Abstract
The mechanisms of the saturable component of long-chain fatty acid (LCFA) transport across the small intestinal epithelium and its regulation by a high-fat diet (HFD) are uncertain. It is hypothesized here that the putative fatty acid translocase/CD36 and intestinal alkaline phosphatases (IAPs) function together to optimize LCFA transport. Phosphorylated CD36 (pCD36) was expressed in mouse enterocytes and dephosphorylated by calf IAP (CIAP). Uptake of fluorescently tagged LCFA into isolated enteroctyes was increased when cells were treated with CIAP; this was blocked with a specific CD36 inhibitor. pCD36 colocalized in enterocytes with the global IAP (gIAP) isozyme and, specifically, coimmunoprecipitated with gIAP, but not the duodenal-specific isozyme (dIAP). Purified recombinant gIAP dephosphorylated immunoprecipitated pCD36, and antiserum to gIAP decreased initial LCFA uptake in enterocytes. Body weight, adiposity, and plasma leptin and triglycerides were significantly increased in HFD mice compared with controls fed a normal-fat diet. HFD significantly increased immunoreactive CD36 and gIAP, but not dIAP, in jejunum, but not duodenum. Uptake of LCFA was increased in a CD36-dependent manner in enterocytes from HFD mice. It is concluded that CD36 exists in its phosphorylated and dephosphorylated states in mouse enterocytes, that pCD36 is a substrate of gIAP, and that dephosphorylation by IAPs results in increased LCFA transport capability. HFD upregulates CD36 and gIAP in parallel and enhances CD36-dependent fatty acid uptake. The interactions between these proteins may be important for efficient fat transport in mouse intestine, but whether the changes in gIAP and CD36 in enterocytes contribute to HFD-induced obesity remains to be determined.
Keywords: intestinal plasticity, phosphorylation, enterocytes, adiposity, obesity
in addition to simple diffusion, long-chain fatty acids (LCFAs) cross plasma membranes by a saturable mechanism that requires one or more proteins (for review see Refs. 16 and 35). One protein implicated in this process is the class B scavenger receptor CD36 in humans and its rodent homolog, also known as fatty acid translocase (FAT). FAT/CD36 (hereafter called CD36) is a cell surface glycoprotein found in many cell types, including platelets, endothelial cells, and monocytes (2), differentiated adipocytes (23), mammary epithelial cells (17), and intestinal enterocytes (13, 41). It is probably ubiquitous among vertebrates, including mice (12), rats (58), humans (53), and other mammals (7). CD36 binds a broad spectrum of extracellular ligands, including thrombospondin-1 (2), oxidized LDL (15), collagen (52), and LCFAs (5, 29). Among numerous functions, CD36 has been demonstrated or believed to play a role in facilitation of the transport of LCFAs into adipocytes (23), platelets (49), skeletal muscle cells (9), cardiomyocytes (6, 8, 28, 32, 43, 51, 59), and enterocytes (41).
The mechanism of action of CD36 in enterocytes is unknown. In all cell types in which it has been examined, however, CD36 is extensively posttranslationally regulated, resulting in a ∼70- to 88-kDa molecule. In stably transfected human embryonic kidney cells, for example, CD36 is palmitoylated on both putative cytoplasmic tails (54); palmitoylation of the protein and its glycosylation state are important in trafficking the protein to and from the plasma membrane (27, 55) and, possibly, in localizing it to lipid rafts in the cell membrane (55). In addition, the extracellular fragment of CD36 contains two consensus phosphorylation sites, including one at Thr92. This residue is recognized by protein kinase C and, to a lesser extent, protein kinase A in 3T3 cells that have been transfected with human CD36 (26). The mechanism and regulation of phosphorylation of CD36 in vivo are uncertain but, in platelets, depend on the action of an unidentified ectokinase (19, 24). Phosphorylation of CD36 is important in determining ligand specificity in platelets (3), increasing adherence of red blood cells to human dermal microvascular endothelial cells (26), and, of particular relevance to the present investigation, decreasing palmitate uptake in platelets (19). Whether CD36 is phosphorylated in enterocytes, however, is unknown.
Dephosphorylation of CD36 is accomplished by alkaline phosphatases (26). COS cells transfected with CD36 express the protein in lipid rafts, identified by cross-linking experiments that revealed CD36 co-patching with placental alkaline phosphatase, a marker of these membranous structures (14). The association of CD36 with lipid rafts is crucial for its ability to increase LCFA uptake (14). Lipid rafts are also present on the brush border of enterocytes (21), where they are enriched in alkaline phosphatases (22); this suggests that enterocyte CD36 and intestinal alkaline phosphatases (IAPs) may interact.
Mammalian alkaline phosphatases comprise up to four distinct families, including IAPs (33, 36, 39, 41). Murine enterocytes express two phosphatases, duodenal IAP (dIAP) and global IAP (gIAP), which are products of the Akp3 and Akp6 genes, respectively; they also express low levels of the embryonic isozyme EAP (37, 39). IAPs have been implicated in the innate immune responses of the mammalian intestine and, in local pH homeostasis, along the brush border (33). They also appear to play a role in mucosal handling of fat. For example, mRNA of all three mouse intestinal phosphatases is increased in the duodenum when the fat content of the diet is changed from very low to moderate or moderately high (41). Interestingly, however, inactivation of the Akp3 gene results in greater weight gain in mice fed a high-fat diet (HFD), suggesting that dIAP may negatively regulate fat absorption (38); however, it is also possible that this may result from the concomitant upregulation of Akp6 that is observed in distal regions of the intestine in these mice (41).
In mouse small intestine, CD36 is primarily expressed in the proximal region (duodenum); more distal regions, including jejunum, express CD36, but at lower levels (41). This is consistent with the relative contributions of proximal and distal regions of the mammalian small intestine in nutrient digestion and absorption. However, Petit et al. (45) reported that the expression of CD36 mRNA in mouse jejunum is increased when the fat content of the diet is increased from 3% to 40% of total calories; moreover, exteriorized jejunal loops from such animals demonstrate an increased ability to absorb LCFAs. Although proximal regions of the intestine were not examined in the study of Petit et al., nor were CD36 protein levels, the data nonetheless suggest that distal regions of the mouse small intestine adapt to increased dietary fat content, which may depend in part on changes in CD36 expression. In humans, a chronic dietary fat content of 3% would rarely, if ever, occur; a typical Western diet may range from ∼15 to ≥45% energy from fat. Thus an animal model with controlled fat consumption that approximates this range would be an advantageous model for understanding the role of CD36 and other proteins in the intestinal adaptations to HFD in humans.
Given the importance of CD36 for LCFA uptake in other cell types, its regulation by dephosphorylation, its expression in the intestinal epithelium, its location in cellular compartments that harbor alkaline phosphatases, and the ability of CD36 to act as a substrate for alkaline phosphatase, we asked the following questions. 1) Is CD36 phosphorylated in enterocytes, and can it be dephosphorylated by IAPs? 2) Does treatment of enterocytes with IAP increase uptake of LCFAs by the cells in a CD36-dependent manner? 3) Does CD36 in enterocytes associate with one or more isozymes of mouse IAP, and, if so, is that isozyme capable of dephosphorylating phosphorylated CD36 (pCD36)? 4) Does chronic HFD induce changes in CD36 and IAP isozyme protein expression in the murine small intestine, and are these changes reflected in increased cellular uptake of LCFA?
MATERIALS AND METHODS
Animals.
Adult male C57BL/6 mice (Taconic Breeding Laboratories, Hudson, NY) were housed singly with lights on from 0700 to 1900 and fed standard rodent chow ad libitum unless otherwise noted. All procedures were approved by the Boston University Institutional Animal Care and Use Committee.
Preparation and purification of recombinant enzymes.
Chinese hamster ovary cells were transfected with pCMV Script expression vector containing a coding sequence of Akp6 in which the glycophosphatidylinositol anchoring site was replaced by a FLAG sequence, as described elsewhere (11), or a coding sequence of human tissue-nonspecific alkaline phosphatase (TNAP) D361V (TNAPD361V) mutant. TNAPD361V enzyme is also FLAG-tagged and has no detectable p-nitrophenyl phosphatase activity. The mutation was introduced by PCR-based site-directed mutagenesis, as previously described (11). Chinese hamster ovary cells stably expressing these secreted forms of gIAP and TNAPD361V were obtained after 3 wk of selection under G418. The media were replaced with Opti-MEM (Invitrogen, Carlsbad, CA) for 48 h prior to collection of the supernate. Expressed enzymes were further purified from the supernate with anti-FLAG M2 column (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's protocol, and the protein concentration was determined with a micro-bicinchoninic acid assay kit (Thermo Fisher Scientific, Waltham, MA). The activity of recombinant gIAP in a p-nitrophenyl phosphate assay was equivalent to 0.0046 U of calf IAP (CIAP) per milliliter.
Detection of pCD36.
A modification of a procedure described for platelets by Ho et al. (26) was used to detect pCD36 from mouse intestinal tissue. Duodenal tissue (considered here as the first 5 cm distal to the pylorus) was homogenized in RIPA buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 10% glycerol, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) for 30 s on ice. The cell lysate was immediately separated into two microcentrifuge tubes. Ten microliters of phosphatase inhibitor cocktail (PIC) 1 (for serine/threonine protein phosphatases and L-isozymes of alkaline phosphatase) and PIC 2 (for tyrosine protein phosphatases, acid, and alkaline phosphatases) (Sigma-Aldrich) were added to one aliquot. Both tubes (with and without PICs) were incubated at 4°C for 1 h. Lysates were centrifuged at 1,000 g for 5 min to pellet unlysed cells. The resulting supernate was added to an equal volume of 2× Laemmli buffer and boiled.
To examine effects of exogenous alkaline phosphatase treatment on CD36 phosphorylation, CD36 was immunoprecipitated from tissue homogenates solubilized in the presence of PIC 1 and 2. After centrifugation to remove unlysed cells, 125 μl of lysate were added to 500 μl of RIPA buffer and 5 μl of anti-CD36 (CD36-H-300 or CD36-N-15 antiserum, Santa Cruz Biotechnology, Santa Cruz, CA). The antibodies were generated against human CD36 and cross-react with mouse CD36 (34, 60). In preliminary studies, using Western blotting, we confirmed that the antisera primarily recognized a single protein of the expected size. In addition, after treatment with the deglycosylating enzyme peptide: N-glycosidase F (PNGaseF, New England Biolabs, Ipswich, MA), a lower ∼55-kDa immunoreactive band appeared (not shown); this is the expected mass of the nascent, nonglycosylated form of CD36 in mice (17). We also confirmed in preliminary studies that the immunoreactive band reacted with antiserum to phosphorylated (phospho) threonine, but not phosphoserine (not shown), as predicted for CD36 (3). Immunoprecipitations were incubated at 4°C overnight with shaking; 20 μl of Protein A/G PLUS-agarose beads (Santa Cruz Biotechnology) were then added for 2 h at 4°C with shaking. The beads were pelleted at 2,000 g for 3 min, washed three times in 1% NP-40 in PBS, and then washed again three times in a second buffer solution containing 0.01 M Tris, pH 7.5, 1 mM EDTA, and PBS with 0.1 M NaCl. The beads were resuspended in alkaline phosphatase buffer (Promega, Madison, WI) and incubated for 25 min at 37°C with or without 100 U of CIAP (Promega) or with 1 or 10 μl of recombinant enzymes. After incubation, the beads were pelleted by centrifugation at 2,000 g for 3 min and resuspended in an equal volume of Laemmli buffer and then boiled for SDS-PAGE.
For immunoblots, 10 μl of sample were separated by 10% Tris·HCl SDS-PAGE and transferred to polyvinylidene difluoride membranes. Membranes were blocked in 5% milk diluted in Tris-buffered saline + Tween 20 (TBST) overnight before detection with anti-CD36 (H-300), anti-phosphothreonine (clone 14B3, Santa Cruz Biotechnology), or anti-pCD36 (gift of Dr. M. Ho, University of Calgary) diluted with blocking buffer. The anti-pCD36 solution also contained a nonphosphorylated peptide (courtesy of Dr. M. Ho) to block nonspecific binding (26). After 2 h of incubation at room temperature, membranes were washed twice in TBST and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h. Membranes were washed twice in PBS before detection with enhanced chemiluminescence. Where appropriate, band densities were quantified by densitometry (UN-SCAN-IT, Silk Scientific, Orem, UT).
Coimmunoprecipitation.
Duodenal tissue was homogenized in buffer [20 mM HEPES, pH 7.4, 1 mM EDTA, 25 mM sucrose, 1% Nonidet P-40 substitute (NP-40), and protease inhibitor cocktail (catalog no. 539134, Calbiochem, Gibbstown, NJ)] and then rotated for 60 min at 4°C. Samples were centrifuged at 2,000 g for 5 min, and the protein concentration of the supernate (whole cell lysate) was determined. Samples were immunoprecipitated with CD36 antibody. Nonimmune goat serum was used as negative control. Whole cell lysate was rotated overnight at 4°C with 5 μg of CD36 antibody or 5 μl of a 1:10 dilution of nonimmune goat serum. On the following morning, Protein G PLUS-agarose beads were added, and the solutions were rotated at 4°C. After 2 h, the supernate was removed and boiled in an equal amount of 2× Laemmli buffer before separation using SDS-PAGE. The beads were washed three times in 1% NP-40 in PBS and then three times in a second buffer solution containing 0.01 M Tris, pH 7.5, 1 mM EDTA, and PBS with 0.1 M NaCl. Antigens were eluted from the beads with 30 μl of 2× sample buffer and 30 μl of 2× Laemmli SDS buffer and boiled for SDS-PAGE.
Cell isolation.
Intestinal epithelial cells were isolated according to the method of Bader et al. (4). Briefly, overnight-fasted mice were euthanized by CO2 exposure followed by decapitation, and the entire small intestine, including the connection with the stomach, was immediately removed. The animals were fasted overnight to prevent any potential acute effects of luminal fat on CD36 expression or internalization. The intestinal lumen was flushed thoroughly with modified Eagle's medium containing PIC 1 and 2 at 37°C. The distal end of the intestinal tube was tied closed with surgical thread, and a syringe was inserted through the pyloric valve to fill the tube with ∼2 ml of physiological saline (37°C) containing PIC 1, PIC 2, and 1 mM DTT to facilitate removal of mucus. The pylorus was tied closed with surgical tubing, and the intestinal tube was submerged in 37°C PBS and kneaded gently for 10 min. After incubation, the buffer in the intestinal tube was discarded and replaced with 2 ml of PBS containing PIC 1, PIC 2, 1.5 mM EDTA, and 0.5 mM DTT and submerged in 37°C PBS. The intestine was kneaded gently during incubation to liberate epithelial cells. Cells were collected by shaking the intestinal tube and washing with MEM into a 1.5-ml Eppendorf tube precooled to 4°C. All cells used for flow cytometry were pooled and counted by trypan blue exclusion to determine yield (∼2.65 × 106 cells/mouse) and viability (∼92%). Cells used for flow cytometry were washed three times in MEM containing 5% BSA; cells used for fatty acid uptake studies were washed twice in MEM. In some experiments, cells were isolated separately from duodenum and jejunum.
Fatty acid uptake.
Isolated cells were treated with one or more concentrations of CIAP (New England Biolabs) or vehicle for 25 min at 37°C and then treated with 0.5 mM sulfosuccinimidyl oleate (SSO) or vehicle (DMSO) for 5 min at 37°C. SSO is a specific, irreversible CD36 inhibitor (23, 42, 46); viability of cells at the end of an experiment was similar regardless of treatment. In one experiment, ∼106 cells were treated on ice with 10 μl of PBS or antiserum to gIAP or dIAP for 30 min, washed once in MEM, and then stored on ice. Fatty acid uptake was measured using the fluorescently labeled LCFA 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-hexadecanoic acid (BODIPY FL C16, Invitrogen; hereafter referred to as C16:bodipy), in combination with the quenching agent trypan blue, as described elsewhere (50). In some cases, a short-chain fatty acid analog, 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-idacene-3-pentanoic acid (BODIPY FL C5, or C5:bodipy), was used as a control, because CD36 specifically binds medium-chain to very long-chain fatty acids (1, 20). Approximately 200,000 live cells were added to each well of optically transparent 96-well plates before addition of 1.25 μM BODIPY FL, 2.1 mM trypan blue (Sigma-Aldrich), and 2.5 μM fatty acid-free BSA (Sigma-Aldrich) as carrier. After excitation at 485 nm, fluorescence at 528 nm was determined using a Victor III plate reader (Perkin Elmer, Waltham, MA) at room temperature. Because trypan blue cannot enter live cells, the only fluorescence detected was intracellular. Statistical significance was determined by two-way ANOVA or by paired t-tests for single time point determinations.
Flow cytometry.
Approximately 8 × 105 cells were used for each flow cytometry assay. In some cases, cells were incubated with 100 U of CIAP for 25 min at 37°C before they were blocked. The cells were blocked in 10% horse serum and labeled with primary antibody in MEM containing 5% BSA. Cells were labeled with antiserum to gIAP, CD36, or pCD36, except for a control group of cells, which were incubated without primary antibody. All cells, including controls, were then labeled with species-appropriate fluorescent secondary antibodies (catalog nos. sc-2090 and sc-3751, Santa Cruz Biotechnology), resuspended in 0.5 ml of MEM, and fixed on ice by addition of an equal volume of 2% paraformaldehyde in the dark. Flow cytometry was performed on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ). Data analyses were performed with FlowJo (Tree Star, Ashland, OR).
Effects of HFD on body weight, adiposity, and protein levels.
Mice were randomly assigned to one of two dietary groups. HFD animals were fed a diet with 45% of kilocalories from fat, 77% of which was saturated fat (Research Diets, New Brunswick, NJ). Normal-fat diet (NFD) controls were fed a diet with 15% of kilocalories from fat, 77% of which was saturated. The amount of protein was identical between the diets (20% of total calories). The complete compositions of the diets (catalog nos. D06061301 and D06061303) are available at the supplier's website (http://www.researchdiets.com). Animals were fed ad libitum.
Body weights were determined approximately twice per week. Food intake was measured weekly, as previously described (56). Energy consumption was determined using the known caloric content of the diets. Statistical significance of body weights and energy consumption was determined by two-way ANOVA followed by Bonferroni's multiple comparison method for pair-wise comparisons.
At 1, 2, 5, or 9 wk after beginning their respective diets, fasted mice were euthanized between 1000 and 1100 by CO2 followed by decapitation. Food was removed from the cages between 1800 and 1900 on the night before the animals were euthanized to eliminate the possibility of acute nutrient-induced changes in enterocyte protein levels. Trunk blood was collected, and the plasma was frozen for triglyceride analysis using the glycerol phosphate oxidase method (Pointe Scientific, Canton, MI). Perirenal and inguinal fat pads were removed and weighed to determine the adiposity index (the ratio of the combined weight of both fat pads to the residual carcass mass), as previously described (18, 56). Statistical significance between dietary groups was determined by Student's unpaired t-test.
Small intestines were removed in two sections. The first 5-cm segment beyond the pyloric valve was considered the proximal segment, or duodenum, and the next 5-cm segment was considered the distal segment, or a portion of the jejunum. All tissue was immediately frozen on dry ice and stored at −80°C for subsequent analysis of CD36 and IAP proteins.
Protein extracts of intestines were boiled for 5 min before separation using SDS-PAGE. The extracts were transferred to polyvinylidene difluoride membranes, which were blocked with 5% milk and then probed with gIAP or dIAP antiserum. Anti-dIAP is highly specific for dIAP; anti-gIAP recognizes gIAP but also dIAP. Membranes were washed, incubated in the appropriate secondary antibody for 60 min, and washed again. Membranes were developed with enhanced chemiluminescence. Membranes that were subsequently probed for β-actin were stripped in Restore Western Blot Stripping Buffer (Thermo Fisher Scientific), probed with a β-actin antibody (Sigma-Aldrich) and appropriate secondary antibody, and then subjected to enhanced chemiluminescence.
RESULTS
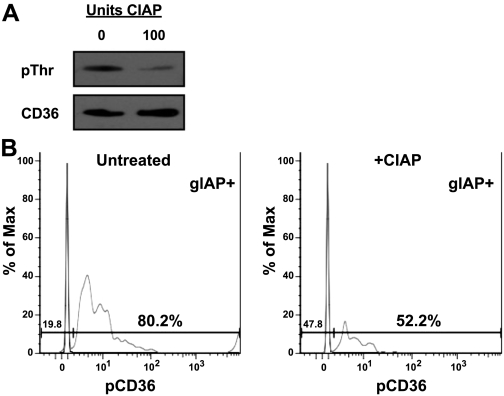
In preliminary experiments with CD36 immunoprecipitated from whole homogenates of mouse intestinal tissue, we observed that antisera to CD36 and to phosphothreonine recognized a 75-kDa band on Western blots and that the intensity of the phosphothreonine band was decreased after treatment of homogenates with CIAP (Fig. 1A) and increased by pretreatment with PIC 1 and PIC 2 (not shown). This suggested that mouse intestine normally expresses CD36 in the phosphorylated and nonphosphorylated forms. Because tissue homogenates may contain lysates of enterocytes and vascular endothelial cells, however, and CD36 is constitutively phosphorylated in human dermal microvascular endothelial cells (26), it was necessary to determine if the immunoreactive pCD36 observed in mouse intestinal lysates was expressed by enterocytes or contaminating endothelial cells. Flow cytometry was used to label cells with antisera that reacted with gIAP, a specific marker of enterocytes in intestinal tissue (40), and with antisera against pCD36 or CD36. The number of gIAP- and CD36-positive cells did not change. The number of gIAP-positive cells did not change after treatment with CIAP, as expected; however, pCD36 labeling of all cells was decreased after treatment with CIAP. When the data were gated to count only cells that expressed the enterocytic marker gIAP, phosphospecific CD36 antiserum colabeled 80.2% of cells that stained with anti-gIAP (Fig. 1B). Colabeling was reduced to 52.2% when cells were pretreated with CIAP (Fig. 1B). Control cells labeled only with fluorescently tagged secondary antibodies did not show significant staining and are shown overlaid with each experiment.
Fig. 1.
Immunoreactive CD36, its sensitivity to dephosphorylation, and its localization to enterocytes in mouse small intestine. A: small intestines from adult male mice were homogenized in the presence of phosphatase inhibitor cocktails (PIC 1 and PIC 2) and then washed to remove the inhibitors. CD36 was immunoprecipitated from the homogenates, and immunoprecipitates were incubated for 25 min at 37°C with or without 100 U of calf intestinal alkaline phosphatase (CIAP); samples were immunoblotted for phosphothreonine (pThr), stripped, and then reprobed for total CD36. CD36 and phosphothreonine bands were identical. B: acutely isolated intestinal epithelial cells pooled from the first 10 cm of intestine were incubated for 25 min at 37°C in the presence or absence of 100 U of CIAP and then incubated with antiserum to the enterocyte marker global intestinal alkaline phosphatase (gIAP) and to phosphorylated CD36 (pCD36). After gating for gIAP-positive cells, pCD36 staining in the absence or presence of CIAP treatment is shown. In both histograms, a gate was set using unstained controls (horizontal line), and percentage of gIAP-positive cells that also stained positive for pCD36 above this control is indicated (80.2 and 52.2%, respectively). Upward spikes represent no-primary-antibody control for pCD36 staining. Results are shown as percentage of maximum staining for pCD36.
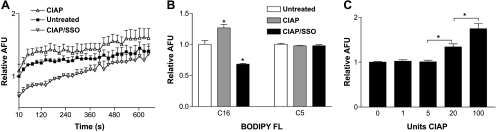
Enterocytes were then isolated for assessment of CD36-dependent LCFA uptake and its sensitivity to CIAP treatment. Cells were first pretreated with CIAP and then incubated with the irreversible CD36 inhibitor SSO or vehicle. All cells were then incubated with the C16:bodipy-labeled LCFA in the presence of the quenching agent trypan blue. Fatty acid uptake was monitored by emission at 528 nm beginning 10 s after addition of the fatty acid analog and trypan blue. Treatment of cells with CIAP significantly increased fatty acid uptake compared with untreated cells; this effect was eliminated by incubation with the CD36 inhibitor SSO (Fig. 2A). At later time points, some of the intracellular fluorescence shifts to a different wavelength when the analog is incorporated into triglycerides, and some of the analog may diffuse out of the cell. To focus strictly on the saturable component of fatty acid uptake, therefore, in subsequent experiments only the initial rate of uptake (i.e., the initial time point) was quantified, as previously described by others (50). There was no effect of CIAP or SSO on the initial uptake of a short-chain analog (C5:bodipy; Fig. 2B). The effect of CIAP on the initial uptake of C16:bodipy was dose-dependent (Fig. 2C).
Fig. 2.
Uptake of a fluorescently labeled long-chain fatty acid (LCFA) analog into isolated mouse enterocytes. A: cells prepared from pools of combined duodenum and jejunum were incubated with vehicle, CIAP (100 U), or CIAP + the CD36 inhibitor sulfosuccinimidyl oleate (SSO, 0.5 mM) and then with a C16:bodipy-labeled LCFA analog. Intracellular fluorescence, shown as arbitrary fluorescence units (AFU), is an indicator of uptake of the analog. Values are means ± SE from 3 pools of mice run separately, each with 3 technical replicates per experiment. Uptake was significantly greater for CIAP- than vehicle- or CIAP/SSO-treated cells. B: no effect of CIAP on initial rate of uptake of a short-chain C5:bodipy analog. Data for the C16 analog are reproduced from the initial time point in A and normalized to untreated values for each analog. *Significantly different (P < 0.001) from respective untreated group. C: effect of CIAP on uptake of C16:bodipy analog was concentration-dependent. *Significantly different from each other (P < 0.001).
Based on the colocalization of pCD36 and gIAP in enterocytes and the effects of CIAP and SSO on fat uptake by these cells, we next determined whether CD36 physically interacted in vivo with a murine IAP and, if so, which IAP(s). As shown in Fig. 3, gIAP, but not dIAP, specifically coimmunoprecipitated with CD36. The antiserum used to detect gIAP cross-reacts with dIAP; however, the lack of staining with the dIAP antiserum (which is specific for dIAP) indicated that gIAP accounted for all the observed signal. The size range of the immunoreactive protein in the supernate was broader than that of the pellet. This was expected because of the varying amounts of posttranslational glycosylation of the protein as it matures through the secretory pathway. The single higher band in the pellet likely corresponds to the fully glycosylated mature protein, not recently translated protein that is still undergoing modification.
Fig. 3.
CD36 coimmunoprecipitates with gIAP, but not duodenal intestinal alkaline phosphatase (dIAP). CD36 was immunoprecipitated (ip) from duodenal tissue homogenates. Nonimmune normal goat serum was used as negative control. Resulting pellets and supernates were prepared for Western blotting. Membranes were stained for gIAP or dIAP. Both phosphatases are extensively posttranslationally modified and show a range in molecular mass from 75 to 110 kDa. The most mature form (110 kDa) of gIAP coimmunoprecipitated with anti-CD36. Bands in supernate lanes represent IAP that did not immunoprecipitate and were therefore not associated with CD36; broad range in molecular mass presumably reflects IAP of varying glycosylation states. Loading amounts between supernate and pellet are not quantitative with respect to each other. Each blot is representative of 3 separate experiments.
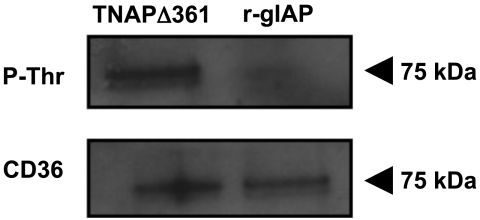
To determine if gIAP could dephosphorylate mouse intestinal CD36, CD36 was immunoprecipitated from mouse small intestine in the presence of PIC 1 and PIC 2. After immunoprecipitation and washing, the beads and their attached CD36 were incubated with purified recombinant gIAP or a recombinant catalytically inactive mutant of TNAPD361V that was prepared in parallel with recombinant gIAP. Samples were then immunoblotted for phosphothreonine. Recombinant gIAP decreased the amount of a 75-kDa threonine-phosphorylated protein compared with beads treated with the catalytically inactive recombinant TNAPD361V mutant (Fig. 4).
Fig. 4.
Recombinant gIAP can dephosphorylate CD36 at a threonine residue. CD36 was immunoprecipitated from duodenal tissue homogenates. Resulting pellets were treated with a recombinant catalytically inactive tissue-nonspecific alkaline phosphatase (TNAPD361V) or recombinant gIAP (r-gIAP) and prepared for Western blotting. Membranes were stained with antiserum that recognizes phosphothreonine residues and then stripped and restained for total CD36 as a loading control. Each blot is representative of 3 separate experiments.
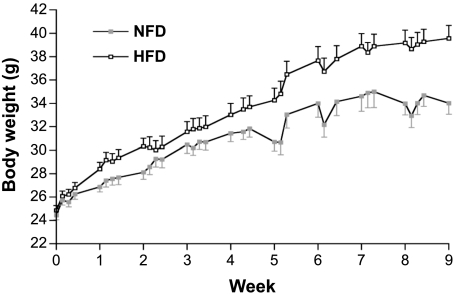
It was next determined whether a chronic HFD altered the expression of CD36, dIAP, and/or gIAP. As expected, HFD mice gained significantly more weight than did NFD controls, with the first significant difference at 5 wk (Fig. 5). HFD animals consumed slightly, but significantly, more energy over the course of the experiment, but there was no individual time point when the two groups differed significantly from each other (not shown). Plasma triglycerides and adiposity index were significantly increased by 2 wk of HFD (Table 1). Protein levels were examined by Western blotting only at week 5, the first time when all three phenotypic markers of HFD-induced adiposity were significantly increased. HFD significantly increased jejunal, but not duodenal, levels of CD36 and gIAP proteins at week 5 (Fig. 6). By contrast, there was no effect of diet on the amount of dIAP protein in duodenum or jejunum; dIAP was not detectable under any conditions in jejunum.
Fig. 5.
Body weights of C57BL/6 mice fed a normal-fat diet (NFD) or a high-fat diet (HFD). Twenty-four mice began the experiment in each diet group; mice were randomly assigned to 1 of 4 groups to be euthanized at weeks 1, 2, 5, and 9 after beginning the diets. Body weights significantly diverged by week 5. Values are means ± SE of 24, 18, 12, and 6 animals at weeks 1, 2, 5, and 9, respectively.
Table 1.
Effect of HFD on plasma triglycerides and adiposity index
| NFD | HFD | |
|---|---|---|
| Plasma triglycerides, mg/dl | ||
| Week 1 | 78.9 ± 7.3 | 59.5 ± 5.8 |
| Week 2 | 98.9 ± 6.2 | 123.4 ± 8.6* |
| Week 5 | 74.0 ± 4.8 | 121.0 ± 6.5* |
| Week 9 | 72.3 ± 6.8 | 112.1 ± 9.6* |
| Adiposity index, % | ||
| Week 1 | 3.4 ± 0.2 | 4.2 ± 0.3 |
| Week 2 | 4.2 ± 0.3 | 5.7 ± 0.3* |
| Week 5 | 5.0 ± 0.3 | 7.3 ± 0.6* |
| Week 9 | 6.9 ± 0.3 | 9.3 ± 0.4* |
Values are means ± SE of 6 animals per group. NFD, normal-fat diet; HFD, high-fat diet. Adiposity index = [(perirenal fat pad weight + inguinal fat pad weight) ÷ residual carcass mass] × 100.
Significantly different (P < 0.05) from NFD.
Fig. 6.
Duodenal and jejunal expression of CD36 (A and B), gIAP (C and D), and dIAP (E and F) in mice fed NFD or HFD for 5 wk. (Animals are those euthanized at week 5 in Fig. 5.) Mice were fasted overnight prior to death. Duodenal (A, C, and E) and jejunal (B, D, and F) tissue homogenates were prepared for Western blotting. Membranes were stained for CD36, gIAP, or dIAP and then stripped and reprobed for β-actin. Densitometry of 6 samples is shown as ratio of each target to β-actin. *P < 0.01. dIAP was not detectable in jejunal samples from mice fed either diet.
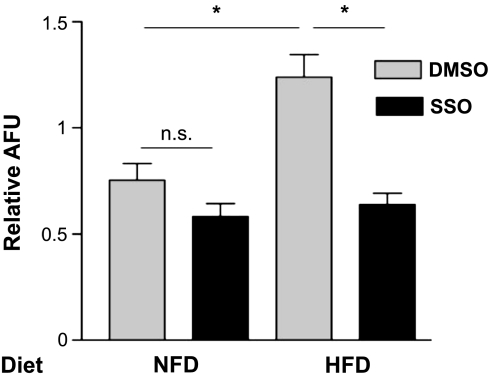
The initial rate of fatty acid uptake was measured in enterocytes isolated from jejunum of another group of mice fed HFD or NFD, as described above. The body weights of the mice after 5 wk on the diet (25.5 ± 1.4 and 33.3 ± 1.9 g for NFD and HFD, respectively) were significantly different and roughly similar to those of the mice in the previous experiment (Figs. 5 and 6). Plasma triglycerides and adiposity index were not determined in these mice, but plasma leptin concentrations, another marker of adiposity, were significantly higher in the HFD mice (5.2 ± 2.2 vs. 19.5 ± 3.4 ng/ml), as expected. HFD significantly increased uptake of the analog in enterocytes compared with NFD; the addition of SSO to cells from HFD animals restored uptake to levels not significantly different from untreated cells from NFD mice (Fig. 7).
Fig. 7.
Effect of HFD on uptake of C16:bodipy fatty acid analog in isolated enterocytes. After 5 wk on NFD or HFD, mice were euthanized, and jejunum was dissected for epithelial cell isolation and fatty acid uptake experiments. Values represent results from 3 replicate experiments. Cells were not treated at any time with IAP, but were treated with the CD36 inhibitor SSO or its vehicle control (DMSO). *Significant effect of HFD. SSO treatment of HFD cells reduced uptake to levels that were no longer significantly different from NFD controls (no SSO). ns, Not significant.
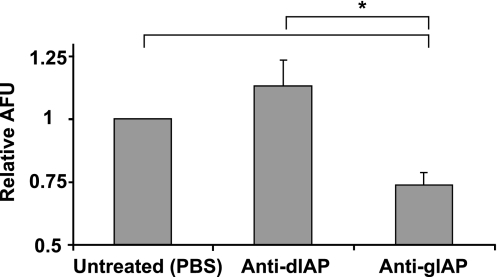
To confirm whether endogenous gIAP participates in the control of the initial rate of fatty acid uptake, enterocytes isolated from duodenum and jejunum were treated with anti-gIAP or anti-dIAP or left untreated, washed to remove serum, and then incubated with C16:bodipy for uptake measurements. Enterocytes treated with antiserum that reacted with gIAP showed decreased uptake of the analog compared with cells treated with anti-dIAP or cells left untreated (Fig. 8).
Fig. 8.
Effect of anti-alkaline phosphatase serum on uptake of C16:bodipy fatty acid analog in isolated enterocytes. Mice were euthanized, and duodenum and jejunum were dissected for epithelial cell isolation and fatty acid uptake experiments. Values represent data from 3 replicate experiments, with untreated control group normalized to 1.0 AFU for comparison across experiments. *P < 0.05.
DISCUSSION
We demonstrate that murine small intestine expresses FAT/CD36 in its phosphorylated and dephosphorylated states and that both forms of the protein are present in enterocytes. Treatment of isolated enterocytes with CIAP results in dephosphorylation of pCD36 and is associated with an increase in fatty acid uptake; this effect is prevented by treatment with a specific inhibitor of CD36. In addition, CD36 specifically interacts with murine gIAP, but not dIAP, under the conditions tested and is dephosphorylated by recombinant gIAP. HFD specifically increases immunoreactive CD36 and gIAP, but not dIAP, protein levels in jejunum, but not duodenum. Finally, HFD also increases the initial rate of fatty acid uptake in enterocytes isolated from the small intestine in a CD36- and gIAP-dependent manner. Thus, pCD36 appears to be a physiological substrate for gIAP in mice. Intestinal expression of CD36 and gIAP proteins is regulated by diet in a parallel, region-specific manner and is associated with diet-driven alterations in the pattern of intestinal fatty acid uptake.
The physiological functions of CD36 in the small intestine are likely to be related to the facilitation of vectorial fat absorption. CD36 expression correlates positively with fatty acid absorption in cultured Ob17PY fibroblasts (29), and uptake of fatty acids appears to be reduced in the proximal intestines of CD36 null mice (42). Whether CD36 directly facilitates LCFA transport is unknown, although the protein can bind LCFAs (5). One model suggests that CD36 acts in concert with other plasma membrane and cytosolic fatty acid-binding proteins to bring LCFAs into close proximity with the plasma membrane. Once there, the LCFAs may be shuttled to intracellular sites, thereby maintaining a steep inwardly directed diffusion gradient (16). However, CD36 may also be significant in the coordination of the intracellular events required for proper chylomicron synthesis (57). The binding of LCFA to membrane CD36 has recently been demonstrated to result in internalization of the protein during the earliest phase of LCFA absorption; this event appears to be critical for initiating the events that regulate the subsequent processing of intracellular triglycerides into properly formed chylomicrons (57). The mechanism of this action is uncertain; however, LCFA activation of ERK1/2 has been shown to be dependent on CD36 and is associated with the expression of proteins required for chylomicron assembly and secretion (57). Our data show that phosphorylation of CD36 inhibits LCFA uptake in the early stage of uptake, providing a possible link between gIAP, CD36, the initial phase of fat transport, and its subsequent processing.
Regardless of the precise mechanisms, the importance of ectophosphorylation in determining CD36 ligand specificity and its ability to mediate LCFA uptake has been established in other systems (3, 19, 26). Specifically, dephosphorylation of an extracellular residue of CD36 has been shown to increase palmitate uptake in platelets (19). In this study, we demonstrate that CD36 is phosphorylated in small intestinal epithelial cells and that pCD36 is subject to dephosphorylation with CIAP and recombinant gIAP. Homogenates of small intestine include the microvasculature and, consequently, endothelial cells (44). Human dermal microvascular endothelial cells constitutively express pCD36 (26), highlighting the possibility that at least a portion of the pCD36 that we observed in preliminary immunoblots of whole tissue homogenates originated from endothelial cells. Using flow cytometry, however, we confirmed that a population of cells that stained for gIAP, which is present only in epithelial cells (25, 39, 41), also stained for pCD36. Treatment of the cells with CIAP had no effect on the number of gIAP-positive cells, as expected, but decreased the proportion of gIAP-positive cells that colabeled with pCD36. These data demonstrate that the phosphorylated ectodomain of CD36 is available as a substrate for an IAP.
Although the proportion of gIAP-positive cells that costained with pCD36 decreased from 80.2% to 52.2%, it is possible that the decrease in CD36 phosphorylation is actually more substantial. Our preliminary Western blots using pCD36 antiserum were performed in conjunction with a nonphosphorylated peptide to reduce nonspecific binding to the nonphosphorylated form of CD36; however, this peptide was not included when intestinal cells were stained for flow cytometry. The absence of this peptide might explain the modest reduction in staining observed by flow cytometry compared with the more robust change in pCD36 staining observed using anti-phosphothreonine (Fig. 1A) or anti-pCD36 (plus blocking peptide) in preliminary Western blots (not shown).
After determining that pCD36 was present on intestinal epithelial cells and that its phosphorylation state could be controlled by CIAP, we assayed fatty acid uptake in cells that were treated with CIAP or left untreated. CIAP-treated cells took up significantly greater amounts of the LCFA analog than did untreated cells in a dose-dependent manner, supporting the hypothesis that dephosphorylation of pCD36 enhances uptake of LCFAs in mouse enterocytes. CIAP may have dephosphorylated other membrane proteins in addition to CD36; however, SSO, a specific and irreversible inhibitor of CD36, completely eliminated the effect of CIAP on fatty acid uptake. Moreover, the effect of CIAP was specific for the long-chain analog, consistent with the known ligand specificity of CD36 (5). Pretreatment of enterocytes with antiserum to gIAP, but not anti-dIAP, decreased initial LCFA uptake, presumably by specifically immunoneutralizing endogenous gIAP and inhibiting CD36 dephosphorylation. It is also interesting that the untreated cells took up more fatty acid than did the SSO-treated cells, suggesting some degree of constitutive CD36-dependent uptake. However, the possibility that SSO may have also inhibited additional proteins or transporters that contribute to LCFA transport cannot be discounted, although SSO has been shown to interact with CD36, but not with other proteins in adipocytes (46), which express some of the same fat-binding and transport proteins that are found on enterocytes.
The uptake assay was performed using cells in suspension, rather than cells cultured as monolayers, raising the possibility that the loss of cell-cell contacts, as well as apical-basolateral polarity, may influence uptake kinetics or integral membrane protein location. An advantage of the cell suspension protocol, however, is that it lacks enzymatic digestions that could perturb membrane-bound protein structure. Cultured enterocytes have also been shown to dedifferentiate in culture, specifically decreasing the activity of alkaline phosphatase (48). Nonetheless, we have observed CIAP- and SSO-dependent uptake of the long-chain analog in acutely plated primary cultures of mouse enterocytes (Lynes and Widmaier, unpublished observations).
The identity of the kinase responsible for phosphorylating CD36 remains unresolved, but preliminary studies of whole tissue lysates revealed an increase in pCD36 staining of lysates treated with inhibitors of alkaline phosphatases and other phosphatases. This observation led us to isolate epithelial cells in the presence of these inhibitors and suggests that kinases capable of phosphorylating CD36 are present in tissue lysates. CD36 also has ecto-NTPase activity, but no studies have demonstrated autophosphorylation of CD36 (30, 31). Identifying this kinase would help determine if a HFD may also alter the rate of constitutive CD36 phosphorylation.
Given our finding that a population of enterocytes coexpressed pCD36 and gIAP and the close proximity of these two proteins in kidney epithelial cell lipid rafts (14), we predicted that intestinal CD36 would coimmunoprecipitate with one or more of the isozymes of IAP in the murine small intestine. gIAP, but not dIAP, coimmunoprecipitated with CD36. This suggests that pCD36 could be an in vivo substrate for gIAP, but possibly not for dIAP. That the two isozymes might have different substrates is not unexpected. The highest degree of dissimilarity between the two isozymes is in the crown region (41), which is known to determine a significant portion of substrate specificity between alkaline phosphatase isozymes and also to potentially mediate interactions between alkaline phosphatases and other protein ligands (10). If pCD36 is, in fact, a specific substrate of gIAP, and not dIAP, the Akp3−/− mouse, which expresses constitutively higher levels of gIAP, could potentially have a higher rate of gIAP-mediated dephosphorylation of pCD36. This, in turn, may increase the activity of CD36 as a translocase or alter its ligand specificity; in either case, it is tempting to speculate that this might partly contribute to the higher rate of fatty acid absorption observed in Akp3−/− mice (41). It is also worth noting that a distinct glycosylation state of gIAP coimmunoprecipitated with CD36. gIAP is a 75- to 110-kDa molecule, and its mass increases when the nascent protein is glycosylated. The mass of the gIAP that coimmunoprecipitated with CD36 in the present experiments suggests that enterocyte CD36 physically interacts with a mature form of gIAP. This is consistent with gIAP that is expressed at the brush border membrane, and not protein that has just been translated. Our results also indicate that recombinant gIAP acts to dephosphorylate a threonine residue in immunoprecipitated CD36, presumably Thr92, supporting the role of this residue in allosterically regulating the activity of CD36.
In the presence of normal biliary flow, fat absorption in the small intestine decreases as a function of the distance from the pylorus (61). However, the absorptive capacity of the distal region of the intestine increases in response to an increase in the proportion of dietary calories from fat (45). This is an example of the well-recognized plasticity of the vertebrate intestine to changes in nutrient consumption. Therefore, we tested the hypothesis that if CD36 and IAPs are part of the coordinated mechanism for maximizing fat transport across the intestinal mucosa, then expression of CD36 and IAPs in the distal regions of the small intestine should increase when mice are chronically fed a HFD. Previous studies have suggested that CD36 expression throughout the intestine is sensitive to the total fat content of the diet (45) and the specific fats in the diet (47). Those studies compared higher-fat diets with standard lab chows that contained no more than ∼5% fat. In the present study, we examined changes in fat content from a low of 15% to a high of 45%; this represents the approximate range of diets prevalent in Western societies and extends beyond the currently recommended daily intake of 30%. Our data indicate that, in the transition from a 15% to a 45% fat diet, the amounts of CD36 and gIAP proteins, but not dIAP protein, were significantly and specifically increased in jejunum of mice. Therefore, regional, parallel changes in CD36 and gIAP proteins may be key events in the adaptation of the intestinal mucosa to HFDs in mice. This is supported by the observation of increased transport of LCFA into isolated enterocytes from HFD mice.
Perspectives and Significance
These observations may explain in part our previous finding that mice consuming a HFD absorb the same percentage of ingested energy as mice fed a regular diet (56). Whether this contributes to the advent of obesity that occurs in response to HFD remains to be determined; notwithstanding, any adaptation that promotes an increase in the absorptive capacity of the distal regions of the intestine may be acutely adaptive, but maladaptive in situations of chronic high-energy intake. Whether the ratio of phosphorylated to dephosphorylated CD36 changes in vivo under these conditions is uncertain. However, because the absolute amounts of CD36 and gIAP proteins are increased with HFD, it is likely that, even in the presence of an unchanged relative amount of pCD36/CD36, the absolute amount of dephosphorylated protein would be increased, accounting in part for the increase in LCFA uptake capacity.
GRANTS
This work was supported by National Science Foundation Grant IOS-0446057 (E. P. Widmaier), National Institutes of Health Grants DE-12889 and AR-47908 (J. L. Millán), and grants from the Boston University Undergraduate Research Opportunities Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Michael Lynes (University of Connecticut) and Dr. Todd Blute (Boston University) for assistance with flow cytometry. We thank Rachel Fogley, Magen Lorenzi, and Mark Wojnarowicz for valuable assistance with animal maintenance and other aspects of the work and Dr. C. Richardson for assistance with statistical analyses.
REFERENCES
- 1. Abumrad NA, Park JH, Park CR. Permeation of long-chain fatty acid into adipocytes. Kinetics, specificity, and evidence for involvement of a membrane protein. J Biol Chem 259: 8945–8953, 1984 [PubMed] [Google Scholar]
- 2. Asch AS, Barnwell J, Silverstein RL, Nachman RL. Isolation of the thrombospondin membrane receptor. J Clin Invest 79: 1054–1061, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asch AS, Liu I, Briccetti FM, Barnwell JW, Kwakye-Berko F, Dokun A, Goldberger J, Pernambuco M. Analysis of CD36 binding domains: ligand specificity controlled by dephosphorylation of an ectodomain. Science 262: 1436–1440, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Bader A, Hansen T, Kirchner G, Allmeling C, Haverich A, Borlak JT. Primary porcine enterocyte and hepatocyte cultures to study drug oxidation reactions. Br J Pharmacol 129: 331–342, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol 153: 75–81, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Bastie CC, Hajri T, Drover VA, Grimaldi PA, Abumrad NA. CD36 in myocytes channels fatty acids to a lipase-accessible triglyceride pool that is related to cell lipid and insulin responsiveness. Diabetes 53: 2209–2216, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Berglund L, Petersen TE, Rasmussen JT. Structural characterization of bovine CD36 from the milk fat globule membrane. Biochim Biophys Acta 1309: 63–68, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Bonen A, Campbell SE, Benton CR, Chabowski A, Coort SL, Han XX, Koonen DP, Glatz JF, Luiken JJ. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc Nutr Soc 63: 245–249, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Bonen A, Luiken JJ, Liu S, Dyck DJ, Kiens B, Kristiansen S, Turcotte LP, Van Der Vusse GJ, Glatz JF. Palmitate transport and fatty acid transporters in red and white muscles. Am J Physiol Endocrinol Metab 275: E471–E478, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Bossi M, Hoylaerts MF, Millán JL. Modifications in a flexible surface loop modulate the isozyme-specific properties of mammalian alkaline phosphatases. J Biol Chem 268: 25409–25416, 1993 [PubMed] [Google Scholar]
- 11. Di Mauro S, Manes T, Hessle L, Kozlenkov A, Pizauro JM, Hoylaerts MF, Millán JL. Kinetic characterization of hypophosphatasia mutations with physiological substrates. J Bone Miner Res 17: 1383–1391, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Dobozy A, Hunyadi J, Bozoky B, Monostori E, Ando I, Kenderessy AS, Tiba A. The mouse erythrocyte binding receptor is expressed on human keratinocytes. Dermatol Monatsschr 176: 615–621, 1990 [PubMed] [Google Scholar]
- 13. Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest 115: 1290–1297, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehehalt R, Sparla R, Kulaksiz H, Herrmann T, Fullekrug J, Stremmel W. Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts). BMC Cell Biol 9: 45, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem 268: 11811–11816, 1993 [PubMed] [Google Scholar]
- 16. Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev 90: 367–417, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Greenwalt DE, Watt KW, So OY, Jiwani N. PAS IV, an integral membrane protein of mammary epithelial cells, is related to platelet and endothelial cell CD36 (GP IV). Biochemistry 29: 7054–7059, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Gregoire FM, Zhang Q, Smith SJ, Tong C, Ross D, Lopez H, West DB. Diet-induced obesity and hepatic gene expression alterations in C57BL/6J and ICAM-1-deficient mice. Am J Physiol Endocrinol Metab 282: E703–E713, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Guthmann F, Maehl P, Preiss J, Kolleck I, Rustow B. Ectoprotein kinase-mediated phosphorylation of FAT/CD36 regulates palmitate uptake by human platelets. Cell Mol Life Sci 59: 1999–2003, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hajri T, Ibrahimi A, Coburn CT, Knapp FF, Jr, Kurtz T, Pravenec M, Abumrad NA. Defective fatty acid uptake in the spontaneously hypertensive rat is a primary determinant of altered glucose metabolism, hyperinsulinemia, and myocardial hypertrophy. J Biol Chem 276: 23661–23666, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Hansen GH, Immerdal L, Thorsen E, Niels-Christiansen LL, Nystrom BT, Demant EJ, Danielsen EM. Lipid rafts exist as stable cholesterol-independent microdomains in the brush border membrane of enterocytes. J Biol Chem 276: 32338–32344, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Hansen GH, Niels-Christiansen LL, Immerdal L, Nystrom BT, Danielsen EM. Intestinal alkaline phosphatase: selective endocytosis from the enterocyte brush border during fat absorption. Am J Physiol Gastrointest Liver Physiol 293: G1325–G1332, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Harmon CM, Abumrad NA. Binding of sulfosuccinimidyl fatty acids to adipocyte membrane proteins: isolation and amino-terminal sequence of an 88-kD protein implicated in transport of long-chain fatty acids. J Membr Biol 133: 43–49, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Hatmi M, Gavaret JM, Elalamy I, Vargaftig BB, Jacquemin C. Evidence for cAMP-dependent platelet ectoprotein kinase activity that phosphorylates platelet glycoprotein IV (CD36). J Biol Chem 271: 24776–24780, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Hinnebusch BF, Siddique A, Henderson JW, Malo MS, Zhang W, Athaide CP, Abedrapo MA, Chen X, Yang VW, Hodin RA. Enterocyte differentiation marker intestinal alkaline phosphatase is a target gene of the gut-enriched Kruppel-like factor. Am J Physiol Gastrointest Liver Physiol 286: G23–G30, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Ho M, Hoang HL, Lee KM, Liu N, MacRae T, Montes L, Flatt CL, Yipp BG, Berger BJ, Looareesuwan S, Robbins SM. Ectophosphorylation of CD36 regulates cytoadherence of Plasmodium falciparum to microvascular endothelium under flow conditions. Infect Immun 73: 8179–8187, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoosdally SJ, Andress EJ, Wooding C, Martin CA, Linton KJ. The human scavenger receptor CD36: glycosylation status and its role in trafficking and function. J Biol Chem 284: 16277–16288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hwang EH, Taki J, Yasue S, Fujimoto M, Taniguchi M, Matsunari I, Nakajima K, Shiobara S, Ikeda T, Tonami N. Absent myocardial iodine-123-BMIPP uptake and platelet/monocyte CD36 deficiency. J Nucl Med 39: 1681–1684, 1998 [PubMed] [Google Scholar]
- 29. Ibrahimi A, Sfeir Z, Magharaie H, Amri EZ, Grimaldi P, Abumrad NA. Expression of the CD36 homolog (FAT) in fibroblast cells: effects on fatty acid transport. Proc Natl Acad Sci USA 93: 2646–2651, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kannan S. E-NTPase/NTPDase: potential role as a regulatory element in inflammation. Med Hypotheses 58: 527–528, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Kannan S. Regulatory role of E-NTPase/NTPDase in FAT/CD36-mediated fatty acid uptake. Cell Biol Int 27: 147–151, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Kintaka T, Tanaka T, Imai M, Adachi I, Narabayashi I, Kitaura Y. CD36 genotype and long-chain fatty acid uptake in the heart. Circ J 66: 819–825, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Lalles JP. Intestinal alkaline phosphatase: multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev 68: 323–332, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest 113: 764–773, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lynes MD, Widmaier EP. Involvement of CD36 and intestinal alkaline phosphatases in fatty acid transport in enterocytes and the response to a high-fat diet. Life Sci 88: 384–391, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Millán JL. Alkaline phosphatases: structure, substrate specificity and functional relatedness to other members of a large superfamily of enzymes. Purinergic Signal 2: 335–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Millán JL. Mammalian Alkaline Phosphatases. From Biology to Applications in Medicine and Biotechnology. Weinheim: Wiley VCH, 2006 [Google Scholar]
- 38. Nakano T, Inoue I, Koyama I, Kanazawa K, Nakamura K, Narisawa S, Tanaka K, Akita M, Masuyama T, Seo M, Hokari S, Katayama S, Alpers DH, Millán JL, Komoda T. Disruption of the murine intestinal alkaline phosphatase gene Akp3 impairs lipid transcytosis and induces visceral fat accumulation and hepatic steatosis. Am J Physiol Gastrointest Liver Physiol 292: G1439–G1449, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Narisawa S, Hoylaerts MF, Doctor KS, Fukuda MN, Alpers DH, Millán JL. A novel phosphatase upregulated in Akp3 knockout mice. Am J Physiol Gastrointest Liver Physiol 293: G1068–G1077, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Narisawa S, Huang L, Iwasaki A, Hasegawa H, Alpers DH, Millán JL. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol 23: 7525–7530, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 282: 19493–19501, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Noushmehr H, D'Amico E, Farilla L, Hui H, Wawrowsky KA, Mlynarski W, Doria A, Abumrad NA, Perfetti R. Fatty acid translocase (FAT/CD36) is localized on insulin-containing granules in human pancreatic beta-cells and mediates fatty acid effects on insulin secretion. Diabetes 54: 472–481, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Nozaki S, Tanaka T, Yamashita S, Sohmiya K, Yoshizumi T, Okamoto F, Kitaura Y, Kotake C, Nishida H, Nakata A, Nakagawa T, Matsumoto K, Kameda-Takemura K, Tadokoro S, Kurata Y, Tomiyama Y, Kawamura K, Matsuzawa Y. CD36 mediates long-chain fatty acid transport in human myocardium: complete myocardial accumulation defect of radiolabeled long-chain fatty acid analog in subjects with CD36 deficiency. Mol Cell Biochem 192: 129–135, 1999 [PubMed] [Google Scholar]
- 44. Ottaway CA, Parrott DM. Regional blood flow and the localization of lymphoblasts in the small intestine of the mouse. I. Examination of normal small intestine. Immunology 41: 955–961, 1980 [PMC free article] [PubMed] [Google Scholar]
- 45. Petit V, Arnould L, Martin P, Monnot MC, Pineau T, Besnard P, Niot I. Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res 48: 278–287, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. FAT/CD36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell 16: 24–31, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poirier H, Degrace P, Niot I, Bernard A, Besnard P. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). Eur J Biochem 238: 368–373, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Rusu D, Loret S, Peulen O, Mainil J, Dandrifosse G. Immunochemical, biomolecular and biochemical characterization of bovine epithelial intestinal primocultures. BMC Cell Biol 6: 42, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Salah-Uddin H, Gordon MJ, Ford I, Tandon NN, Greaves M, Duttaroy AK. Surface expression of fatty acid translocase (FAT/CD36) on platelets in myeloproliferative disorders and non-insulin dependent diabetes mellitus: effect on arachidonic acid uptake. Mol Cell Biochem 239: 203–211, 2002 [PubMed] [Google Scholar]
- 50. Sandoval A, Fraisl P, Arias-Barrau E, Dirusso CC, Singer D, Sealls W, Black PN. Fatty acid transport and activation and the expression patterns of genes involved in fatty acid trafficking. Arch Biochem Biophys 477: 363–371, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, Shimamoto K, Itakura K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res 42: 751–759, 2001 [PubMed] [Google Scholar]
- 52. Tandon NN, Kralisz U, Jamieson GA. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem 264: 7576–7583, 1989 [PubMed] [Google Scholar]
- 53. Tandon NN, Lipsky RH, Burgess WH, Jamieson GA. Isolation and characterization of platelet glycoprotein IV (CD36). J Biol Chem 264: 7570–7575, 1989 [PubMed] [Google Scholar]
- 54. Tao N, Wagner SJ, Lublin DM. CD36 is palmitoylated on both N- and C-terminal cytoplasmic tails. J Biol Chem 271: 22315–22320, 1996 [DOI] [PubMed] [Google Scholar]
- 55. Thorne RF, Ralston KJ, de Bock CE, Mhaidat NM, Zhang XD, Boyd AW, Burns GF. Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum. Biochim Biophys Acta 1803: 1298–1307, 2010 [DOI] [PubMed] [Google Scholar]
- 56. Townsend KL, Lorenzi MM, Widmaier EP. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocr J 33: 176–188, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Tran TT, Poirier H, Clement L, Nassir F, Pelsers MM, Petit V, Degrace P, Monnot MC, Glatz JF, Abumrad NA, Besnard P, Niot I. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem 286: 25201–25210, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vega MA, Segui-Real B, Garcia JA, Cales C, Rodriguez F, Vanderkerckhove J, Sandoval IV. Cloning, sequencing, and expression of a cDNA encoding rat LIMP II, a novel 74-kDa lysosomal membrane protein related to the surface adhesion protein CD36. J Biol Chem 266: 16818–16824, 1991 [PubMed] [Google Scholar]
- 59. Watanabe K, Ohta Y, Toba K, Ogawa Y, Hanawa H, Hirokawa Y, Kodama M, Tanabe N, Hirono S, Ohkura Y, Nakamura Y, Kato K, Aizawa Y, Fuse I, Miyajima S, Kusano Y, Nagamoto T, Hasegawa G, Naito M. Myocardial CD36 expression and fatty acid accumulation in patients with type I and II CD36 deficiency. Ann Nucl Med 12: 261–266, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Weng S, Zemany L, Standley KN, Novack DV, La Regina M, Bernal-Mizrachi C, Coleman T, Semenkovich CF. β3-Integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc Natl Acad Sci USA 100: 6730–6735, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wollaeger EE. Role of the ileum in fat absorption. Mayo Clin Proc 48: 836–843, 1973 [PubMed] [Google Scholar]