Abstract
Tetherin, a recently identified interferon (IFN)-inducible, type 2 transmembrane protein, has been shown to be a cellular antiviral restriction factor that retains newly formed virions in infected cells. Thus, tetherin plays an important role in the innate cell-autonomous immune response. The aim of this study was to examine the antiviral activities of tetherin in vesicular stomatitis virus infections of murine neuronal cells. Both IFN-β and IFN-γ induce the expression of tetherin mRNA and protein. Tetherin knockdown experiments were carried out by transfection of tethrin shRNA into murine neuroblastoma cells using a vector containing the pCMV-driven tGFP gene. The efficiency of transfection was monitored through GFP expression by the transfected cells. Selected transfected cells were used for further mRNA and protein analysis, fluorescent immunocytolocalization, and viral infection to study the impact of tetherin knockdown. Our research indicates that tetherin is expressed on the outer face of the plasma membrane of murine neuroblastoma cells, its expression can be induced with both IFN-γ and IFN-β, and tetherin restricts progeny virus release up to 100-fold in mammalian neurons, thus contributing to a potent antiviral state within the host cell.
Introduction
The critical role interferons (IFNs) play in innate antiviral immunity has been studied in detail (Goodbourn et al., 2000; Levy and Garcia-Sastre, 2001; Samuel, 2001; Pestka et al., 2004). Pathogen recognition molecules, including Toll-like receptors (TLR), retinoic acid-inducible gene-I (RIG-I)-like receptors, and NOD-like receptors, are actively involved in induction of IFNs soon after viruses infect cells (Roberts et al., 2003; van Pesch et al., 2004; Akira et al., 2006; Pichlmair and Reis e Sousa, 2007; Kumagai et al., 2008; Takeuchi and Akira, 2008). Subsequently, IFNs bind their receptor and signal via the JAK-STAT pathway, and induce expression of a variety of antiviral IFN-stimulated genes (ISGs) that are highly critical for antiviral responses (Levy and Darnell, 2002; Haller et al., 2007). Mice deficient in any component of the IFN pathway are more susceptible to lethal viral infection (Hwang et al., 1995; Durbin et al., 1996; Meraz et al., 1996).
Among important ISGs with well-established antiviral activities are RNaseL/2′,5′-oligoadenylate synthase, ISG15, myxovirus resistance (Mx), protein kinase R (PKR), nitric oxide synthase, and RNA-specific adenosine deaminase that inhibit virus infection by different mechanisms (Chesler and Reiss, 2002; Sadler and Williams, 2008; Reiss, 2009; Kuhl et al., 2011). Thus, the replication of viruses in eukaryotic cells is inhibited by a variety of ISGs. An important molecule among these is the recently identified cellular antiviral restriction factor tetherin, which is an IFN-inducible, 28- to 36-kDa transmembrane protein. It has two membrane anchors: a transmembrane domain near the N-terminus and a glycophosphatidylinositol-linked anchor at the C-terminus. This protein is also known as Bst-2 and CD317. The ectodomain of the protein features three cysteine residues that may mediate homodimerization: two N-linked glycosylation sites and a coiled-coil domain (Neil et al., 2008). To restrict release of the newly assembled viral particles from the infected cells, tetherin retains the virions at the cell surface (Schubert et al., 2010). Expression of tetherin is strongly induced by IFN due to the presence of IFN response elements (IREs) and a binding site for STAT3 in the promoter region (Blasius et al., 2006). Additional response elements are present in tetherin promoter consistent with induction by other inflammatory cytokines such as IL-6 and TNF-α (Sauter et al., 2010). The level of tetherin expression appears to be cell-type dependent (Miyagi et al., 2009).
In this study, we have extended our investigations of the mechanisms of IFN-induced antiviral pathways in neurons. We have observed IFN-dependent tetherin upregulation in mouse neuroblastoma cells. We use vesicular stomatitis virus (VSV) infections. A member of Rhabdoviridae, order Mononegavirales, VSV encodes five major proteins in <12 kb of RNA: the glycoprotein (GP) G, nucleocapsid protein N, phosphoprotein P, matrix protein M, and large protein L (Rose and Whitt, 2001). VSV replicates readily in IFN pathway-defective cells, but poorly in IFN-responsive normal tissues due to its exquisite sensitivity to the antiviral pathways induced by IFNs (Stojdl et al., 2000; Chesler and Reiss, 2002; Trottier et al., 2005; Zhang et al., 2010).
Among the pattern recognition receptor (PRR) families, TLRs are one of the best characterized. They are responsible for sensing the invading pathogens outside the cell, and in intracellular endosomes and lysosomes as well (Akira, 2006). Nucleic acids derived from viruses are recognized by TLR3, TLR7, TLR8, and TLR9. Endosomal TLR7 and TLR 8 recognize ssRNA, and TLR3 is activated by dsRNA from RNA viruses (Akira, 2006; Baum and Garcia-Sastre, 2010). Type I IFNs are produced as a result of a MyD88-dependent pathway (Negishi et al., 2006; Takeuchi and Akira, 2010). RIG-I and melanoma differentiation-associated gene 5 (MDA5) are important for cell signaling activated by cytoplasmic RNAs (Takeuchi and Akira, 2008; Wilkins and Gale, 2010). Lack of RIG-I in mouse fibroblasts and cDCs results in defective production of type I IFNs and inflammatory cytokines in response to various RNA viruses, including VSV (Kato et al., 2006). VSV produces dsRNA in infected cells (Kato et al., 2008) and IFN-γ-inducing activity is highly reduced when these dsRNAs are disrupted; to evade RIG-I recognition, the dsRNA particles produced by VSV are much shorter in size than genomic RNA (Takeuchi and Akira, 2010). VSV G is essential for the induction of CD14/TLR4-dependent response pathway, which gives the adapter TRAM prime importance. Downstream of TRAM cascade, IRF7 activation leads to type 1 IFN production (Georgel et al., 2007).
Injection of VSV into immunocompetent mice via many routes results only in the induction of type I IFNs (Trottier et al., 2007; Reiss, 2009), and strong innate and adaptive immune responses with no viral replication or disease. Intranasal inoculation in mice results in infection of the olfactory bulb and retrograde progression in the central nervous system, resulting in acute encephalitis, break down of the blood–brain barrier with high rate of mortality (Olitsky et al., 1933; Huneycutt et al., 1993; Plakhov et al., 1995; Chauhan et al., 2010). Experiments with VSV infections of IFN-β-treated primary neurons and NB41A3 cells are consistent with a highly attenuated infection, associated with production of viral RNA and proteins, dysregulation of viral protein phosphorylation, and impaired viral assembly and release (Trottier et al., 2005; D'Agostino et al., 2009a).
In this study, to understand the role played by tetherin in mammalian neurons, we studied the expression of tetherin in murine neuroblastoma cells. We observed that expression was upregulated with both IFN-γ and IFN-β treatment. We silenced the expression of tetherin in murine neuroblastoma cells using shRNA. Tetherin-silenced cells were characterized and then subjected to VSV infection to compare the difference in viral titers with that of tetherin expressing cells.
Materials and Methods
Cell culture: virus
Murine NB41A3 cells (ATCC) maintained in F12K (Mediatech, Inc.) medium, supplied with 15% horse serum, 2.5% fetal bovine serum, and 1% penicillin/streptomycin (Trottier et al., 2005; D'Agostino and Reiss, 2010). VSV (Indiana serotype, San Juan strain) was the gift from Alice S. Huang (then at The Children's Hospital, Boston). Plaque assay on L929 adipocyte monolayers was used to measure the viral titer of supernatants obtained after VSV infection (Trottier et al., 2007), and was modified with an overlay of Methyl cellulose (Sigma) followed by incubation at 37°C overnight (Robin et al., 1982). Plates were then washed and plaques were counted and viral titer calculated. IFN-β, purchased from Cell Sciences, Inc., was used at 400 U/mL for 24 h treatment, as previously published (Trottier et al., 2005). Mouse IFN-γ (R&D Systems) treatments were done in complete media at 20 ng/mL and incubated at 37°C for 72 h, as previously published (Chesler et al., 2004).
Expression assays
Western blot assays, RNA extraction, RT-PCR, and immunofluorescence were performed as previously published (Ireland et al., 2005; D'Agostino and Reiss, 2010). For mRNA, purified RNA (5 μg) was amplified with tetherin forward and reverse primers by RT-PCR having sequences 5′ TCAGGAGTCCCTGGAGAAGA 3′ and 5′ ATTCTCCAGCTCCTGGTTCA 3′ (100 bp product). β-actin forward primer sequence was 5′ AAGAGCTATGAGCTGCCTGA 3′, and reverse primer sequence was 5′ TACGGATGTCAACGTCACAC 3′ (200 bp product). Primary antibodies for western blot included rabbit anti-BST2 (1:1,000; for tetherin; Sigma Aldrich), and rabbit anti-β-actin (1:2500; Abcam); and horseradish peroxidase-labeled donkey anti-rabbit IgG (1:2500; Zymax). For confocal microscopy on a Leica TCS SP5, the primary antibodies used were rabbit anti-Bst2 antibody (1: 500) for tetherin, mouse anti-NMDA receptor subunit 1 antibody (1:1000) (Abcam), and Alexa Fluor 633 (1:500; red; Invitrogen) and Alexa Fluor 488 (1:500; green; Invitrogen) as the secondary antibodies. Cells (unfixed) were treated with a primary antibody, and then permabilized and stained with DAPI to identify nuclei (D'Agostino and Reiss, 2010). In western blots and RT-PCR studies, individual bands on scanned films or gels were analyzed using UN-SCAN-IT™ gel software version 5.1.
Transfection with shRNA plasmids
Synthetic shRNA constructs (HuSH plasmid constructs from Origene™) used to silence the expression of tetherin include GFP and puromycin resistance as detection and selection markers, respectively. Turbofectin 8.0 (Invitrogen) solution, shRNA plasmid (100 ng/μL), and serum-free DMEM were mixed together and incubated at room temperature for 45 min and added to murine neuroblastoma cells (3×105), in a six-well culture dish followed by incubation of the cells at 37°C for an hour. A control plasmid containing scrambled shRNA (100 ng/μL) was also transfected same as above. After the incubation period, the medium was replaced with complete F12K medium and incubated at 37°C overnight. The medium was then replaced with medium containing puromycin (10 μg/mL). The expression of GFP was monitored by fluorescence microscopy (Olympus BH2; Olympus) to ensure the efficiency of transfection.
Flow cytometry
A BD FACS ARIA II Flow cytometer was used for both analyzing and positively selecting GFP expression of the tetherin shRNA-transfected cells. Cells were harvested after 2–3 passages and were re-suspended in chilled incomplete medium to prevent the division of cells during the assay. This was repeated several times to collect the maximum number of GFP-expressing transfected cells. Positively sorted tetherin knockdown cells were cultured as above.
Statistical analyses
All experimental samples were prepared in triplicate in at least 3 separate experiments. Sample t-values were calculated using Satterwaite's method for independent samples of unequal variances, and hypothesis testing was employed to determine whether or not quantities were equal, yielding p-values indicative of these tests. All error bars represent 95% confidence intervals of a particular dataset, unless otherwise stated.
Results
Tetherin is expressed in murine neuroblastoma cells and is upregulated by IFN-γ and IFN-β
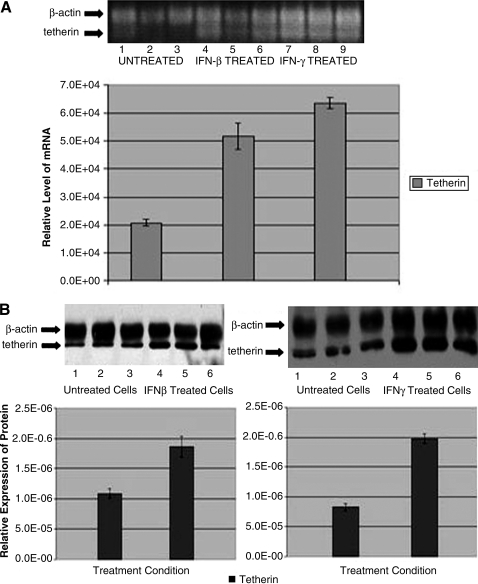
To study the expression of tetherin mRNA in murine neuroblastoma cells (NB41A3), RT-PCR was performed; this revealed the presence of tetherin and β-actin, a house keeping gene (Figs. 1A and 2A). Several previous studies have demonstrated IFN-β induction of tetherin in many different cell types (Ohtomo et al., 1999; Blasius et al., 2006; Neil et al., 2007; Kawai et al., 2008; Miyagi et al., 2009; Weidner et al., 2010; Dietrich et al., 2011). To determine whether type I (IFN-β) or type II (IFN-γ) regulate tetherin mRNA expression in murine neuroblastoma cells, we treated the cells overnight with IFN-β (400 U/mL) or for 72 h with IFN-γ (20 ng/mL). Figure 1A indicates that the expression of tetherin mRNA was upregulated in IFN-treated neurons. There was approximately a 2.5-fold increase in expression of tetherin mRNA in IFN-β-treated neuroblastoma cells (lanes 4–6) compared to the medium-treated neuroblastoma cells and 3-fold by type II IFN (lanes 7–9).
FIG. 1.
Tetherin is expressed in neuroblastoma cells and is upregulated by both interferon (IFN)-β and IFN-γ. (A) Tetherin mRNA was measured in lysates from control, IFN-β−, or IFN-γ-treated neuronal cells. Replicate cultures were treated with the medium (Control, lanes 1–3), IFN-β (400 U/mL for 24 h, lanes 4–6), or IFN-γ (20 ng/mL for 72 h, lanes 7–9). Tetherin (100 bp) and β-actin (200 bp) bands were detected, and intensities normalized. (B) Western blot analyses were performed on cell lysates isolated from medium-treated (lanes 1–3 on both left and right blots and bar graphs), IFN-β-treated (lanes 4–6, left panels), and IFN-γ-treated (lanes 4–6, right panels) samples. Tetherin (28 kDa) and β-actin (36 kDa) were detected. Data of the intensities of the tetherin bands were normalized to β-actin. All experiments were done in triplicates and replicated at least three times.
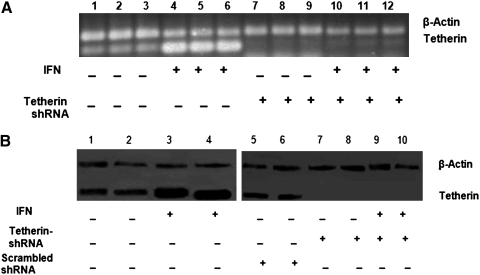
FIG. 2.
IFN-β upregulates tetherin expression in neuroblastoma cells, but has no impact on tetherin shRNA-treated cultures. (A) Tetherin mRNA expression (100 bp) was detected after either medium or IFN-β treatment, of control neuroblastoma cells and tetherin shRNA cultures. Cell lysates were prepared from medium-treated control neuronal cells (lanes 1–3), IFN-β-treated control cells (lanes 4–6), medium-treated tetherin shRNA cells (lanes 7–9), and IFN-β-treated tetherin shRNA cells (lanes 9–12). β-Actin was used as a housekeeping gene (200 bp). All experiments were performed in triplicate and replicated at least three times. (B) Western blot analyses were conducted to study tetherin expression. Cell lysates were prepared from medium-treated control neuronal cells (lanes 1–2), IFN-β-treated control cells (lanes 3 and 4), medium-treated scrambled shRNA-treated neuronal cells (lanes 5 and 6) medium-treated tetherin shRNA cells (lanes 7 and 8) and IFN-β-treated tetherin shRNA cells (lanes 9 and 10). Tetherin was observed at 28 kDa and β-actin at 36 kDa. All experiments were performed in triplicate and replicated at least three times.
To investigate if tetherin protein levels were induced by IFN-γ or IFN-β treatment, we performed western blot analysis on cell lysates. These data revealed a doubling of tetherin after IFN-β induction as compared to the medium-treated cells (left set of blots and bar graphs). There was more than a 2-fold increase in protein expression of tetherin in neuroblastoma IFN-γ-treated neuronal cells as compared to the medium-treated cells (right set of blots and graphs, Fig. 1B).
Silencing the expression of tetherin in neuroblastoma cells using shRNA vectors for infection studies with VSV
We silenced tetherin expression in neuroblastoma cells using synthetic shRNA constructs containing the tGFP gene, the expression of which was monitored continuously in the progeny cells to check the efficiency and success of transfection. Two different shRNA cassettes were used in this study: a purified and sequence verified expression plasmid with tetherin-specific shRNA and a purified and sequence verified plasmid containing noneffective 29-mer scrambled shRNA cassette. Transfectants expressing GFP were sorted (Supplementary Figs. S1 and S2, tetherin shRNA and scrambled shRNA, respectively; Supplementary Data are available online at www.liebertonline.com/dna) and expanded. RNA and protein from the transfected cells were isolated and subjected to RT-PCR (Fig. 2A) and western blot analysis (Fig. 2B). The expression of tetherin was completely silenced in tetherin-shRNA-transfected cells, whereas the scrambled shRNA-transfected cells expressed normal levels of tetherin; scrambled shRNA-treated cells were used as controls in further experiments. IFN-β treatment of silenced cells did not induce tetherin mRNA or protein expression.
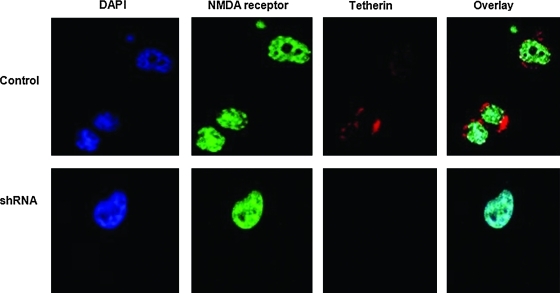
Morphologically, the tetherin-knockdown cells were smaller and rounder than the control lines. A confocal microscopic analysis also revealed the presence of tetherin in punctate clusters on the outside of the plasma membrane of control neuroblastoma cells (Fig. 3). The tetherin-silenced cells did not exhibit surface tetherin expression, but were positive for expression of a control surface GP, the NMDA receptor. These experiments indicate that we have established tetherin-deficient neuronal cells.
FIG. 3.
Detection of tetherin on the outer surface of neuronal cells but not on the silenced neuronal cells. Control NB41A3 (top row) and the tetherin-silenced neuronal cell line (bottom row) were incubated with anti-tetherin (red secondary antibody) and anti-NMDA-R1 (green secondary antibody) antibodies and afterward fixed and permeabilized and nuclei were detected with DAPI stain (blue). Slides were examined using a Leica confocal microscope. Images were overlaid. Images shown are representative of many fields from three replicate experiments. Color images available online at www.liebertonline.com/dna
Upregulation of tetherin restricts VSV release in neuroblastoma cells but tetherin knockdown cells are IFN unresponsive for suppressing infectious VSV progeny
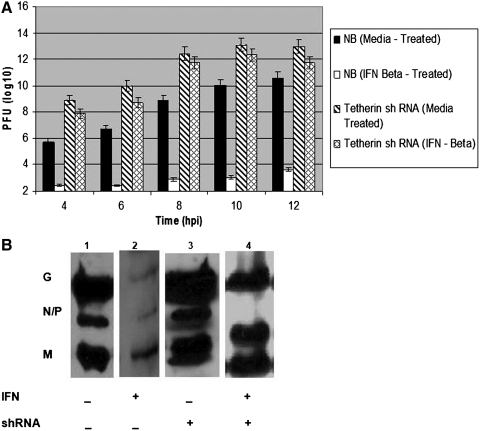
After silencing the expression of tetherin in neuroblastoma cells, we examined the replication restriction of VSV virion release in IFN-treated control cells as compared to tetherin-silenced cells. Supernatants were collected at 4, 6, 8, 10, and 12 h postinfection (hpi), and assayed for viral titer on L929 monolayers (Fig. 4A). There was a steady increase in viral titers in supernatants up to 10 hpi in medium-treated neuroblastoma cells (solid bars). In IFN-β-treated control cells, the yield was 10,000-fold less than pfu with medium-treated cells (empty bars), recapitulating our published data (Trottier et al., 2005; D'Agostino and Reiss, 2010).
FIG. 4.
Impact of tetherin expression on vesicular stomatitis virus (VSV) replication. (A) Supernatants from VSV-infected neuronal cells were assayed for infectious virus by plaque assay. Control neuronal cells or tetherin-silenced cells were incubated with the medium (solid fill or slashed fill, respectively) or with IFN-β (empty bars or cross-hatched bar fill, respectively) for 24 h before infection at 3 multiplicity of infection (moi). Supernatants from triplicate cultures were collected at 4, 6, 8, 10, and 12 h postinfection (hpi) and serially diluted before adding to L929 cells. The bars represent mean plaque forming units detected. Error bars represent 3 SD; the experiment was replicated three times. (B) Expression of VSV G, N, P, and M proteins was determined by western blot analyses performed with cell lysates (8–9 hpi) of neuroblastoma cells (lanes 1 and 2) and tetherin-silenced cells (lanes 3 and 4) pretreated with the medium (lanes 1 and 3) or with IFN-β-treated (lanes 2 and 4) for 24 h. The bands were 55 kDa for VSV G protein, 45 kDa for VSV N and P proteins, and 25 kDa for VSV M protein. Mouse GAPDH was used as loading control. Data are representative of three replicate experiments, each with triplicate cultures at each condition.
We performed the VSV infection in medium-treated tetherin-silenced NB41A3 cells and observed that there is 100-fold increase in infectious virus released into the supernatants when tetherin is silenced in neuroblastomas as compared to the normal tetherin-expressing cells (Fig. 4A, slashed bar fill) and there was ∼10-fold IFN-mediated inhibition in the tetherin-silenced cells (cross-hatched bar fill). This is consistent with a central role for tetherin in the IFN-mediated antiviral effect in neuronal cells.
Morphological examination of the cells after VSV infection showed a profound difference between IFN-β-pretreated and medium-treated control cells. The medium-treated neuroblastoma cells showed clumping of the cells with a slightly rounded morphology, a cytopathic effect, reflecting the development of apoptosis, as early as 4 hpi and continuing until 12 hpi with dead cells floating in the medium. In contrast, the IFN-β-pretreated control neuronal cells exhibited a spread out morphology with few dead cells, even at 12 hpi. Tetherin-silenced cells were more clumped and exhibited a rounded appearance both in uninfected and VSV-infected conditions. The number of floating cells was higher in the infected-silenced cells, and there was no difference in the appearance of IFN-treated, VSV-infected knockdown cells (data not shown).
We had previously observed an inhibition, but not ablation, of viral protein expression in IFN-treated neuronal cells (Trottier et al., 2005; D'Agostino et al., 2009a; D'Agostino and Reiss, 2010). Western blot analysis for expression of viral proteins (G, N and P, and M) was performed after extracting the cell lysates 8–9 hpi from neuroblastoma cells and tetherin-silenced cells (both IFN-β-treated and medium-treated). The results showed the presence of VSV proteins in tetherin-silenced cells (both medium-treated and IFN-β treated), and tetherin-expressing neuroblastoma cells (both medium-treated and IFN-β treated). GAPDH was used as loading control. The intensity of VSV protein bands was comparatively higher in tetherin-silenced cells than tetherin-expressing neuroblastoma cells and much greater in mock-treated than in IFN-treated cells, whether control or tetherin-silenced (Fig. 4B). Thus, IFN treatment was effective in inhibiting some of the viral protein synthesis, but not in preventing virus release from tetherin-silenced cells.
Discussion
Murine neuroblastoma cells used in this research are originally derived from neoplastic tumors and also they are similar in responses to IFN treatment of primary olfactory neurons (Schubert et al., 1969; Breakfield et al., 1975; Chesler et al., 2003). In this research we demonstrated that tetherin is expressed in neuronal cells and is upregulated by both IFN-γ and IFN-β (Fig. 1). The mRNA and protein studies demonstrated upregulation of tetherin by IFN-β approximately 2.5 and 2-fold, respectively. After IFN-γ treatment, a 3-fold increase in mRNA and greater than 2-fold increase in protein were observed. Induction of tetherin by IFNs in non-neuronal cells has been studied by many authors in different cell types and concluded to be cell type-dependant (Neil et al., 2007; Van Damme et al., 2008; Miyagi et al., 2009; Rong et al., 2009).
We used shRNA expressed on a plasmid to transfect the neuronal cells and develop tetherin knocked down lines. They were positively selected by puromycin and flow cytometry (Supplementary Fig. S1) and expanded before characterization. RNA and protein analysis (Fig. 2A, B) confirmed the successful knockdown in the expression of tetherin, with mRNA and protein undetectable in cell lysates, and on the neuronal cell surface (Fig. 3).
Published immunofluorescence studies using 293T cells indicated punctate cell surface expression of tetherin (Hinz et al., 2010), we saw the same distribution of tetherin in our confocal microscopic analysis of neuroblastoma cells (Fig. 3). Confocal microscopic images show the presence of NMDA receptor uniformly expressed on control and shRNA-transfected cells, but not tetherin, which was silenced (Fig. 3). Expression of tetherin was below the level of detection on transfected, GFP-expressing cells, even after treatment with IFN-β, consistent with gene silencing (not shown). Morphological examination of tetherin-silenced cells in our study indicated smaller and rounder cells as compared to the neuroblastoma cells. An explanation for the altered cell appearance may be the interaction of tetherin with the cytoskeleton (Rollason et al., 2009).
In humans, chromosome number 19p13.2 carries a single copy of the tetherin gene, which is organized as four exons and is nonpolymorphic (Sugiyama et al., 1995). Orthologs of this occurs in Old World monkeys and placental mammals. Several cell types, including T cells, B cells, monocytes, macrophages, and plasmacytoid dendritic cells (PDC), express tetherin constitutively (Sugiyama et al., 1995; Vidal-Laliena et al., 2005). Before this report, tetherin expression has not been described on neurons.
The virions retained by tetherin undergo endocytosis followed by lysosomal degradation (Neil et al., 2008; Hammonds and Spearman, 2009; Sato et al., 2009). The destruction of virions has been shown by biochemical and immunoelectron microscopic methods (Evans et al., 2010). Tetherin forms a bridge between virions and the plasma membrane (Sato et al., 2009). Tetherin contributes to antiviral activity against various enveloped viruses, including Ebola, Marburg, Lassa, KSHV, HIV-1, HIV-2, SIV, MLV, E1AV, VSV, HTLV-1, XMRV, and spumaviruses (Jouvenet et al., 2009; Kaletsky et al., 2009; Le Tortorec and Neil, 2009; Mansouri et al., 2009; Miyakawa et al., 2009; Sakuma et al., 2009; Groom et al., 2010; Weidner et al., 2010; Xu et al., 2011).
Many viruses have evolved pathways to evade host antiviral mechanisms by disabling the IFN pathway (Versteeg and Garcia-Sastre, 2010) or by using proteins to antagonize tetherin activity and remove it from the plasma membrane. The coimmunoprecipitation of HIV-1 vpu and tetherin results from the interaction of their transmembrane domains, and this association was found to be critical for reducing cell-surface tetherin expression (Dube et al., 2010; Jolly et al., 2010). Tetherin and HIV-1 Gag accumulate at the contact zone between infected and target cells, but tetherin cannot prevent the formation of viral synapses; together, tetherin and HIV-1 virions are transferred to target cells as abnormally large patches that are impaired in their fusion capacities (Casartelli et al., 2010).
Studies with the Ebola virus GP show evasion the antiviral effect of both murine and human tetherin and coimmunoprecipitation. Studies indicate that Ebola GP is active against an artificial tetherin construct without removing the protein from the cell surface, suggesting that it is possible to overcome this restriction by mechanisms other than blocking tetherin's cell surface expression (Lopez et al., 2010).
Similarly, the K5 protein of KSHV (HHV8) antagonizes conserved lysine residues of primate tetherin by targeting it for ubiquitination (Pardieu et al., 2010). K5 induces a species-specific downregulation of human tetherin and endosomal degradation as well. SIV negative regulatory factor (Nef) antagonizes nonhuman primate orthologs of tetherin (Bartee et al., 2006; Gupta et al., 2009; Jia et al., 2009; Zhang et al., 2009).
Cotranslational movement of tetherin into endoplasmic reticulum, transportation through the Golgi apparatus in COPII-coated vesicles to the plasma membrane comprises its expression pattern (Rollason et al., 2007). In PDCs, tetherin inhibits the expression of type 1 IFNs and other proinflammatory cytokines to prevent hyper activation of immune system by binding to immunoglobulin-like transcript 7 (ILT7) (Cao et al., 2009). Experiments using artificial tetherin suggest that it may be possible to develop tetherin-based restriction factors to prevent a wide range of viral infections and which could also overcome the evasion of antagonists produced by many viruses (Perez-Caballero et al., 2009).
As we have previously demonstrated, neuroblastoma cells pretreated with IFN-β showed a considerable decrease in both viral proteins in lysates and viral yields from supernatants (Trottier et al., 2005; D'Agostino et al., 2009a) than medium-treated cells, consistent with the powerful antiviral cell-autonomous response induced by IFN-β. There was approximately a 10,000-fold decrease in viral titers obtained from IFN-β-treated samples as compared to medium-treated cells (Fig. 4A). After knocking down the expression of tetherin, medium-treated neuronal cells were found to yield more than 100-fold more virus after VSV infection. Our observation was consistent with the results from Ju-Tao Guo's laboratory obtained from a BHK-21 FLP-IN T Rex-derived stable tetherin knockdown cell line (Weidner et al., 2010); they did not assess the role of IFN treatment on the tetherin-deficient line. We found that there was no significant difference between the viral yield in medium-treated tetherin shRNA cells and IFN-β-treated tetherin-silenced neuronal cells (Fig. 4A). This unexpected finding is consistent with a primary role of tetherin in the antiviral effect of IFN in neuronal cells.
Previously, we had observed that IFN treatment has a significant effect on the levels of VSV viral proteins produced in neuronal cells, but that PKR was not induced (Trottier et al., 2005); we assessed the impact of IFN treatment of the knockdown cells by western blot analyses of cell lysates at 8–9 hpi. VSV proteins G, N, P, and M were expressed more highly in neuroblastoma (medium-treated) cells, than the IFN-β pretreated control neuroblastoma cell lysates. The expression of VSV proteins were substantially higher in tetherin knocked down cells than control neuronal cells, but IFN treatment did (modestly) inhibit VSV protein synthesis in the tetherin shRNA cells (Fig. 4B).
Clearly, additional cell autonomous antiviral pathways, including altered kinase/phosphatase activity, are regulated by IFN-γ and impede viral protein expression (D'Agostino et al., 2009b). Thus, IFN treatment reduced viral protein production in the tetherin-silenced cells, but was unable to suppress release of assembled infectious progeny virions.
Conclusions
We have demonstrated that tetherin is constitutively expressed by murine neuronal cells. Data obtained from this research clearly indicate the importance of tetherin in contributing to the cell autonomous antiviral immune response in mammalian neuronal cells by restricting the release of virions. Tetherin expression in neurons is positively regulated by both type I and type II IFN. Tetherin is expressed in a punctate pattern on the surface of neurons. Tetherin-silenced cells were functionally unresponsive to the antiviral actions of IFN treatment.
Supplementary Material
Acknowledgments
We thank Ms. Pui-Leng (NYU, Biology Department and Center for Genomics and Systems Biology) for assisting us with FACS analysis and Dr. Ignatius Tan (NYU, Biology department) for training in confocal microscopy, Ben tenOever (Mt Sinai School of Medicine, NYC) for advice on the methyl cellulose plaque assay procedure. This work was supported by an M.S. Biology student research grant from New York University (to S.S.), a CAS Dean's Undergraduate Research Fund award (to T.T.), a grant from the NIH NS039746 (to C.S.R.), and M7543, a grant from the NYU FAS Dean for Science (to C.S.R.).
Disclosure Statement
No competing financial interests exist.
References
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- Akira S. Uematsu S. Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bartee E. McCormack A. Fruh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2006;2:e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum A. Garcia-Sastre A. Induction of type I interferon by RNA viruses: cellular receptors and their substrates. Amino Acids. 2010;38:1283–1299. doi: 10.1007/s00726-009-0374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius A.L. Giurisato E. Cella M. Schreiber R.D. Shaw A.S. Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- Breakfield X.O. Neale E.A. Neale J.H. Jacobowitz D.M. Localized catecholamine storage associated with granules in murine neuroblastoma cells. Brain Res. 1975;92:237–256. doi: 10.1016/0006-8993(75)90273-5. [DOI] [PubMed] [Google Scholar]
- Cao W. Bover L. Cho M. Wen X. Hanabuchi S. Bao M., et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casartelli N. Sourisseau M. Feldmann J. Guivel-Benhassine F. Mallet A. Marcelin A.G., et al. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog. 2010;6:e1000955. doi: 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V.S. Furr S.R. Sterka D.G., Jr. Nelson D.A. Moerdyk-Schauwecker M. Marriott I., et al. Vesicular stomatitis virus infects resident cells of the central nervous system and induces replication-dependent inflammatory responses. Virology. 2010;400:187–196. doi: 10.1016/j.virol.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Chesler D.A. McCutcheon J.A. Reiss C.S. Posttranscriptional regulation of neuronal nitric oxide synthase expression by IFN-gamma. J Interferon Cytokine Res. 2004;24:141–149. doi: 10.1089/107999004322813381. [DOI] [PubMed] [Google Scholar]
- Chesler D.A. Munoz-Jordan J.L. Donelan N. Garcia-Sastre A. Reiss C.S. PKR is not required for interferon-gamma inhibition of VSV replication in neurons. Viral Immunol. 2003;16:87–96. doi: 10.1089/088282403763635474. [DOI] [PubMed] [Google Scholar]
- Chesler D.A. Reiss C.S. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 2002;13:441–454. doi: 10.1016/s1359-6101(02)00044-8. [DOI] [PubMed] [Google Scholar]
- D'Agostino P.M. Amenta J.J. Reiss C.S. IFN-beta-induced alteration of VSV protein phosphorylation in neuronal cells. Viral Immunol. 2009a;22:353–369. doi: 10.1089/vim.2009.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino P.M. Yang J. Reiss C.S. Distinct mechanisms of inhibition of VSV replication in neurons mediated by type I and type II IFN. Virus Rev Res. 2009b;14:20–29. [PMC free article] [PubMed] [Google Scholar]
- D'Agostino P.M. Reiss C.S. A confocal and electron microscopic comparison of interferon beta-induced changes in vesicular stomatitis virus infection of neuroblastoma and nonneuronal cells. DNA Cell Biol. 2010;29:103–120. doi: 10.1089/dna.2009.0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich I. McMonagle E.L. Pelit S.J. Vijayakrishnan S. Logan N. Chari C.N., et al. Feline tetherin efficiently restricts release of feline immunodeficiency virus but not spreading of infection. J Virol. 2001;85:5840–5852. doi: 10.1128/JVI.00071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M. Roy B.B. Guiot-Guillain P. Binette J. Mercier J. Chiasson A., et al. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 2010;6:e1000856. doi: 10.1371/journal.ppat.1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin J.E. Hackenmiller R. Simon M.C. Levy D.E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- Evans D.T. Serra-Moreno R. Singh R.K. Guatelli J.C. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010;18:388–396. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgel P. Jiang Z. Kunz S. Janssen E. Mols J. Hoebe K., et al. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Goodbourn S. Didcock L. Randall R.E. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- Groom H.C. Yap M.W. Galao R.P. Neil S.J. Bishop K.N. Susceptibility of xenotropic murine leukemia virus-related virus (XMRV) to retroviral restriction factors. Proc Natl Acad Sci U S A. 2010;107:5166–5171. doi: 10.1073/pnas.0913650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K. Mlcochova P. Pelchen-Matthews A. Petit S.J. Mattiuzzo G. Pillay D., et al. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc Natl Acad Sci U S A. 2009;106:20889–20894. doi: 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O. Kochs G. Weber F. Interferon, Mx, and viral countermeasures. Cytokine Growth Factor Rev. 2007;18:425–433. doi: 10.1016/j.cytogfr.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammonds J. Spearman P. Tetherin is as tetherin does. Cell. 2009;139:456–457. doi: 10.1016/j.cell.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Hinz A. Miguet N. Natrajan G. Usami Y. Yamanaka H. Renesto P., et al. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe. 2010;7:314–323. doi: 10.1016/j.chom.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneycutt B.S. Bi Z. Aoki C.J. Reiss C.S. Central neuropathogenesis of vesicular stomatitis virus infection of immunodeficient mice. J Virol. 1993;67:6698–6706. doi: 10.1128/jvi.67.11.6698-6706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.Y. Hertzog P.J. Holland K.A. Sumarsono S.H. Tymms M.J. Hamilton J.A., et al. A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons alpha and beta and alters macrophage responses. Proc Natl Acad Sci U S A. 1995;92:11284–11288. doi: 10.1073/pnas.92.24.11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland D.D. Palian B.M. Reiss C.S. Interleukin (IL)-12 receptor beta1 or IL-12 receptor beta 2 deficiency in mice indicates that IL-12 and IL-23 are not essential for host recovery from viral encephalitis. Viral Immunol. 2005;18:397–402. doi: 10.1089/vim.2005.18.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B. Serra-Moreno R. Neidermyer W. Rahmberg A. Mackey J. Fofana I.B., et al. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5:e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C. Booth N.J. Neil S.J. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J Virol. 2010;84:12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N. Neil S.J. Zhadina M. Zang T. Kratovac Z. Lee Y., et al. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83:1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky R.L. Francica J.R. Agrawal-Gamse C. Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci U S A. 2009;106:2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H. Takeuchi O. Mikamo-Satoh E. Hirai R. Kawai T. Matsushita K., et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H. Takeuchi O. Sato S. Yoneyama M. Yamamoto M. Matsui K., et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kawai S. Azuma Y. Fujii E. Furugaki K. Ozaki S. Matsumoto T., et al. Interfeson-alpha enhances CD317 expression and the antitumor activity of anti-CD317 monoclonal antibody in renal cell carcinoma xenograft models. Cancer Sci. 2008;99:2461–2466. doi: 10.1111/j.1349-7006.2008.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl B.D. Cheng V. Wainberg M.A. Liang C. Tetherin and its viral antagonists. J Neuroimmune Pharmacol. 2011;6:188–201. doi: 10.1007/s11481-010-9256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai Y. Takeuchi O. Akira S. Pathogen recognition by innate receptors. J Infect Chemother. 2008;14:86–92. doi: 10.1007/s10156-008-0596-1. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A. Neil S.J. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J Virol. 2009;83:11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D.E. Darnell J.E., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- Levy D.E. Garcia-Sastre A. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 2001;12:143–156. doi: 10.1016/s1359-6101(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Lopez L.A. Yang S.J. Hauser H. Exline C.M. Haworth K.G. Oldenburg J., et al. Ebola virus glycoprotein counteracts BST-2/Tetherin restriction in a sequence-independent manner that does not require tetherin surface removal. J Virol. 2010;84:7243–7255. doi: 10.1128/JVI.02636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M. Viswanathan K. Douglas J.L. Hines J. Gustin J. Moses A.V., et al. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J Virol. 2009;83:9672–9681. doi: 10.1128/JVI.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraz M.A. White J.M. Sheehan K.C. Bach E.A. Rodig S.J. Dighe A.S., et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- Miyagi E. Andrew A.J. Kao S. Strebel K. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc Natl Acad Sci U S A. 2009;106:2868–2873. doi: 10.1073/pnas.0813223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa K. Ryo A. Murakami T. Ohba K. Yamaoka S. Fukuda M., et al. BCA2/Rabring7 promotes tetherin-dependent HIV-1 restriction. PLoS Pathog. 2009;5:e1000700. doi: 10.1371/journal.ppat.1000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi H. Fujita Y. Yanai H. Sakaguchi S. Ouyang X. Shinohara M., et al. Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program. Proc Natl Acad Sci U S A. 2006;103:15136–15141. doi: 10.1073/pnas.0607181103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil S.J. Sandrin V. Sundquist W.I. Bieniasz P.D. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2:193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil S.J. Zang T. Bieniasz P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Ohtomo T. Sugamata Y. Ozaki Y. Ono K. Yoshimura Y. Kawai S., et al. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem Biophys Res Commun. 1999;258:583–591. doi: 10.1006/bbrc.1999.0683. [DOI] [PubMed] [Google Scholar]
- Olitsky P.K. Cox H.R. Syverton J.T. Infection in mice following instillation of Vesicular Stomatitis virus. Science. 1933;77:611–612. doi: 10.1126/science.77.2008.611. [DOI] [PubMed] [Google Scholar]
- Pardieu C. Vigan R. Wilson S.J. Calvi A. Zang T. Bieniasz P., et al. The RING-CH ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 2010;6:e1000843. doi: 10.1371/journal.ppat.1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D. Zang T. Ebrahimi A. McNatt M.W. Gregory D.A. Johnson M.C., et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139:499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Krause C.D. Walter M.R. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- Pichlmair A. Reis e Sousa C. Innate recognition of viruses. Immunity. 2007;27:370–383. doi: 10.1016/j.immuni.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Plakhov I.V. Arlund E.E. Aoki C. Reiss C.S. The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology. 1995;209:257–262. doi: 10.1006/viro.1995.1252. [DOI] [PubMed] [Google Scholar]
- Reiss C.S. VSV infection elicits distinct host responses in the periphery and the brain. In: Yang D., editor. RNA Viruses: Host Gene Responses to Infection. World Scientific Publishing; Hackensack, NJ: 2009. pp. 229–246. [Google Scholar]
- Roberts R.M. Ezashi T. Rosenfeld C.S. Ealy A.D. Kubisch H.M. Evolution of the interferon tau genes and their promoters, and maternal-trophoblast interactions in control of their expression. Reprod Suppl. 2003;61:239–251. [PubMed] [Google Scholar]
- Robin J. Lariviere-Durand C. Berthiaume L. Plaque assay of bluegill virus using a methylcellulose overlay. J Virol Methods. 1982;5:351–354. doi: 10.1016/0166-0934(82)90027-1. [DOI] [PubMed] [Google Scholar]
- Rollason R. Korolchuk V. Hamilton C. Jepson M. Banting G. A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J Cell Biol. 2009;184:721–736. doi: 10.1083/jcb.200804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollason R. Korolchuk V. Hamilton C. Schu P. Banting G. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J Cell Sci. 2007;120:3850–3858. doi: 10.1242/jcs.003343. [DOI] [PubMed] [Google Scholar]
- Rong L. Zhang J. Lu J. Pan Q. Lorgeoux R.P. Aloysius C., et al. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by human immunodeficiency virus type 1 Vpu. J Virol. 2009;83:7536–7546. doi: 10.1128/JVI.00620-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J.K. Whitt M.A. Rhabdoviridae: the viruses and their replication. In: Knipe D.M., editor; Howley P.M., editor. Fields Virology. Lippincott, Williams & Wilkins; Philadephia, PA: 2001. pp. 1221–1244. [Google Scholar]
- Sadler A.J. Williams B.R. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T. Noda T. Urata S. Kawaoka Y. Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J Virol. 2009;83:2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel C.E. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. Yamamoto S.P. Misawa N. Yoshida T. Miyazawa T. Koyanagi Y. Comparative study on the effect of human BST-2/Tetherin on HIV-1 release in cells of various species. Retrovirology. 2009;6:53. doi: 10.1186/1742-4690-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D. Specht A. Kirchhoff F. Tetherin: holding on and letting go. Cell. 2010;141:392–398. doi: 10.1016/j.cell.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Schubert D. Humphreys S. Baroni C. Cohn M. In vitro differentiation of a mouse neuroblastoma. Proc Natl Acad Sci U S A. 1969;64:316–323. doi: 10.1073/pnas.64.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert H.L. Zhai Q. Sandrin V. Eckert D.M. Garcia-Maya M. Saul L., et al. Structural and functional studies on the extracellular domain of BST2/tetherin in reduced and oxidized conformations. Proc Natl Acad Sci U S A. 2010;107:17951–17956. doi: 10.1073/pnas.1008206107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojdl D.F. Lichty B. Knowles S. Marius R. Atkins H. Sonenberg N., et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y. Odaka H. Itokawa S. Ishikawa E. Tomari Y. Ikeda H. TMP-153, a novel ACAT inhibitor, lowers plasma cholesterol through its hepatic action in golden hamsters. Atherosclerosis. 1995;118:145–153. doi: 10.1016/0021-9150(95)05601-r. [DOI] [PubMed] [Google Scholar]
- Takeuchi O. Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Takeuchi O. Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Trottier M.D., Jr. Palian B.M. Reiss C.S. VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology. 2005;333:215–225. doi: 10.1016/j.virol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Trottier M.D. Lyles D.S. Reiss C.S. Peripheral, but not central nervous system, type I interferon expression in mice in response to intranasal vesicular stomatitis virus infection. J Neurovirol. 2007;13:433–445. doi: 10.1080/13550280701460565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N. Goff D. Katsura C. Jorgenson R.L. Mitchell R. Johnson M.C., et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pesch V. Lanaya H. Renauld J.C. Michiels T. Characterization of the murine alpha interferon gene family. J Virol. 2004;78:8219–8228. doi: 10.1128/JVI.78.15.8219-8228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg G.A. Garcia-Sastre A. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol. 2010;13:508–516. doi: 10.1016/j.mib.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Laliena M. Romero X. March S. Requena V. Petriz J. Engel P. Characterization of antibodies submitted to the B cell section of the 8th Human Leukocyte Differentiation Antigens Workshop by flow cytometry and immunohistochemistry. Cell Immunol. 2005;236:6–16. doi: 10.1016/j.cellimm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Weidner J.M. Jiang D. Pan X.B. Chang J. Block T.M. Guo J.T. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J Virol. 2010;84:12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins C. Gale M., Jr Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22:41–47. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F. Tan J. Liu R. Xu D. Li Y. Geng Y., et al. Tetherin Inhibits Prototypic Foamy Virus Release. Virol J. 2011;8:198. doi: 10.1186/1743-422X-8-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. Wilson S.J. Landford W.C. Virgen B. Gregory D. Johnson M.C., et al. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6:54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.X. Matsui Y. Hadaschik B.A. Lee C. Jia W. Bell J.C., et al. Down-regulation of type I interferon receptor sensitizes bladder cancer cells to vesicular stomatitis virus-induced cell death. Int J Cancer. 2010;127:830–838. doi: 10.1002/ijc.25088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.