Abstract
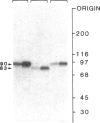
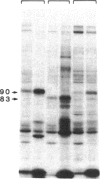
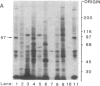
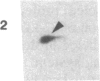
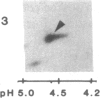
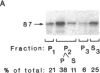
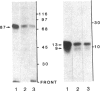
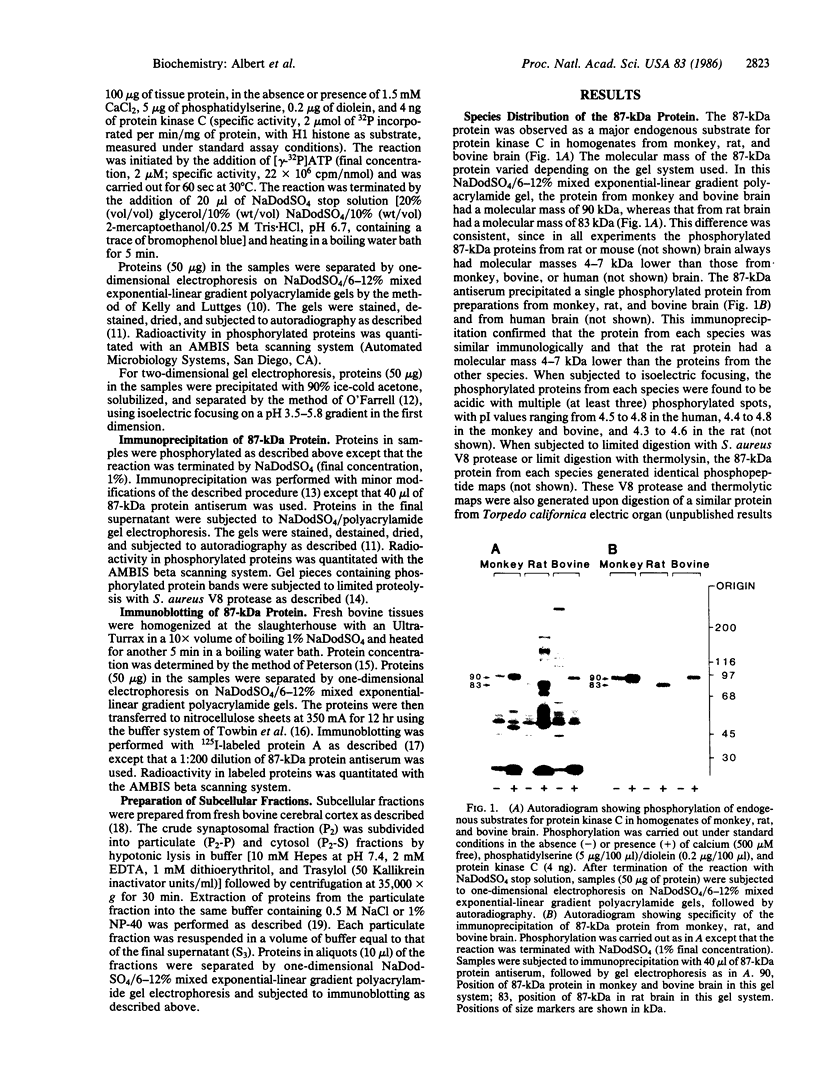
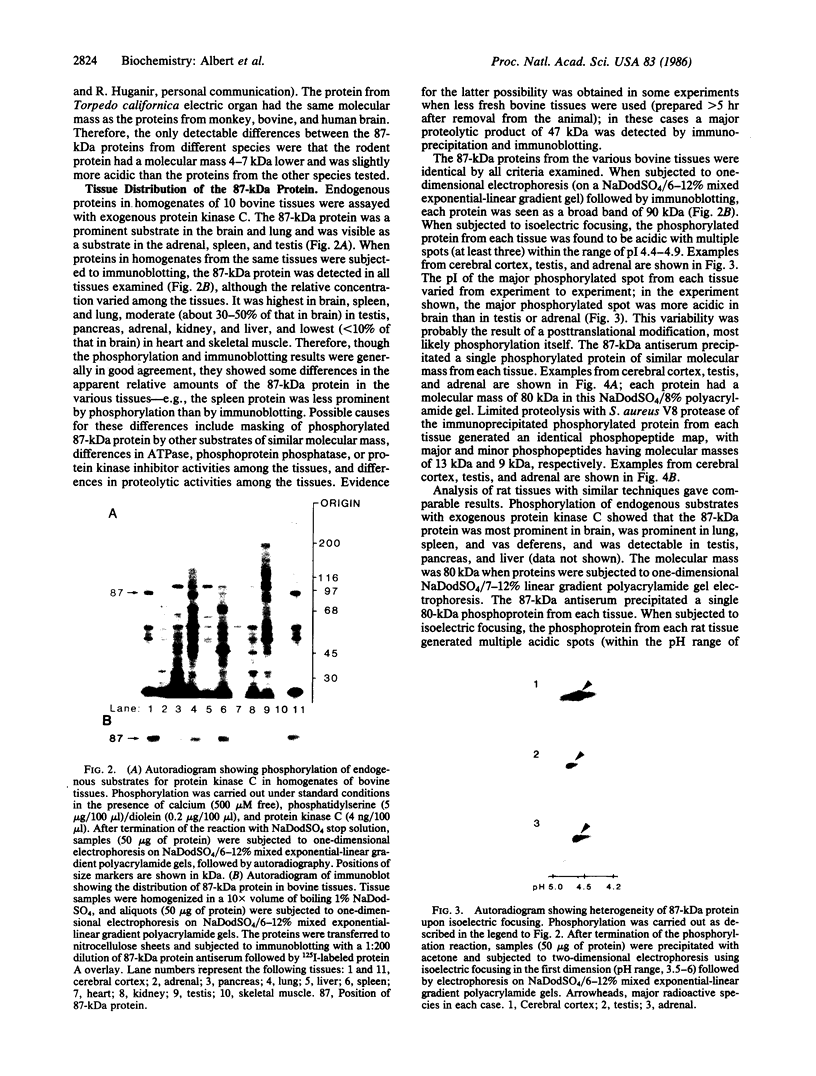
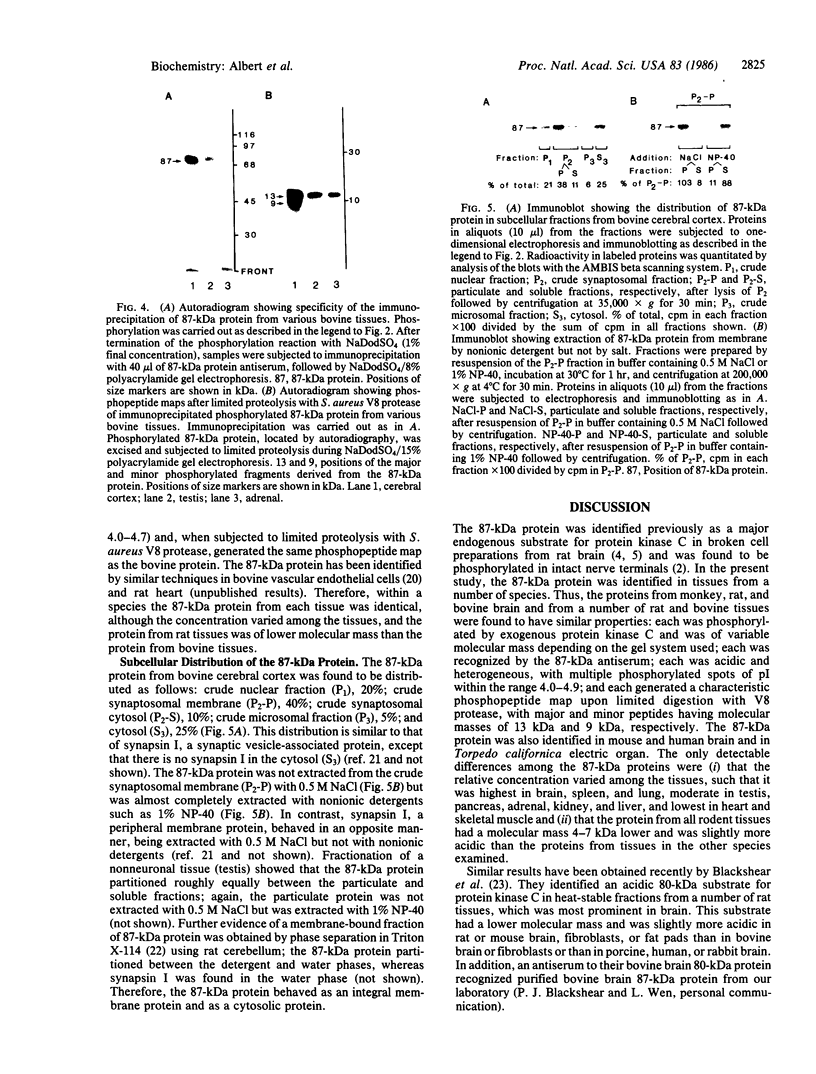
An 87-kDa phosphoprotein, identified previously as a major, specific substrate for Ca2+/phospholipid/diacylglycerol-dependent protein kinase (protein kinase C) in broken cell preparations from rat brain, has been characterized with respect to its species, tissue, and subcellular distribution. A similar protein was present in monkey, human, mouse, and bovine brain and in Torpedo californica electric organ. The protein was also identified in a variety of nonneuronal rat and bovine tissues. The rat protein had an apparent molecular mass 4-7 kDa lower, and was slightly more acidic, than the protein in bovine tissues. The 87-kDa proteins from various bovine tissues were identical by the following criteria: each was phosphorylated by exogenous protein kinase C, was of comparable molecular mass, generated multiple spots within the pH range of 4.4-4.9 upon isoelectric focusing, yielded identical patterns upon digestion with Staphylococcus aureus V8 protease, and was recognized by a specific 87-kDa antiserum. The relative concentrations of the 87-kDa protein in bovine tissues were highest in brain, spleen, and lung, moderate in testis, pancreas, adrenal, kidney, and liver, and lowest in heart and skeletal muscle. In the brain, the 87-kDa protein was concentrated in the synaptosomal membrane and in the cytosol. The membrane-bound protein was extractable with nonionic detergents but not with NaCl. This species, tissue, and subcellular distribution of the 87-kDa protein is similar to that of protein kinase C.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert K. A., Wu W. C., Nairn A. C., Greengard P. Inhibition by calmodulin of calcium/phospholipid-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3622–3625. doi: 10.1073/pnas.81.12.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Blackshear P. J., Witters L. A., Girard P. R., Kuo J. F., Quamo S. N. Growth factor-stimulated protein phosphorylation in 3T3-L1 cells. Evidence for protein kinase C-dependent and -independent pathways. J Biol Chem. 1985 Oct 25;260(24):13304–13315. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Nestler E. J., Chehrazi B., Greengard P. Distribution of protein I in mammalian brain as determined by a detergent-based radioimmunoassay. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2130–2134. doi: 10.1073/pnas.78.4.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa K., Nishizuka Y. Phosphatidylinositol turnover in receptor mechanism and signal transduction. Annu Rev Pharmacol Toxicol. 1985;25:147–170. doi: 10.1146/annurev.pa.25.040185.001051. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh N., Kuo J. F. Subcellular distribution of phospholipid-sensitive calcium-dependent protein kinase in guinea pig heart, spleen and cerebral cortex, and inhibition of the enzyme by Triton X-100. Biochem Biophys Res Commun. 1982 May 31;106(2):590–595. doi: 10.1016/0006-291x(82)91151-2. [DOI] [PubMed] [Google Scholar]
- Kelly P. T., Luttges M. W. Electrophoretic separation of nervous system proteins on exponential gradient polyacrylamide gels. J Neurochem. 1975 May;24(5):1077–1079. doi: 10.1111/j.1471-4159.1975.tb03680.x. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kuo J. F., Andersson R. G., Wise B. C., Mackerlova L., Salomonsson I., Brackett N. L., Katoh N., Shoji M., Wrenn R. W. Calcium-dependent protein kinase: widespread occurrence in various tissues and phyla of the animal kingdom and comparison of effects of phospholipid, calmodulin, and trifluoperazine. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7039–7043. doi: 10.1073/pnas.77.12.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson J. M., Whitehouse S., Walsh D. A. Possibility of shape conformers of the protein inhibitor of the cyclic adenosine monophosphate dependent protein kinase. Biochemistry. 1979 Oct 30;18(22):4835–4845. doi: 10.1021/bi00589a011. [DOI] [PubMed] [Google Scholar]
- Minakuchi R., Takai Y., Yu B., Nishizuka Y. Widespread occurrence of calcium-activated, phospholipid-dependent protein kinase in mammalian tissues. J Biochem. 1981 May;89(5):1651–1654. doi: 10.1093/oxfordjournals.jbchem.a133362. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Rodriguez-Pena M., Smith K. A. Phorbol esters, phospholipase C, and growth factors rapidly stimulate the phosphorylation of a Mr 80,000 protein in intact quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7244–7248. doi: 10.1073/pnas.80.23.7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Nakayama T., Kobayashi T. Effect upon mitogenic stimulation of calcium-dependent phosphorylation of cytoskeleton-associated 350,000- and 80,000-mol-wt polypeptides in quiescent 3Y1 cells. J Cell Biol. 1985 Mar;100(3):748–753. doi: 10.1083/jcb.100.3.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- Walaas S. I., Nairn A. C., Greengard P. Regional distribution of calcium- and cyclic adenosine 3':5'-monophosphate-regulated protein phosphorylation systems in mammalian brain. I. Particulate systems. J Neurosci. 1983 Feb;3(2):291–301. doi: 10.1523/JNEUROSCI.03-02-00291.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas S. I., Nairn A. C., Greengard P. Regional distribution of calcium- and cyclic adenosine 3':5'-monophosphate-regulated protein phosphorylation systems in mammalian brain. II. Soluble systems. J Neurosci. 1983 Feb;3(2):302–311. doi: 10.1523/JNEUROSCI.03-02-00302.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. C., Walaas S. I., Nairn A. C., Greengard P. Calcium/phospholipid regulates phosphorylation of a Mr "87k" substrate protein in brain synaptosomes. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5249–5253. doi: 10.1073/pnas.79.17.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]