Abstract
The intracerebral injection of β-amyloid-containing brain extracts can induce cerebralβ-amyloidosis and associated pathologies in susceptible hosts. We found that intraperitoneal inoculation with β-amyloid-rich extracts inducesβ-amyloidosis in the brains of β-amyloid precursor protein transgenic mice after prolonged incubation times.
Intracerebral (i.c.) inoculation with minute amounts of brain extract containing misfolded β-amyloid (Aβ) from patients with Alzheimer’s Disease or from amyloid-bearing β-amyloid precursor protein (APP) transgenic (tg) mice induces cerebral β-amyloidosis and related pathologies in APP tg mice in a time- and concentration-dependent manner (1). However, oral, intravenous, intraocular, or intranasal inoculations have failed to induce cerebral β-amyloidosis in APP tg hosts (2). These findings suggest that Aβ-containing brain material in direct contact with the brain can induce cerebral β-amyloidosis, but that, unlike prions, either the inducing agent is not readily conveyed from peripheral sites to the brain or a higher concentration or longer incubation period is required for peripherally delivered Aβ seeds.
Intraperitoneal administration of prion-rich material is more efficient at transmitting prion disease than is oral administration (3,4). To test whether intraperitoneal inoculation of Aβ-rich material might similarly trigger Aβ misfolding and deposition in the brain, we administered two intraperitoneal injections (100 μl each, one week apart) of Aβ-laden (10–20 ng/μl) brain extract from aged APP23 tg mice (Tg extract) to a cohort of young (2-month-old) female APP23 tg mice (5). After a 7-month incubation period, cerebral β-amyloidosis was robustly induced in all intraperitoneally inoculated mice compared with untreated littermate controls (Fig. 1). To confirm this finding, we inoculated a second cohort of 2-month-old female APP23 mice with a different batch of Tg brain extract in another laboratory (cohort 2: Tübingen, vs. cohort 1: Basel). After 6 to 7 months, mice injected intraperitoneally with the Tg extract exhibited robust cerebral β-amyloidosis, whereas intraperitoneal inoculation with phosphate-buffered saline (PBS) or brain extract from age-matched, non-tg wild-type mice (Wt extract) was ineffective (Fig. 1).
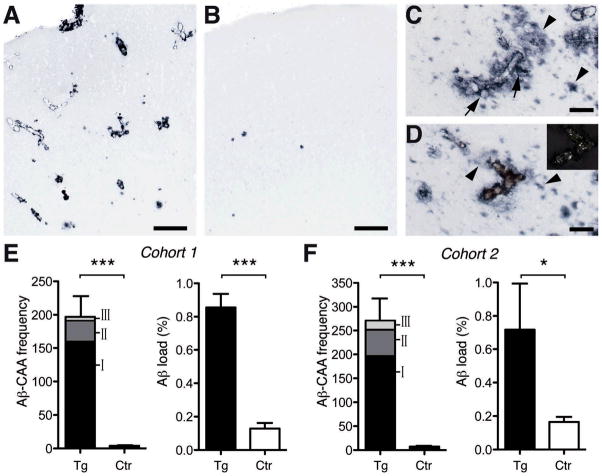
Fig. 1. Induced Aβ deposition.
(A, B) Aβ-immunostained frontal cortex of APP23 mice inoculated intraperitoneally with Tg extract (A) or Wt extract- (B). (C, D) Most induced β-amyloid was vascular (Aβ-CAA), with Aβ-immunoreactivity extending into the brain parenchyma (arrows). Amyloid-laden vessels were congophilic [red in (D); birefringent under cross-polarized light in insert] and often were surrounded by diffuse, Congo red-negative Aβ deposits (arrowheads). (E, F) Analysis of the entire neocortex for Aβ-CAA frequency [indicated are all three (I-III) CAA severity grades (5)], and for total Aβ load in Tg extract-inoculated mice compared with control (Ctr) mice. Cohort 1 consisted of six Tg extract-inoculated mice versus seven untreated control mice. Aβ-CAA: t(11)=6.78 (all severity grades combined), ***p<0.0001; Aβ load: t(11)=8.79, ***p<0.0001. Cohort 2 consisted of five Tg extract-inoculated mice versus five Wt extract-inoculated mice and four PBS-injected mice. These latter two (control) groups did not differ significantly and were combined for analysis. Aβ-CAA: t(12)=7.79, ***p<0.0001; Aβ load t(12)=2.71, *p<0.05. The occasional parenchymal Aβ-deposits in control mice are normal for 9-month-old APP23 mice. Error bars, means ±SEM. Scale bars: [(A) and (B)] 200μm; [0(C) and (D)] 50μm.
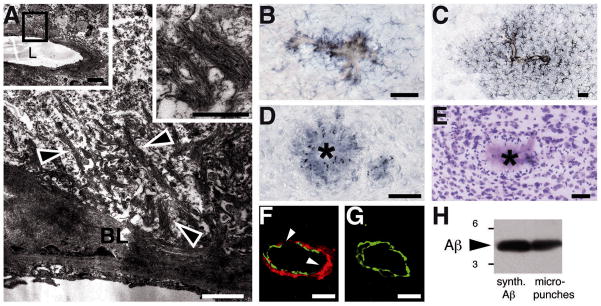
Induced β-amyloidosis was strongest in the anterior and entorhinal cortices, with additional deposition in the hippocampus, resembling the regional development of endogenous β-amyloidosis in aged APP23 mice (6). However, whereas normal aged APP23 mice manifest mostly parenchymal deposits, the induced β-amyloid in intraperitoneally seeded mice was predominantly associated with blood vessels [cerebral β-amyloid angiopathy (CAA)], often with massive spreading into the neighboring brain parenchyma (Fig. 1). The presence of Aβ was confirmed by immunoblotting, and amyloid fibrils were evident ultrastructurally; in addition, the induced β-amyloidosis was linked to gliosis, hyperphosphorylated tau, and other associated pathologies (Fig. 2), reminiscent of the cerebral β-amyloid deposition in aged APP23 mice (6,7).
Fig. 2. Induced Aβ deposition was linked to multiple associated pathologies.
(A) Ultrastructural analysis showed amyloid deposition within the vascular basal lamina (BL), with typical amyloid fibrils (arrowheads) extending into the brain parenchyma. Insets are low- and high-magnification views of the examined vessel (L, lumen) and the typical nonbranching amyloid fibrils. (B to E) Vascular amyloid [stained by Congo Red in (B) and (C)] and parenchymal plaques were surrounded by hypertrophic, Iba1-positive microglia (B), Glial fibrillary acidic protein (GFAP)-positive astrocytes (C), hyperphosphorylated tau-positive neurites [(D); asterisk indicates amyloid core], but a paucity of proximate neurons [cresyl-violet stain (E)]. (F and G) Vessels with CAA types II and III showed smooth muscle cell loss at the site of amyloid deposition (arrowheads; confocal image, maximum projection of 5μm z-stack: red, Aβ; green, smooth muscle actin). A normal vessel (G) has a complete ring of smooth muscle cells. (H) Immunoblotting of micropunches of Aβ-immunoreactive material revealed the expected Aβ band. Synthetic Aβ40/42 is shown as control. Markers, 3 and 6 kD. Scale bars: (A) 1μm (insets, 5 and 0.5 μm); [(B) to (E)] 50μm; [(F) and (G)] 10μm.
To compare the efficiency and time course of intraperitoneal versus intracerebral inoculation, 2-month-old female APP23 mice were inoculated either intraperitoneally (2 x 100 μl) or intracerebrally (2.5 μl into the hippocampus) with Tg extract, and then analyzed 4 months later. No cerebral β-amyloid induction was found in any of the four intraperitoneally inoculated mice, whereas all six intracerebrally inoculated mice revealed β-amyloid induction identical to that previously reported (1,2). From this observation, together with previous time course and 1:20 dilution experiments for intracerebral inoculations (1), we estimate that intraperitoneal inoculations with 1000 times as much Aβ take 2 to 5 months longer to induce cerebral β-amyloidosis than do intracerebral inoculations.
The replication of peripherally applied prions and their translocation into the central nervous system depend on hematopoietic and stromal immune cells, in combination with sympathetic innervation of abdominal lymphoid organs (8). Both activation of the immune system and chronic inflammation promote prion replication (9,10). To assess the immune response to Aβ-rich brain extracts, additional APP23 mice were given single intraperitoneal injections of 200 μl Tg or Wt extract and sacrificed 1 hour, 1 week, or 1 month after injection (5). An acute immune activation to the injected brain material was indicated by transient increases in plasma chemokines and cytokines [interleukin-6 (IL6), IL10, tumor necrosis factor-α, monocyte chemotactic protein-1, and macrophage inflammatory protein-1β] in both Tg and Wt extract-inoculated mice after 1 hour, with IL-6 still mildly elevated in Tg extract-injected mice 1 week after inoculation (fig. S1). However, no signs of chronic inflammation in various peripheral organs (e.g., liver, pancreas, kidney, and lung) or plasma titers of antibodies to Aβ were found in any mice investigated at 1 or 7 months after seeding (5). Moreover, no β-amyloid deposition was found in any of the peripheral tissues at any time point studied.
Thus, like prion disease, cerebral β-amyloidosis can be seeded in the brain by homologous protein aggregates delivered into the peritoneal cavity, although the intraperitoneal route required more time and was less efficient than was direct injection into the brain (1, 2). The amyloid-inducing factor in the Tg extract is probably a species of misfolded Aβ that is generated in its most effective form or composition in vivo (1). Because the expression of tg (human) APP is restricted to the nervous system in APP23 mice (7), in this model it is likely that the seed carried to the brain was the injected material itself, rather than Aβ aggregates that were first amplified in peripheral tissues.
There is now persuasive evidence that the aggregation of Aβ is a key pathogenic feature of Alzheimer’s disease and Aβ-CAA (11–14), although the majority of these cases are initiated by unknown causes. The possibility that mechanisms exist allowing for the transport of Aβ aggregates (and possibly other seeds) from the periphery to the brain justifies further studies to better understand the cellular and molecular origin of these diseases and to clarify the basis of infectious vs. noninfectious proteopathies (15,16).
Supplementary Material
Acknowledgments
We thank M.-J. Runser, L. Jacobson (Basel), F. Langer, J. Coomaraswamy, S. Grathwohl, N. Varvel, T. Hamaguchi, C. Schäfer, A. Bosch, G. Frommer-Kästle, U. Scheurlen (Tübingen) for experimental help and A. Aguzzi (Zürich) for insightful comments. This work was supported by the Competence Network on Degenerative Dementias (BMBF-01GI0705), the Bundesministerium für Bildung und Forschung as part of ERA-Net NEURON (MIPROTRAN), the Center for Integrative Neuroscience (Deutsche Forschungsgemeinschaft) and NIH RR-00165.
REFERENCES & NOTES
- 1.Meyer-Lühmann M, et al. Science. 2006;131:1781. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 2.Eisele YS, et al. Proc Natl Acad Sci USA. 2009;106:12926. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prusiner SB. Prion Biology and Diseases. 2. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2004. [Google Scholar]
- 4.Kimberlin RH, Walker CA. J Comp Path. 1978;88:39. doi: 10.1016/0021-9975(78)90059-2. [DOI] [PubMed] [Google Scholar]
- 5.Materials and methods are available as supporting material on Science Online.
- 6.Sturchler-Pierrat C, et al. Proc Natl Acad Sci USA. 1997;94:13287. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun ME, et al. Proc Natl Acad Sci USA. 1999;96:14088. doi: 10.1073/pnas.96.24.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguzzi A, Sigurdson C, Heikenwälder M. Annu Rev Pathol Mech Dis. 2008;3:11. doi: 10.1146/annurev.pathmechdis.3.121806.154326. [DOI] [PubMed] [Google Scholar]
- 9.Heikenwalder M, et al. Science. 2005;307:1107. doi: 10.1126/science.1106460. [DOI] [PubMed] [Google Scholar]
- 10.Bremer J, et al. PloS One. 2009;4:e7160. doi: 10.1371/journal.pone.0007160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy J, Selkoe DJ. Science. 2002;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 12.Sorandt M, Mintun MA, Head D, Morris JC. Arch Neurol. 2009;66:1476. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JC, et al. Arch Neurol. 2009;66:1469. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang-Nunes SX, et al. Brain Pathol. 2006;16:30. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LC, LeVine H, 3rd, Mattson MP, Jucker M. Trends Neurosci. 2006;29:438. doi: 10.1016/j.tins.2006.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguzzi A, Rajendran L. Neuron. 2009;64:783. doi: 10.1016/j.neuron.2009.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.