medicine.
Abbreviations
- ZFP
zinc finger protein
- WCE
whole cell extract
Supplementary Material
References
- 1.Taylor JH. Nucleic acid synthesis in relation to the cell division cycle. Ann NY Acad Sci. 1960;90:409–421. doi: 10.1111/j.1749-6632.1960.tb23259.x. [DOI] [PubMed] [Google Scholar]
- 2.Prescott DM, Bender MA. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp Cell Res. 1962;26:260–268. doi: 10.1016/0014-4827(62)90176-3. [DOI] [PubMed] [Google Scholar]
- 3.Gottesfeld JM, Forbes DJ. Mitotic repression of the transcriptional machinery. Trends Biochem Sci. 1997;22:197–202. doi: 10.1016/S0968-0004(97)01045-1. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Balbás MA, Dey A, Rabindran SK, Ozato K, Wu C. Displacement of sequence-specific transcription factors from mitotic chromatin. Cell. 1995;83:29–38. doi: 10.1016/0092-8674(95)90231-7. [DOI] [PubMed] [Google Scholar]
- 5.Güttinger S, Laurell E, Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat Rev Mol Cell Biol. 2009;10:178–191. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 6.Bellier S, Dubois MF, Nishida E, Almouzni G, Bensaude O. Phosphorylation of the RNA polymerase II largest subunit during Xenopus laevis oocyte maturation. Mol Cell Biol. 1997;17:1434–1440. doi: 10.1128/mcb.17.3.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gebara MM, Sayre MH, Corden JL. Phosphorylation of the carboxy-terminal repeat domain in RNA polymerase II by cyclin-dependent kinases is sufficient to inhibit transcription. J Cell Biochem. 1997;64:390–402. doi: 10.1002/(SICI)1097-4644(19970301)64:3<390::AID-JCB6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Long JJ, Leresche A, Kriwacki RW, Gottesfeld JM. Repression of TFIIH transcriptional activity and TFIIH-associated cdk7 kinase activity at mitosis. Mol Cell Biol. 1998;18:1467–1476. doi: 10.1128/mcb.18.3.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lüscher B, Eisenman RN. Mitosis-specific phosphorylation of the nuclear oncoproteins Myc and Myb. J Cell Biol. 1992;118:775–784. doi: 10.1083/jcb.118.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segil N, Roberts SB, Heintz N. Mitotic phosphorylation of the Oct-1 homeodomain and regulation of Oct-1 DNA binding activity. Science. 1991;254:1814–1816. doi: 10.1126/science.1684878. [DOI] [PubMed] [Google Scholar]
- 11.Caelles C, Hennemann H, Karin M. M-phase-specific phosphorylation of the POU transcription factor GHF-1 by a cell cycle-regulated protein kinase inhibits DNA binding. Mol Cell Biol. 1995;15:6694–6701. doi: 10.1128/mcb.15.12.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dovat S, Ronni T, Russell D, Ferrini R, Cobb BS, Smale ST. A common mechanism for mitotic inactivation of C2H2 zinc finger DNA-binding domains. Genes Dev. 2002;16:2985–2990. doi: 10.1101/gad.1040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tupler R, Perini G, Green MR. Expressing the human genome. Nature. 2001;409:832–833. doi: 10.1038/35057011. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe SA, Nekludova L, Pabo CO. DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct. 2000;29:183–212. doi: 10.1146/annurev.biophys.29.1.183. [DOI] [PubMed] [Google Scholar]
- 15.Laity JH, Lee BM, Wright PE. Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol. 2001;11:39–46. doi: 10.1016/S0959-440X(00)00167-6. [DOI] [PubMed] [Google Scholar]
- 16.Wuttke DS, Foster MP, Case DA, Gottesfeld JM, Wright PE. Solution structure of the first three zinc fingers of TFIIIA bound to the cognate DNA sequence: determinants of affinity and sequence specificity. J Mol Biol. 1997;273:183–206. doi: 10.1006/jmbi.1997.1291. [DOI] [PubMed] [Google Scholar]
- 17.Choo Y, Klug A. A role in DNA binding for the linker sequences of the first three zinc fingers of TFIIIA. Nucleic Acids Res. 1993;21:3341–3346. doi: 10.1093/nar/21.15.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiesen HJ, Bach C. DNA recognition of C2H2 zinc-finger proteins. Evidence for a zinc-finger-specific DNA recognition code. Ann NY Acad Sci. 1993;684:246–249. doi: 10.1111/j.1749-6632.1993.tb32299.x. [DOI] [PubMed] [Google Scholar]
- 19.Cui X, Ji D, Fisher DA, Wu Y, Briner DM, Weinstein EJ. Targeted integration in rat and mouse embryos with zinc-finger nucleases. Nat Biotechnol. 2011;29:64–67. doi: 10.1038/nbt.1731. [DOI] [PubMed] [Google Scholar]
- 20.Jamieson AC, Miller JC, Pabo CO. Drug discovery with engineered zinc-finger proteins. Nat Rev Drug Discov. 2003;2:361–368. doi: 10.1038/nrd1087. [DOI] [PubMed] [Google Scholar]
- 21.Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu Rev Biochem. 2010;79:213–231. doi: 10.1146/annurev-biochem-010909-095056. [DOI] [PubMed] [Google Scholar]
- 22.Santiago Y, Chan E, Liu PQ, Orlando S, Zhang L, Urnov FD, et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc Natl Acad Sci USA. 2008;105:5809–5814. doi: 10.1073/pnas.0800940105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thukral SK, Morrison ML, Young ET. Alanine scanning site-directed mutagenesis of the zinc fingers of transcription factor ADR1: residues that contact DNA and that transactivate. Proc Natl Acad Sci USA. 1991;88:9188–9192. doi: 10.1073/pnas.88.20.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clemens KR, Zhang P, Liao X, McBryant SJ, Wright PE, Gottesfeld JM. Relative contributions of the zinc fingers of transcription factor IIIA to the energetics of DNA binding. J Mol Biol. 1994;244:23–35. doi: 10.1006/jmbi.1994.1701. [DOI] [PubMed] [Google Scholar]
- 25.Laity JH, Dyson HJ, Wright PE. DNA-induced alpha-helix capping in conserved linker sequences is a determinant of binding affinity in Cys(2)-His(2) zinc fingers. J Mol Biol. 2000;295:719–727. doi: 10.1006/jmbi.1999.3406. [DOI] [PubMed] [Google Scholar]
- 26.Jantz D, Berg JM. Reduction in DNA-binding affinity of Cys2His2 zinc finger proteins by linker phosphorylation. Proc Natl Acad Sci USA. 2004;101:7589–7593. doi: 10.1073/pnas.0402191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizkallah R, Hurt MM. Regulation of the transcription factor YY1 in mitosis through phosphorylation of its DNA-binding domain. Mol Biol Cell. 2009;20:4766–4776. doi: 10.1091/mbc.E09-04-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, et al. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen RQ, Yang QK, Lu BW, Yi W, Cantin G, Chen YL, et al. CDC25B mediates rapamycin-induced oncogenic responses in cancer cells. Cancer Res. 2009;69:2663–2668. doi: 10.1158/0008-5472.CAN-08-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayya V, Lundgren DH, Hwang SI, Rezaul K, Wu L, Eng JK, et al. Quantitative phosphoproteomic analysis of T cell receptor signaling reveals system-wide modulation of protein-protein interactions. Sci Signal. 2009;2:46. doi: 10.126/scisignal.2000007. [DOI] [PubMed] [Google Scholar]
- 31.Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- 32.Moritz A, Li Y, Guo A, Villen J, Wang Y, MacNeill J, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3:64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan M, Morgan DO. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol. 2007;8:894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]
- 34.Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. Biochim Biophys Acta. 1997;1332:49–66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 35.Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- 36.Castellano G, Torrisi E, Ligresti G, Malaponte G, Militello L, Russo AE, et al. The involvement of the transcription factor Yin Yang 1 in cancer development and progression. Cell Cycle. 2009;8:1367–1372. doi: 10.4161/cc.8.9.8314. [DOI] [PubMed] [Google Scholar]
- 37.Zaravinos A, Spandidos DA. Yin yang 1 expression in human tumors. Cell Cycle. 2010;9:512–522. doi: 10.4161/cc.9.3.10588. [DOI] [PubMed] [Google Scholar]
- 38.Bushmeyer S, Park K, Atchison ML. Characterization of functional domains within the multifunctional transcription factor, YY1. J Biol Chem. 1995;270:30213–30220. doi: 10.1074/jbc.270.50.30213. [DOI] [PubMed] [Google Scholar]
- 39.Austen M, Luscher B, Luscher-Firzlaff JM. Characterization of the transcriptional regulator YY1. The bipartite transactivation domain is independent of interaction with the TATA box-binding protein, transcription factor IIB, TAFII55 or cAMP-responsive element-binding protein (CPB)-binding protein. J Biol Chem. 1997;272:1709–1717. doi: 10.1074/jbc.272.3.1709. [DOI] [PubMed] [Google Scholar]
- 40.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin D, Pantoja C, Minan AF, Valdes-Quezada C, Molto E, Matesanz F, et al. Genome-wide CTCF distribution in vertebrates defines equivalent sites that aid the identification of disease-associated genes. Nat Struct Mol Biol. 2011;18:708–714. doi: 10.1038/nsmb.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene. 2001;20:3021–3027. doi: 10.1038/sj.onc.1204326. [DOI] [PubMed] [Google Scholar]
- 43.Nowak SJ, Corces VG. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–220. doi: 10.1016/j.tig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Johansen KM, Johansen J. Regulation of chromatin structure by histone H3S10 phosphorylation. Chromosome Res. 2006;14:393–404. doi: 10.1007/s10577-006-1063-4. [DOI] [PubMed] [Google Scholar]
- 45.Egli D, Birkhoff G, Eggan K. Mediators of reprogramming: transcription factors and transitions through mitosis. Nat Rev Mol Cell Biol. 2008;9:505–516. doi: 10.1038/nrm2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brayer KJ, Segal DJ. Keep your fingers off my DNA: protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem Biophys. 2008;50:111–131. doi: 10.1007/s12013-008-9008-5. [DOI] [PubMed] [Google Scholar]
- 47.Jones DR, Prasad AA, Chan PK, Duncker BP. The Dbf4 motif C zinc finger promotes DNA replication and mediates resistance to genotoxic stress. Cell Cycle. 2010;9:2018–2026. doi: 10.4161/cc.9.10.11752. [DOI] [PubMed] [Google Scholar]
- 48.Dudekula S, Lee MH, Hsu LJ, Chen SJ, Chang NS. Zfra is a small wizard in the mitochondrial apoptosis. Aging (Albany NY) 2010;2:1023–1029. doi: 10.18632/aging.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He S, Davie JR. Sp1 and Sp3 foci distribution throughout mitosis. J Cell Sci. 2006;119:1063–1070. doi: 10.1242/jcs.02829.. [DOI] [PubMed] [Google Scholar]
- 50.Whitfield ML, George LK, Grant GD, Perou CM. Common markers of proliferation. Nat Rev Cancer. 2006;6:99–106. doi: 10.1038/nrc1802. [DOI] [PubMed] [Google Scholar]
- 51.Baak JP, Gudlaugsson E, Skaland I, Guo LH, Klos J, Lende TH, et al. Proliferation is the strongest prognosticator in node-negative breast cancer: significance, error sources, alternatives and comparison with molecular prognostic markers. Breast Cancer Res Treat. 2009;115:241–254. doi: 10.1007/s10549-008-0126-y. [DOI] [PubMed] [Google Scholar]
- 52.Colman H, Giannini C, Huang L, Gonzalez J, Hess K, Bruner J, et al. Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. Am J Surg Pathol. 2006;30:657–664. doi: 10.1097/01.pas.0000202048.28203.25. [DOI] [PubMed] [Google Scholar]
- 53.Kim YJ, Ketter R, Steudel WI, Feiden W. Prognostic significance of the mitotic index using the mitosis marker anti-phosphohistone H3 in meningiomas. Am J Clin Pathol. 2007;128:118–125. doi: 10.1309/HXUNAG34B3CEFDU8. [DOI] [PubMed] [Google Scholar]
- 54.van Diest PJ, van der Wall E, Baak JP. Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol. 2004;57:675–681. doi: 10.1136/jcp.2003.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.