Abstract
GABA projection neurons (GABA neurons) in the substantia nigra pars reticulata (SNr) and dopamine projection neurons (DA neurons) in substantia nigra pars compacta (SNc) have strikingly different firing properties. SNc DA neurons fire low-frequency, long-duration spikes, whereas SNr GABA neurons fire high-frequency, short-duration spikes. Since voltage-activated sodium (NaV) channels are critical to spike generation, the different firing properties raise the possibility that, compared with DA neurons, NaV channels in SNr GABA neurons have higher density, faster kinetics, and less cumulative inactivation. Our quantitative RT-PCR analysis on immunohistochemically identified nigral neurons indicated that mRNAs for pore-forming NaV1.1 and NaV1.6 subunits and regulatory NaVβ1 and Navβ4 subunits are more abundant in SNr GABA neurons than SNc DA neurons. These α-subunits and β-subunits are key subunits for forming NaV channels conducting the transient NaV current (INaT), persistent Na current (INaP), and resurgent Na current (INaR). Nucleated patch-clamp recordings showed that INaT had a higher density, a steeper voltage-dependent activation, and a faster deactivation in SNr GABA neurons than in SNc DA neurons. INaT also recovered more quickly from inactivation and had less cumulative inactivation in SNr GABA neurons than in SNc DA neurons. Furthermore, compared with nigral DA neurons, SNr GABA neurons had a larger INaR and INaP. Blockade of INaP induced a larger hyperpolarization in SNr GABA neurons than in SNc DA neurons. Taken together, these results indicate that NaV channels expressed in fast-spiking SNr GABA neurons and slow-spiking SNc DA neurons are tailored to support their different spiking capabilities.
Keywords: basal ganglia, substantia nigra, transient sodium current, persistent sodium current, resurgent sodium current
as a key component of the basal ganglia motor circuitry, substantia nigra (SN) is populated largely by two types of projection neurons: GABA neurons in SN pars reticulata (SNr) and dopamine (DA) neurons in SN pars compacta (SNc) and also in SNr (Bolam et al. 2000; Deniau et al. 2007; Gonzalez-Hernandez and Rodriguez, 2000; Nelson et al. 1996; Parent et al. 2000). SNr GABA neurons and SNc DA neurons have strikingly different firing properties. SNr GABA neurons fire high-frequency, brief action potentials (Atherton et al. 2005; Zhou et al. 2006), whereas SNc DA neurons fire low-frequency, long-duration spikes (Hyland et al. 2002; Zhou et al. 2006), indicating potential differences in voltage-gated potassium (Kv) and sodium (NaV) channels in these two cell types (Ding et al. 2011; Seutin and Engel 2010). Indeed, our laboratory's recent study showed that a Kv3-like current is essential to the sustained high-frequency firing in SNr GABA neurons (Ding et al. 2011).
NaV channels are composed of one α-subunit and two β-subunits (Catterall 2000). The α-subunit forms the channel pore, whereas the two auxiliary β-subunits regulate the kinetics and function of the α-subunit. Four of the nine identified α-subunits, NaV1.1, NaV1.2, NaV1.3, and NaV1.6, and all four β-subunits, NaVβ1, NaVβ2, NaVβ3, and NaVβ4, are expressed in the central nervous system (Catterall et al. 2005; Goldin 2001; Yu et al. 2003). NaV channels formed by different α- and β-subunits conduct the classical fast transient Na current (INaT) with different kinetics (Goldin 2001; Hille 2001; Smith and Goldin 1998). Consequently, different neuron types expressing NaV channels comprised of different subunits may have different action potential waveforms and patterns (Bean 2007). For example, INaT in hippocampal fast-spiking interneurons recovers rapidly from inactivation and thus promotes fast spiking (Martina & Jonas 1997). NaV channels containing NaV1.6 and/or NaVβ4 may have a resurgent property that generates a resurgent Na current (INaR) after the inactivation of the INaT and upon repolarization that may facilitate fast spiking (Bant and Raman 2010; Khaliq et al. 2003; Mercer et al. 2007; Raman and Bean 1997). Additionally, NaV channels also conduct a small but long-lasting or persistent Na current (INaP) that increases neuronal excitability and promotes pacemaking firing (Bean 2007; Crill 1996).
mRNAs for NaV1.1, NaV1.2, NaV1.3, and NaV1.6 α-subunits, NaVβ3 and NaVβ4 β-subunits, and also NaV1.1 and NV1.2 α-subunit proteins have been detected in the nigral region (Burbidge et al. 2002; Chen et al. 2000; Furuyama et al. 1993; Gong et al. 1999; Morgan et al. 2000; Yu et al. 2003). Furthermore, mRNA for NaVβ4, a key β-subunit for NaV channels with resurgent kinetics, is expressed at a high level in the SNr but not in SNc (Yu et al. 2003). Since the major neuron types in SNr and SNc are the GABA projection neurons and DA neurons, respectively, SNr GABA neurons may express more NaVβ4 than SNc DA neuron. It also raises the possibility that gene expression levels for other NaV α- and β-subunits are different in SNr GABA and SNc DA neurons, leading to functional differences in NaV channels in SNr GABA and SNc DA neurons. More specifically, we reasoned that, compared with SNc DA neuron, NaV α- and β-subunits expressed in SNr GABA neurons may form NaV channels that conduct a larger INaT with faster kinetics and less cumulative inactivation, and also a larger INaP and INaR.
To test these ideas, we first used single-cell RT-PCR (scRT-PCR) on electrophysiologically characterized SNr GABA and nigral DA neurons and quantitative RT-PCR (qRT-PCR) on laser capture-microdissected immunohistochemically identified SNr GABA and SN DA neurons to profile the mRNA expression of Nav channels. Then we used electrophysiological techniques to compare NaV currents in SNr GABA neurons and SNc DA neurons. Consistent with our hypothesis, we found that 1) mRNAs for NaV1.1 and NaV1.6 and NaVβ1 and NaVβ4 are more abundant in SNr GABA neurons than in SNc DA neurons; 2) INaT was larger and activated and deactivated more quickly in SNr GABA neurons than in SNc DA neurons; the recovery of INaT from inactivation was faster and thus the cumulative inactivation was smaller in SNr GABA neurons than in nigral DA neurons; and 3) SNr GABA neurons had a larger INaP and also a larger INaR than SNc DA neurons. These differences in NaV currents support the different spiking behaviors in fast-spiking SNr GABA neurons and slow-spiking nigral DA neurons. A preliminary analysis of these results has appeared in an abstract (Ding and Zhou 2010).
METHODS
Preparation of brain slices.
Sixteen- to 24-day old male and female Sprague-Dawley rats were used. All procedures were carried out in accordance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care Committee of The University of Tennessee Health Science Center. Midbrain slices were prepared as previously described (Atherton & Bevan 2005; Ding et al. 2011; Zhou et al. 2006, 2008, 2009). In brief, rats were deeply anesthetized with urethane. After transcardiac perfusion with an oxygenated ice-cold high-sucrose cutting solution (see below), their brains were quickly dissected out and immersed in the ice-cold oxygenated cutting solution for 2 min. Coronal midbrain slices (300-μm thickness) containing the midrostral part of the SN were prepared in an ice-cold, oxygenated high-sucrose cutting solution using a Vibratome 1000 Plus (Vibratome, St. Louis MO) or Leica Zero Z VT1200S vibratome (Leica Microsystems, Wetzlar, Germany). Slices were transferred to a holding chamber containing the normal extracellular solution (see below) at 30°C for 45 min and then kept at room temperature.
Electrophysiological cell identification and recording of Nav currents in nucleated membrane patches.
Recordings were made under visual guidance of a video microscope (Olympus BX51WI and Zeiss Axiocam MRm digital camera) equipped with Nomarski optics and a ×60 water immersion lens. Patch pipettes with resistances of 1–3 MΩ were pulled from borosilicate (KG-33) glass capillary tubing (1.10 mm ID, 1.65 mm OD; King Precision Glass, Claremont, CA) using a PC-10 puller (Narishige, Tokyo, Japan). A Multiclamp 700B amplifier, pClamp 9.2 software, and Digidata 1322A interface (Molecular Devices, Sunnyvale, CA) were used to acquire and analyze data. After electrophysiologically fingerprinting nigral GABA and DA neurons with conventional whole cell patch clamp within the first 10 s after obtaining access to the cell interior, gentle negative pressure was applied, and the patch pipette was withdrawn slowly to isolate nucleated membrane patches (Ding et al. 2011; Martina and Jonas 1997).
Voltage-clamp waveforms were generated by pClamp 9.2 software. Signals were digitized at 50 kHz and filtered at 10 kHz using the built-in low-pass Bessel filter in the patch clamp amplifier. This 10-kHz filtering, often necessitated by reducing noise, plus stray capacitance-induced filtering, was likely to have affected the measurements of the activation and deactivation time courses, since these two kinetic parameters were very fast. Cell membrane capacitive transients were compensated with the autocompensation function of the Multiclamp 700B. The capacitance readings were taken as the capacitance estimates of the nucleated membrane patch to calculate current and conductance density. Series resistance was not corrected, since it was estimated to be between 4 and 8 MΩ, and the current amplitude was under 500 pA such that the voltage error caused by series resistance was minimal. Since both electrode capacitance and cell capacitance were compensated, the filtering from residual equivalent electrode-cell resistor-capacitor circuit should also be minimal. However, unknown stray capacitance may still exist in the recording system, leading to underestimation of the rapid activation and deactivation kinetics. Leak currents were subtracted online with a P/4 protocol. K+ channels were blocked by internal Cs+ and/or external tetraethylammonium chloride (TEA, 20 mM) and 4-aminopyridine (5 mM). Ca2+ currents were eliminated by replacing extracellular Ca2+ with Mg2+. GABAA receptors and ionotropic glutamate receptors were routinely blocked by 100 μM picrotoxin and 20 μM D-(−)-2-amino-5-phosphonopentanoic acid and 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione disodium, respectively. Nav currents were isolated by offline digital subtraction following application of 1 μM tetrodotoxin (TTX). Test pulses were applied every 5 s. Most recordings were made at 30°C. Additional recordings (see Figs. 12 and 13) were obtained at room temperature (25°C), filtered at 30 kHz, and sampled at 200 kHz.
Fig. 12.
Voltage-dependent activation of INaT in SNr GABA and nigral DA neurons at room temperature (25°C). A and B: representative traces of INaT evoked in nucleated membrane patches isolated from a SNr GABA neuron (A) and a nigral DA neuron (B). The same voltage protocol used in Fig. 6A was used. C: current-voltage plots for INaT in SNr GABA neurons (n = 9) and nigral DA neurons (n = 11). D: INaT activation curves in SNr GABA neurons (n = 9) and nigral DA neurons (n = 11). Continuous lines are Boltzmann fits.
Fig. 13.
INaT deactivation is faster in SNr GABA neurons than in SNc DA neurons at room temperature (25°C). A and B: representative traces of INaT deactivation in nucleated membrane patches from a SNr GABA neurons (A) and a SNc DA neuron (B). The traces at −40 mV are displayed in A′ and B′ to show more clearly INaT deactivation at −40 mV. The smooth gray curves are single-exponential fits. C: pooled data for deactivation time constants plotted against deactivation potentials. Filled circles, SNr GABA neurons (n = 4); open circles, SNc DA neurons (n = 5). The time constants were shorter at −80 to −40 mV, P < 0.05. D: voltage waveform used to evoke INaT deactivation. Holding potential, −90 mV; 50-ms pulse to −120 mV; 200-μs pulse to 0 mV; 50-ms test pulse from −100 mV to −40 mV at 10-mV increase.
Composition of solutions.
The high-sucrose cutting solution contained the following (in mM): 220 sucrose, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 20 d-glucose. The normal extracellular solution contained the following (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2.5 CaCl2, 1.3 MgCl2, 10 d-glucose, maintaining pH at 7.4 by continuously bubbling with 95% O2 and 5% CO2. During recording of Na+ currents, 2.5 mM CaCl2 were substituted by 2.5 mM MgCl2, and extracellular NaCl was reduced on an equal molar basis when 20 mM TEA and 5 mM 4-aminopyridine were used. The Cs-based intracellular solution contained the following (in mM): 135 CsCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, 4 Na2-phosphocreatine. pH was adjusted to 7.25 with CsOH. To record normal membrane potential and action potentials, a KCl-based intracellular solution was used, containing the following (in mM): 135 KCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 4 Na2-phosphocreatine. pH was adjusted to 7.25 with KOH. The osmolarity for the intracellular solutions was 280–290 mosM.
Data analysis.
The liquid junction potential between the 135 mM KCl- or CsCl-based intracellular solution and 100 mM NaCl- and 20 mM TEA-Cl-based extracellular bathing solution, estimated by the liquid junction potential calculator in Clampex, was 4.6 and 4.7 mV, respectively, and not corrected in the data presented below. To obtain activation curves, Na+ conductance was calculated with the equation gNa = I/(V − Erev), where Erev is the reverse potential and was estimated to be +50 mV under our recording conditions, following the method of Safronov and Vogel (1995) and Raman et al. (2000) (see Fig. 5), gNa is Na+ conductance, I is current, and V is voltage. Activation and inactivation curves were fitted with Boltzmann equation: f = 1/{1 + exp[±(V − V1/2)/k]}, where V is the membrane potential, V1/2 is the potential at which the value of the Boltzmann function is 0.5, and k is the slope factor. Data are reported as means ± SE. Two-sample independent t-tests were used to make comparisons, with P value < 0.05 being considered statistically significant.
Fig. 5.
Higher transient NaV current (INaT) density in SNr GABA neurons than in nigral DA neurons. INaT was evoked from a holding potential of −100 mV by a test potential ranging from −80 mV to +30 mV in 5-mV steps. INaT started to appear at −55 mV. For example current traces, see Fig. 6A, because Figs. 5 and 6A were derived from the same data set. The reversal potential was obtained by linear-fitting the data points between 0 mV and 30 mV (dotted lines), according to the method of Safronov and Vogel (1995) and Raman et al. (2000). The estimated reversal potential was 49.8 mV for GABA neurons and 49.9 mV for DA neurons.
scRT-PCR.
scRT-PCR procedures generally followed well-established principles and methods (Ding et al. 2011; Liss et al. 2001; Surmeier et al. 1996; Zhou et al. 2008, 2009;). Patch pipettes were autoclaved to eliminate RNase. The intracellular solution for scRT-PCR contained the following (in mM): 135 KCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 4 Na2-phosphocreatine, and was prepared using DNase-RNase-free water. After electrophysiologically fingerprinting SNr GABA neurons and nigral DA neurons, gentle suction was applied to aspirate the cytoplasm without disrupting the seal. The aspirated cell content was expelled into a 0.2-ml PCR tube and treated with DNase I (5 min at 25°C) to remove genomic DNA contamination. cDNA was synthesized using SuperScript III reverse transcriptase-based Cells-Direct cDNA Synthesis kit (Invitrogen). The synthesized cDNA was amplified using a hot-start Platinum PCR SuperMix (Invitrogen). RT-minus controls, in which the reverse transcriptase was omitted, while all other reaction components were exactly the same, were performed to verify complete removal of genomic DNA. Negative controls were performed by lowering patch pipettes into the tissue and taking them out without seal formation and suction to exclude nonspecific harvesting of surrounding tissue components.
Two-stage PCR amplification was used as previously described (Ding et al. 2011; Zhou et al. 2008, 2009). Briefly, 5 μl of 30-μl cDNA were amplified for 45 cycles in the presence of primers for the first stage (see Table 1 for primer pair sequences). The thermal cycling protocol was 2 min at 94°C for the initial denaturation, than 45 cycles of 15 s at 94°C to denature, 30 s at 48°C to anneal, and 50 s at 72°C to extend, followed by a 10-min final extension. In the second-stage PCR, the product of 1 μl from the first-stage PCR amplification was used as template, and the same primer pair that was used in the first stage was used, and 40 cycles were run.
Table 1.
Single-cell RT-PCR primer pairs for rat neuronal Nav channels, TH, and GAD1 mRNAs
| mRNA (Accession No.) | Start Position | Primers (5′ to 3′) | |
|---|---|---|---|
| Nav1.1 (NM_030875) | F | 4659 | ggctcgttcttcactctaaa |
| R | 5106 | ttcctacaatggagaggatg | |
| Nav1.2 (NM_012647) | F | 1641 | aggcgggataggtgttttct |
| R | 1991 | gcaaagtcattttcggaacc | |
| Nav1.3 (NM_013119) | F | 3291 | ggaaggatgcaaaagggaat |
| R | 3586 | acagcaattggcacagtcac | |
| Nav1.6 (NM_019266) | F | 2808 | ggccatcatcgtcttcatct |
| R | 3058 | ggttgccaatgaccataacc | |
| Navβ1 (NM_017288) | F | 820 | ctggccattacttccgagag |
| R | 1118 | gccatattgcttcacccatc | |
| Navβ2 (NM_012877) | F | 403 | gttcctccagttccgaatga |
| R | 751 | gtcatccgtgctcagcttct | |
| Navβ3 (NM_139097) | F | 1850 | ctgggccagagatgactttc |
| R | 2149 | acacgcccatctttgtctct | |
| Navβ4 (AF544988.1) | F | 380 | cccaaactgttctttctgag |
| R | 729 | caatcccagagttctagtgc | |
| TH (NM_012740) | F | 1130 | cactgtggaattcgggctat |
| R | 1329 | cattgaagctctcggacaca | |
| GAD (NM_017007) | F | 1567 | caagttctggctgatgtgga |
| R | 1826 | actccatcatcagggctttg |
Nav, voltage-activated sodium; TH, tyrosine hydroxylase; GAD, glutamate decarboxylase; F, forward; R, reverse.
We first detected glutamate decarboxylase 1 and TH (tyrosine hydroxylase) mRNA to identify GABA and DA neurons and to confirm the success of cytoplasm aspiration and cDNA synthesis. Then we used 5 μl of the remaining cDNA from the original 30-μl cDNAs to detect the target genes. The products from the second-stage amplification were separated by 1.5% agrose gel electrophoresis, visualized by ethidium bromide (0.05 mg/100 ml gel) or Gelgreen under UV light and photographed. The positive bands were then cut out and extracted using a Qiagen extraction kit. The extracted products were sequenced at the Molecular Resource Center of University of Tennessee Health Science Center in Memphis, Tennessee, and positively identified.
The web-based Primer3 software (http://fokker.wi.mit.edu/primer3/input.htm) (MIT, Cambridge, MA) was used to design PCR primers, according to sequences published in GenBank. The sequences of these primers are listed in Table 1. All primers were synthesized by Integrated DNA Technologies (Coralville, IA). The effectiveness of the primers was positively confirmed by using whole brain total RNA. All primers yielded amplicons of expected sizes with correct sequences.
qRT-PCR assay on laser-captured nigral neurons.
Rats were deeply anesthetized with urethane (1.5 g/kg). Brains were rapidly removed and frozen with Freeze Spray. Cryostat coronal midbrain sections (10 μm) were collected and stored at −80°C. TH and parvalbumin (PV) were used as markers for DA and GABA neurons, respectively (Gonzalez-Hernandez and Rodriguez 2000; Zhou et al. 2009). To quickly immunostain DA neurons or GABA neurons, slide-mounted cryostat sections of unfixed rat midbrain sections were removed from −80°C storage and allowed to thaw before fixation in ice-cold 100% methanol for 3 min. The slide-mounted sections were briefly dipped in cold 0.02 M PBS and incubated for 3 min with 1:25 rabbit anti-TH polyclonal antibody or 1:25 mouse anti-PV monoclonal antibody diluted in PBS. This was followed by four brief rinses in cold PBS. The tissue sections were incubated for 3 min in red fluorescent secondary donkey anti-rabbit or donkey anti-mouse antibody diluted at 1:50 in PBS. The slide-mounted sections were washed four times in cold PBS and dehydrated (1 min each in 75%, 95%, and 100% EtOH), followed by 5 min in xylene twice. After air-drying for 10 min, brain sections were visualized on the ArcturursXT fluorescent Laser Capture Microdissection (LCM) System (Applied Biosystems). TH-positive or PV-positive cells were picked by LCM and collected on the Arcturus plastic sample caps. Approximately 300–400 TH-positive or PV-positive neurons from six coronal sections of SNc or SNr were collected and pooled into a single cap. Six caps were used to collect TH-positive or PV-positive neurons for each animal. RNA was extracted and purified using the PicoPure RNA isolation kit (Applied Biosystems). RNA samples were further treated with DNase to remove any potential genomic DNA contamination and then used as template to generate cDNA using SuperScript III CellsDirect cDNA Synthesis kit (InVitrogen). Levels of mRNA for Nav1.1, Nav1.2, Nav1.3, Nav1.6, Navβ1–4, and β-actin (internal control) were measured by using a Roche LightCycler 480 (LC 480) qRT-PCR system and the Universal ProbeLibrary probes and primers (Roche Applied Science, Indianapolis, IN). The sequences for these primers are listed in Table 2. Using β-actin mRNA as internal control, Nav mRNA quantification was performed employing the comparative crossing point (Cp) method in the form of 2−ΔCp (Luu-The et al. 2005). The Cp values of the real-time fluorescence intensity curve were calculated using the second derivative method. The calculation was performed by the built-in software on LC 480. For Nav mRNA levels in DA neurons, ΔCp,Nav,DA neuron = Cp,Nav,DA neuron − Cp,β-actin,DA neuron. For Nav mRNA levels in GABA neurons, ΔCp,Nav,GABA neuron = Cp,Nav,GABA neuron − Cp,β-actin,GABA neuron. Finally, 2−ΔCp values for Nav mRNAs were normalized to those in SNr GABA neurons.
Table 2.
Quantitative RT-PCR primer pairs and UPL probes for rat neuronal Nav channel and β-actin mRNAs
| mRNA (Accession No.) | Start Position | Primer (5′ to 3′) | UPL Probe No. | |
|---|---|---|---|---|
| Nav1.1 (NM_030875) | F | 2748 | aatatctttgatggtttcattgtgac | 64 |
| R | 2858 | caacttgaagactcggagca | ||
| Nav1.2 (NM_012647) | F | 2168 | tggtgtccctggttggag | 74 |
| R | 2256 | ccttatttctgtctcagtagttgtgc | ||
| Nav1.3 (NM_013119) | F | 295 | gcaccgtccattctaaccat | 56 |
| R | 387 | tttagcttcttgcataagaattgc | ||
| Nav1.6 (NM_019266) | F | 3951 | ctggtgctggttggacttc | 5 |
| R | 4017 | gcccagggcattagctataa | ||
| Navβ1 (NM_017288) | F | 629 | gcgtcgtcaagaagatccac | 10 |
| R | 691 | cgatggatgccatatctctg | ||
| Navβ2 (NM_012877) | F | 365 | tggacttaccaggagtgtagca | 82 |
| R | 424 | cttcattcggaactggagga | ||
| Navβ3 (NM_139097) | F | 576 | gagggcggtaaagatttcct | 49 |
| R | 643 | tggaaggggctctccact | ||
| Navβ4 (AF544988.1) | F | 154 | agaagctcatcactttcatcctg | 126 |
| R | 224 | cccagaggaactcacgagac | ||
| β-Actin (NM_031144.2) | F | 416 | cccgcgagtacaaccttct | 115 |
| R | 479 | cgtcatccatggcgaact |
UPL, Universal Probe Library.
RESULTS
SNr GABA neurons fire faster spikes with larger amplitude than nigral DA neurons.
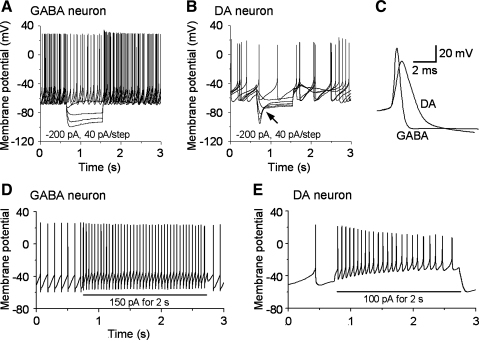
As shown in Fig. 1, A–C, putative SNr GABA neurons exhibited high-frequency spontaneous spiking with brief action potential duration and weak or no “sag” in response to hyperpolarizing current injections (Fig. 1, A and C). In contrast, the presumed DA neurons in SNc and SNr (nigral DA neurons hereafter) spiked spontaneously at low frequency with long action potential duration and displayed a pronounced hyperpolarization-activated current (Ih)-induced sag in response to hyperpolarizing current injection (Fig. 1, B and C, Table 3). These characteristics are consistent with published studies for these two neuron types (Atherton and Bevan 2005; Blythe et al. 2007; Lee and Tepper 2007; Richards et al. 1997; Tepper et al. 1995; Zhou et al. 2006, 2008). Our laboratory has previously shown that a Kv3-like channel-mediated potassium current is critical to the fast-repolarization, short-spike duration and high-frequency firing in SNr GABA neurons (Ding et al. 2011). We also noticed that, compared with nigral DA neurons, the action potentials of SNr GABA neurons have more negative threshold (−40.3 ± 0.5 vs. −36.2 ± 0.4 mV), a larger amplitude (69.5 ± 1.6 vs. 59.3 ± 1.4 mV), shorter duration (1.1 ± 0.1 vs. 2.9 ± 0.2 ms), shorter 10–90% rise time (0.27 ± 0.02 vs. 0.69 ± 0.05 ms), and faster rise rate (183.8 ± 17.3 vs. 71.8 ± 8.8 mV/ms) (Fig. 1, P values < 0.05, see Table 3). Also, SNr GABA neurons showed no or little adaptation in both firing frequency and spike amplitude, whereas nigral DA neurons were adaptive in both firing frequency and spike amplitude (Fig. 1, D and E). These results indicate that, in addition to differences in Kv channel-mediated repolarization that we have already characterized (Ding et al. 2011), SNr GABA neurons and nigral DA neurons may also have qualitative and/or quantitative differences in Nav channel expression.
Fig. 1.
Electrophysiological characteristics of substantia nigra pars reticulata (SNr) GABA neurons and nigral dopamine projection (DA) neurons. A: electrophysiological properties of SNr GABA neurons. Current-clamp recordings show spontaneous high-frequency activity and minimal hyperpolarization-activated current (Ih)-mediated depolarizing sag in response to hyperpolarizing current injection. B: electrophysiological properties of nigral DA neurons. Current-clamp recordings show spontaneous low-frequency pacemaker activity and prominent Ih-mediated depolarizing sag (arrow) in response to hyperpolarizing current injection. C: overlay of a GABA neuron spike and a DA neuron spike, showing the clear differences in the spike waveform between the two cell types. D: SNr GABA neurons show little firing frequency and amplitude adaptation to depolarizing current injection (150 pA). E: nigral DA neurons show prominent firing frequency and spike amplitude adaptation to depolarizing current injection (100 pA). Recordings were made with a 135 mM KCl-based intracellular solution.
Table 3.
Action potential and Nav current parameters in substantia nigra pars reticulata GABA neurons and nigral DA neurons
| Parameters | GABA Neuron | DA Neuron | P |
|---|---|---|---|
| Action potential | |||
| Spontaneous firing rate, Hz | 9.4 ± 0.6 | 1.9 ± 0.2 | <0.01 |
| Spike threshold, mV | −40.3 ± 0.5 | −36.2 ± 0.4 | <0.05 |
| Amplitude, mV | 69.5 ± 1.6 | 59.3 ± 1.4 | <0.01 |
| Base duration, ms | 1.1 ± 0.1 | 2.9 ± 0.2 | <0.01 |
| 10–90% spike rise time, ms | 0.27 ± 0.02 | 0.69 ± 0.05 | <0.01 |
| Average rise rate, mV/ms | 183.8 ± 17.3 | 71.8 ± 8.8 | <0.01 |
| INaT | |||
| Peak INaT density (at −10 mV), pA/pF | 148.9 ± 17.8 | 86.0 ± 14.1 | <0.01 |
| Activation 10–90% rise time (at 0 mV), μs | 85 ± 5 | 84 ± 5 | >0.05 |
| Activation V1/2, mV | −30.2 ± 0.2 | −26.7 ± 0.5 | <0.05 |
| Activation slope K | 6.2 ± 0.2 | 7.3 ± 0.3 | <0.05 |
| Inactivation 10−90% decay time (at 0 mV), μs | 191 ± 12 | 195 ± 15 | >0.05 |
| Steady-state inactivation V1/2, mV | −63.3 ± 1.3 | −61.3 ± 2.5 | >0.05 |
| Steady-state inactivation slope K | 8.1 ± 0.5 | 10.1 ± 1.6 | >0.05 |
| Recovery from inactivation | |||
| τf, ms | 0.59 ± 0.07 | 1.15 ± 0.1 | <0.05 |
| τs, ms | 35.1 ± 6.4 | 79.7 ± 13.1 | <0.05 |
| INaR | |||
| Peak density (at −40 mV), pA/pF | 3.3 ± 0.5 | 1.4 ± 0.6 | <0.01 |
| INaP | |||
| Whole cell peak amplitude (at −40 mV), pA | 185.4 ± 17.5 | 121.3 ± 12.7 | <0.01 |
Values are means ± SE. DA, dopamine projection; INaT, transient voltage-activated sodium current; INaP, persistent Na current; INaR, resurgent Na current; V1/2, the potential at which the value of the Boltzmann function is 0.5; τf, fast recovery; τs, slow recovery. Spike threshold was measured at the point that had the sharpest inflection of membrane potential before the full spike. Base duration starts at the spike threshold point and ends when the membrane potential returns and crosses the spike threshold level.
NaV channel gene expression in identified SNr GABA and nigral DA neurons.
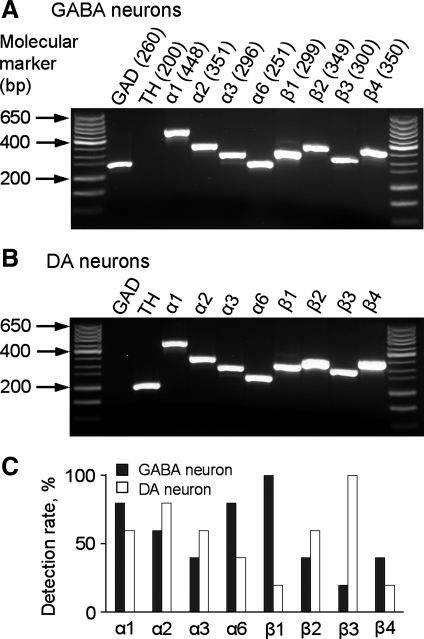
After electrophysiologically fingerprinting SNr GABA and nigral DA neurons with a 135 mM KCl-based intracellular solution, we performed scRT-PCR to profile mRNAs for NaVα and NaVβ subunits. As illustrated in Fig. 2, mRNAs for NaV1.1, NaV1.2, NaV1.3, NaV1.6, NaVβ1, NaVβ2, NaVβ3, and NaVβ4 were detected in both SNr GABA neuron and SNc DA neuron, although there were apparent differences in the rate of detection. Since scRT-PCR is a qualitative method, these results indicate that SNr GABA neurons and SNc DA neurons may express identical types of NaV subunits at different levels. Testing this idea requires quantitative comparison of NaV subunit mRNA levels in SNr GABA neurons and SN DA neurons. However, the quantity of mRNA obtained in a single neuron was not sufficient for qRT-PCR. To overcome this difficulty, we collected and pooled SNr GABA neurons and SN DA neurons, separately, by using LCM and performed qRT-PCR analysis on NaV channel subunit mRNAs isolated from these neurons. SNr GABA neurons and nigral DA neurons were identified by PV and TH fluorescent immunoreactivity, respectively (Gonzalez-Hernandez and Rodriguez 2000; Zhou 2009). As shown in Fig. 3, we found that the mRNA expression level of NaV1.1, Nav1.6, NaVβ1, and NaVβ4 in SNr GABA neurons was 2.07-, 2.03-, 2.15-, and 1.96-fold of that in SNc DA neurons, respectively (P < 0.005). In contrast, the level of NaV1.2, NaVβ2, and NaVβ3 mRNAs in SNc DA neurons was 1.65-, 1.69-, and 3.26-fold of that in SNr GABA neurons (P < 0.005). As in scRT-PCR, NaV1.3 was detectable in qRT-PCR, but the Cp values (defined in methods) of SNr GABA neurons and SNc DA neurons were too high (more than 40), indicating low abundance and potentially unreliable quantification. Thus NaV1.3 was excluded from quantitative comparison in this study.
Fig. 2.
Single-cell RT-PCR scRT-PCR detection of Nav mRNAs in SNr GABA neurons and nigral DA neurons. A: voltage-activated sodium (NaV) mRNAs detected in electrophysiologically identified SNr GABA neurons. No tyrosine hydroxylase (TH) mRNA was detected in any of these neurons. Numbers in the parentheses are the expected amplicon sizes in base pair. B: NaV mRNAs detected in electrophysiologically identified nigral DA neurons. The expected amplicon sizes are the same as in A. C: summary of scRT-PCR detection ratio of Nav channel mRNAs in SNr GABA neurons and nigral DA neurons. For each NaV mRNA, 5–10 TH mRNA-positive DA neurons and 5–10 glutamate decarboxylase 1 (GAD1) mRNA-positive neurons were tested. In this figure and Fig. 3, the NaV α-subunits are denoted as α1, α2, α3, and α6, while the standard nomenclature (NaV1.1, NaV1.2, NaV1.3, and NaV1.6) is used in the main text (Catterall et al. 2005).
Fig. 3.
Quantitative RT-PCR (qRT-PCR) analysis of Nav channel mRNAs in laser microdissection-captured, immunofluorescence-identified SNr GABA neurons and nigral DA neurons. β-Actin mRNA was used as internal control. mRNA semiquantification was performed using the comparative crossing point (Cp) method in the form of 2−ΔCp. 2−ΔCp values for NaV mRNAs were normalized to those in SNr GABA neurons. NaV1.1, NaV1.6, NaVβ1, and NaVβ4 mRNAs were higher in SNr GABA neurons than in nigral DA neurons, whereas NaV1.2, NaVβ2, and NaVβ3 mRNAs were lower in SNr GABA neurons than in nigral DA neurons. The difference in the expression of these NaV genes between SNr GABA neurons and nigral DA neurons was significant with P < 0.005.
Based on these qRT-PCR data on NaV mRNAs (Fig. 3), the differences in action potential waveforms in SNr GABA neurons and SNc DA neurons (Fig. 1), and the different electrophysiological characteristics of NaV subunits in heterologous expression systems (Goldin 2001), we made the following prediction: compared with NaV channels in SNc DA neurons, Nav channels in SNr GABA neurons 1) are expressed at a higher density; 2) activate at a lower threshold and/or with a steeper voltage dependence, and 3) recover faster from inactivation and thus have less cumulative inactivation; and 4) conduct larger INaP and INaR. These NaV channel properties would support the sustained fast spiking in SNr GABA neurons.
Higher INaT density in SNr GABA neurons than in DA neuron.
To study NaV currents in SNr GABA neurons and nigral DA neurons, we performed nucleated patch-clamp recordings in rat brain slices (Ding et al. 2011; Martina and Jonas 1997). The key advantage of nucleated patch clamp is the substantially reduced spatial and voltage-clamp problems because of the elimination of axonal and dendritic processes. To help isolate NaV currents, a 135 mM CsCl-based intracellular solution was used to block potassium channels. Cs+ also blocks the Ih that is prominent in nigral DA neurons, but lacking or small in SNr GABA neurons (Fig. 1). Thus we performed electrophysiological identification of SNr GABA and nigral DA neurons within the first 10 s after obtaining access to the cell interior. A similar method was used by Martina and Jonas (1997). As shown in Fig. 4, A and B, during this short time window, SNr GABA neurons and nigral DA still displayed distinct electrophysiological properties. SNr GABA neurons exhibited spontaneous firing at frequencies ≥10 Hz and little or no “sag” in response to hyperpolarizing current injection. In contrast, nigral DA neurons exhibited spontaneous firing at frequencies ≤5 Hz and prominent “sag” in response to hyperpolarizing current injection. After electrophysiological identification, nucleated membrane patches were isolated from these neurons (Fig. 4, C–E).
Fig. 4.
Electrophysiological identification of SNr GABA neurons and nigral DA neurons with 135 mM CsCl-based intracellular solution and nucleated patch formation. A: electrophysiological properties of SNr GABA neurons. Current-clamp recordings within 10 s after obtaining access to the cell interior show spontaneous high-frequency activity and no Ih-mediated sag in response to hyperpolarizing current injection (−200 pA). Due to Cs+ infusion, the spikes were broadened, and the firing frequency was increased. B: electrophysiological properties of nigral DA neurons. Current-clamp recordings within 10 s after obtaining access to the cell interior show spontaneous low-frequency activity and prominent Ih-mediated sag in response to hyperpolarizing current injection. The spikes were broadened, and the firing frequency was increased due to Cs+ infusion. Despite Cs+ infusion, the differences in membrane properties are still striking within the first 10 s. C, D, and E: sequential images demonstrating the formation of nucleated somatic membrane patch. C shows conventional whole cell patch clamp to electrophysiologically fingerprint SNr GABA and nigral DA neurons. D shows that gentle negative pressure was being applied, and the patch pipette was being withdrawn slowly to form nucleated patches. Arrow indicates the remaining part of the neuron. Arrowhead points to the round nucleated membrane patch. E: spherical nucleated patches free of axon and dendritic processes was formed, as demonstrated by including 20 μM fluorescent Alexa 594 in the pipette solution.
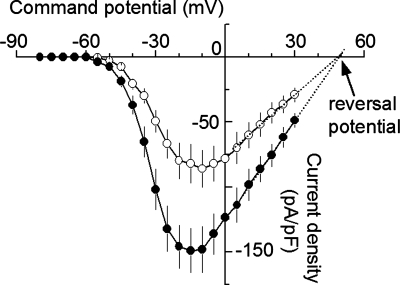
We first compared INaT density in nucleated membrane patches from SNr GABA neurons and nigral DA neurons. INaT was evoked from a holding potential of −100 mV to test potentials ranging −80 to +30 mV in 5-mV steps. As shown in Fig. 5, INaT reached its peak amplitude around −10 mV in both SNr GABA neurons and nigral DA neurons. The peak INaT and thus conductance density were clearly higher in SNr GABA neurons than in nigral DA neurons: 148.9 ± 17.8 pA/pF and 2481.8 ± 296.2 pS/pF for SNr GABA neurons (n = 12) and 86.0 ± 14.1 pA/pF and 1433.5 ± 234.2 pS/pF for nigral DA neurons (n = 9, P < 0.05, Table 3). If we assume the specific membrane capacitance to be 1 μF/cm2 (Hille 2001), then INaT conductance density was 24.8 ± 3.0 pS/μm2 for SNr GABA neurons and 14.3 ± 2.3 pS/μm2 for nigral DA neurons. The reversal potential used for calculating the conductance was obtained by linear fitting the data points between 0 mV and 30 mV (Fig. 5), following the method of Safronov and Vogel (1995) and Raman et al. (2000). The estimated reversal potential was 49.8 mV for GABA neurons and 49.9 mV for DA neurons. The higher INaT density may contribute to the faster rise and larger amplitude of the action potentials in SNr GABA neurons.
Steeper voltage-dependent INaT activation in SNr GABA neurons than in nigral DA neurons.
We next compared the voltage dependence and kinetics of INaT in SNr GABA neurons and nigral DA neurons. The voltage-dependent activation of INaT was studied by holding the nucleated membrane patch at −100 mV and then stepping to test membrane potentials between −80 mV and 30 mV (Fig. 6A1). Representative INaT traces at these test membrane potentials and averaged activation curves were shown in Fig. 6, A2–A4. INaT started to activate around −50 mV in both cell types. However, with increasing depolarization, the increase of INaT conductance was faster and reached the maximum earlier in SNr GABA neurons than in DA neurons, indicating that INaT had a steeper voltage-dependent activation in SNr GABA neurons than in SN DA neurons (Fig. 6A4). Boltzmann equation fitting revealed that the activation slope factor K was 6.2 ± 0.2 for SNr GABA neurons (n = 12) and 7.3 ± 0.3 for nigral DA neurons (n = 9, P < 0.05). The activation midpoint potential V1/2 was also different for the two cell types: V1/2 was −30.2 ± 0.6 mV for SNr GABA neurons (n = 12) and −26.7 ± 0.5 mV for nigral DA neurons (n = 9, P < 0.05, Table 3). The steeper activation curve with a more negative midpoint indicates that INaT in SNr GABA neurons can be activated more readily than those in nigral DA neurons, contributing to the more negative spike threshold and faster spike rise rate in SNr GABA neurons.
Fig. 6.
Voltage-dependent activation and steady-state inactivation of INaT in SNr GABA and nigral DA neurons. A, A1: activation voltage waveform to evoke INaT. A2 and A3: representative traces of INaT evoked in nucleated membrane patches isolated from a SNr GABA neuron (A2) and a nigral DA neuron (A3). A4: activation curves of INaT in SNr GABA neurons (filled circles) (n = 12) and nigral DA neurons (open circles) (n = 9). Continuous lines are Boltzmann fits. Data points below −60 mV and above 5 mV are not displayed in order to show the difference between the two curves more clearly. B, B1: voltage waveform to induce and determine INaT steady-state inactivation. B2 and B3: representative traces of INaT evoked by the steady-state inactivation protocol in nucleated membrane patches isolated from a SNr GABA neuron (B2) and a nigral DA neuron (B3). B4: steady-state inactivation curves of INaT in SNr GABA neurons (filled circles) (n = 12) and nigral DA neurons (open circles) (n = 9). Continuous lines are Boltzmann fits. G/Gmax, normalized conductance; I/Imax, normalized current.
As expected for INaT, both the activation (measured by 10–90% rise time) and inactivation (measured by 10–90% decay time) of INaT were fast (Fig. 6, A2, A3, B2, B3, Table 3). However, we did not detect any significant difference in INaT rise time and decay time in these two cell types. For example, when stepped from −100 mV to 0 mV, the 10–90% rise time was 85 ± 5 μs for SNr GABA neurons (n = 12) and 84 ± 5 μs for nigral DA neurons (n = 9); the 10–90% decay time was 191 ± 12 μs for SNr GABA neurons and 195 ± 15 μs for nigral DA neurons.
Similar steady-state inactivation of INaT in SNr GABA neurons and nigral DA neurons.
Next, we studied the steady-state inactivation of INaT in SNr GABA and nigral DA neurons. Representative traces evoked by a steady-state inactivation protocol were shown in Fig. 6B. INaT in SNr GABA neurons and nigral DA neurons showed a similar steady-state inactivation. Boltzmann equation fitting revealed that the slope factor of steady-state inactivation was 8.1 ± 0.5 for SNr GABA neurons (n = 12) and 10.1 ± 1.6 for nigral DA neurons (n = 9), respectively (P > 0.05). The midpoint potential of steady-state inactivation was −63.3 ± 1.3 mV for SNr GABA neurons (n = 12) and −61.3 ± 2.5 mV for nigral DA neurons (n = 9), respectively (P > 0.05). These results show that steady-state inactivation of INaT did not differ between the two types of neurons.
INaT recovery from inactivation is faster in SNr GABA neurons than in nigral DA neurons.
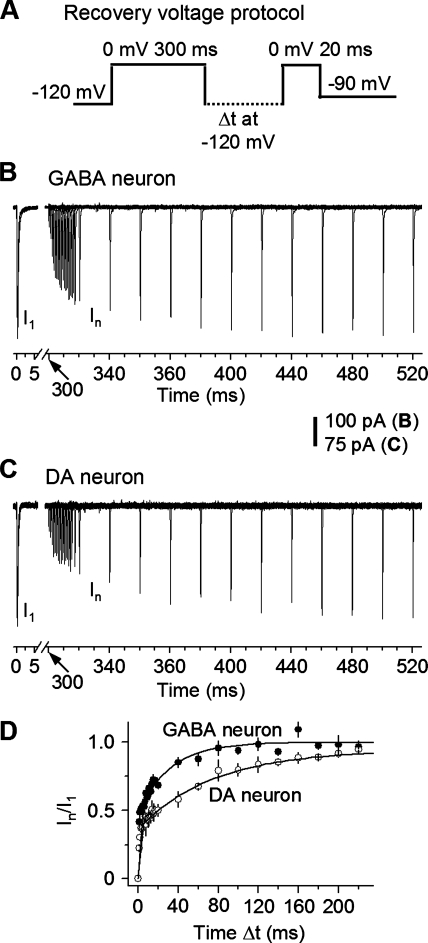
Fast recovery (τf) of INaT from inactivation is essential for high-frequency repetitive firing. Based on the fact that the spike amplitudes decline less in SNr GABA neurons than in nigral DA neuron (Fig. 1, D and E), we hypothesized that INaT may recover more rapidly from inactivation such that more functional NaV channels are available in SNr GABA neurons than in nigral DA neurons. We examined the recovery time course from inactivation with a double-pulse protocol (Fig. 7A): the holding potential was −90 mV, then a 50-ms prepulse at −120 mV, then a 300-ms pulse at 0 mV to completely inactivate INa, then stepping to −120 mV for increasing duration (Δt) to allow INaT recover, and finally a 20-ms test pulse at 0 mV (Fig. 7A). We found that the time course for INaT to recover from inactivation was biexponential in both SNr GABA neurons and SNc DA neurons (Fig. 7, B–D). The τf component contributed 52.6 ± 5.7 and 35.8 ± 3.4% (recovered within 5 ms) to the total current in SNr GABA neurons and nigral DA neurons, respectively. Mostly importantly, INaT recovered faster in SNr GABA neurons than in SNc DA neurons. The τf was 0.59 ± 0.07 ms for SNr GABA neurons (n = 5) and 1.15 ± 0.1 ms for SNc DA neurons (n = 5, P < 0.05). The slow recovery (τs) was 35.1 ± 6.4 ms for SNr GABA neurons (n = 5) and 79.7 ± 13.1 ms for SNc DA neurons (n = 5, P < 0.05, Table 3). These results indicate that INaT in the fast spiking SNr GABA neurons recovered more rapidly from inactivation than that in the slow spiking SNc DA neurons.
Fig. 7.
INaT recovered more rapidly from inactivation in SNr GABA neurons than in substantia nigra pars compacta (SNc) DA neurons. A: voltage protocol for recovery from a 300-ms-induced inactivation. B and C: representative traces of recovery from inactivation in a SNr GABA neuron nucleated patch (B) and a SNc DA neuron nucleated patch (C). D: pooled data on INaT recovery time course from inactivation by a 300-ms conditioning pulse (I). Curves represent sums of two exponential fits. Filled circles are nucleated patches from SNr GABA neuron (n = 5). Open circles are nucleated patches from SNc DA neuron (n = 5).
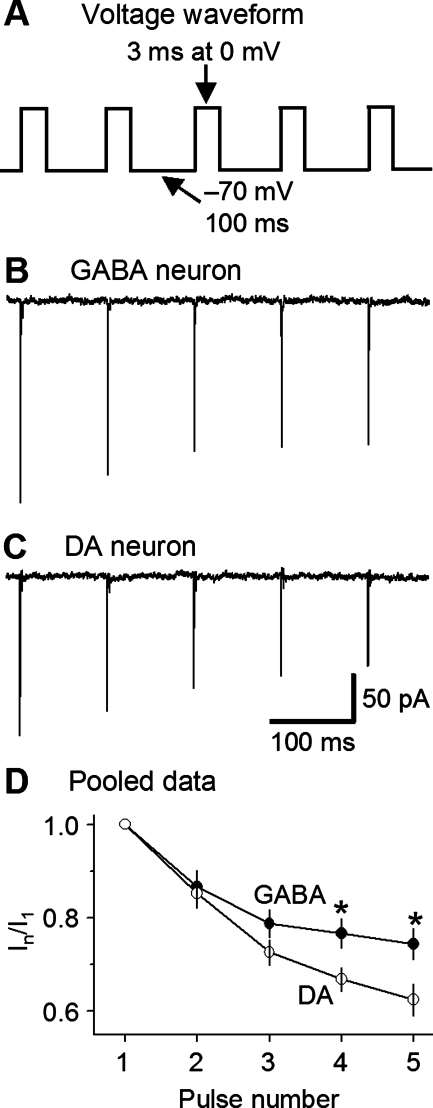
Because of the different recoveries from inactivation, we reasoned that, compared with nigral DA neurons, SNr GABA neurons may incur less cumulative INaT inactivation during repetitive depolarization. To test this idea, we performed the experiment shown in Fig. 8. The membrane patch was held at −70 mV, then stepped to a train of five pulses (3 ms at 0 mV with an interpulse interval of 100 ms at −70 mV, Fig. 8A). The ratio of the INaT evoked by a pulse over that evoked by the first pulse, In/I1, was used as a measure of cumulative inactivation. The ratio becomes small when cumulative inactivation is large. As illustrated in Fig. 8, B–D, by the third pulse, the ratio was already significantly larger in SNr GABA neurons (n = 6) than in SNc DA neurons (n = 5), although without reaching statistical significance. By the fourth pulse, the difference was statistically significant (P < 0.05). These results indicate that, during repetitive depolarization such as spike firing, NaV channels undergo less cumulative activation in SNr GABA neurons than in nigral DA neurons. This difference may contribute to the nonadaptive spiking in SNr GABA neurons and the adaptive spiking in nigral DA neurons, respectively (Fig. 1, D and E).
Fig. 8.
Less cumulative INaT inactivation in SNr GABA neurons than in SN DA neurons. A: voltage protocol: holding potential, −70 mV; 3-ms test pulses to 0 mV at 100-ms interval. B and C: representative traces of cumulative INaT inactivation in nucleated membrane patches from a SNr GABA neuron (B) and SNc DA neuron (C). D: pooled data on INaT cumulative inactivation. Peak INaT was normalized to the INaT evoked by the first test pulse I1. *Significant difference at P < 0.05.
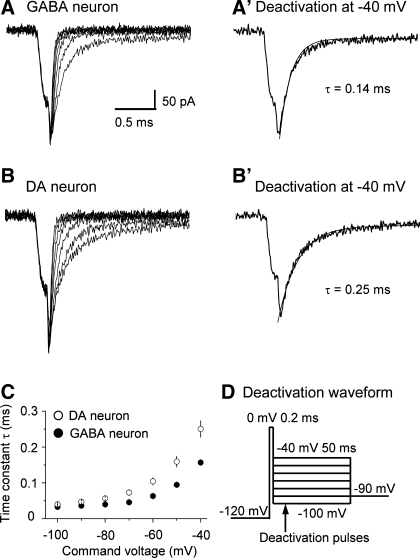
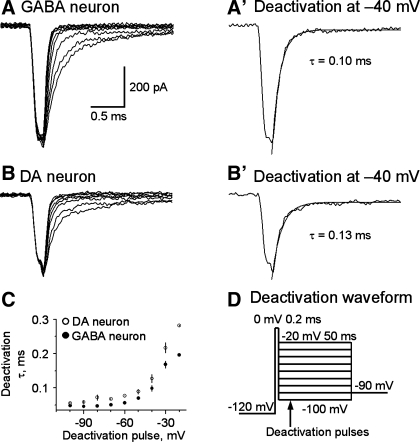
INaT deactivation is faster in SNr GABA neurons than in nigral DA neurons.
NaV channels with slower deactivation kinetics can conduct longer-lasting inward current upon membrane repolarization and prolong action potential duration. We compared the deactivation properties of INaT in SNr GABA neurons and nigral DA neurons. As illustrated in Fig. 9D, INaT was evoked by a 200-μs pulse to 0 mV. Based on our data on INaT activation (Fig. 6, A2 and A3), at the end of a 200-μs pulse at 0 mV, INaT and thus NaV channel opening were at or near their peak. To deactivate NaV channels, the membrane was stepped to −100 mV through −20 mV in a 10-mV step. As shown in Fig. 9, A and B, INaT deactivated very quickly, as reflected by the rapid decay of the tail currents in both neuron types. These tail currents were well fitted with a single exponential function. At very negative membrane potentials (−100 mV and −90 mV), the deactivation was only slightly faster in SNr GABA neurons than in nigral DA neurons. For example, the deactivation time constant at −100 mV was 48.1 ± 2.6 μs for SNr GABA neurons (n = 7) and 54.9 ± 5.3 μs for SNc DA neurons (n = 6), but the difference was not statistically significant (P > 0.05). When the deactivation pulse became more positive, particularly at −50 mV to −20 mV, which is in the range of action potential repolarization, the deactivation time course became significantly faster in SNr GABA neurons than in SNc DA neurons (Fig. 9, A–C). For example, at −40 mV, the deactivation time constant was 99 ± 8.9 μs for SNr GABA neurons (n = 7) and 127.8 ± 12.2 μs for nigral DA neurons (n = 6, P < 0.01). The faster deactivation of INaT, together with a robust Kv3-like current (Ding et al. 2011), can thus repolarize the membrane more quickly in the fast-spiking SNr GABA neurons than in slow-spiking SNc DA neurons (see Fig. 1).
Fig. 9.
INaT deactivation is faster in SNr GABA neurons than in SNc DA neurons. A and B: representative traces of INaT deactivation in nucleated membrane patches from a SNr GABA neurons (A) and a SNc DA neuron (B). The two traces at −40 mV are displayed in A′ and B′ to show more clearly INaT deactivation at −40 mV. The smooth gray curves are single-exponential fits. C: pooled data for deactivation time constants plotted against deactivation potentials. Filled circle: SNr GABA neurons. Open circles: SNc DA neurons. The time constants were shorter at −80 to −20 mV, P < 0.05. D: voltage waveform used to evoke INaT deactivation. Holding potential, −90 mV; 50-ms pulse to −120 mV; 200-μs pulse to 0 mV; 50-ms test pulse from −100 mV to −20 mV at 10-mV increase.
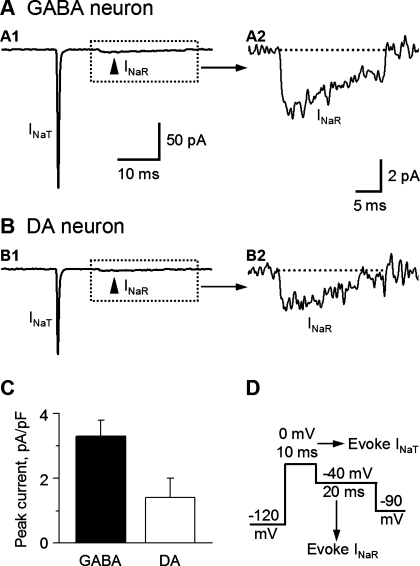
INaR is larger in SNr GABA neurons than in nigral DA neurons.
INaR is generated during membrane repolarization after depolarization and inactivation of INaT (Bant and Raman 2010; Grieco et al. 2005; Raman and Bean 1997). INaR is much smaller but longer-lasting than INaT. NaV1.6 (α6) and NaVβ4 (β4) are critical for INaR generation (Bant and Raman 2010; Do and Bean 2004; Grieco and Raman 2004; Grieco et al. 2005). In cerebellar Purkinje neurons and granule neurons and the GABA neurons in the globus pallidus, the Nav channels containing NaV1.6 and/or Navβ4 have a resurgent property that generates a INaR after the inactivation of the INaT and upon repolarization (Bant and Raman 2010; Khaliq et al. 2003; Mercer et al. 2007; Raman and Bean 1997). INaR has been suggested to facilitate fast spiking. Our qRT-PCR data (Fig. 3) indicated that NaVβ4 mRNA is expressed at a higher level in SNr GABA neurons than in nigral DA neurons. Using a voltage protocol illustrated in Fig. 10D, we observed INaR in both SNr GABA neurons and SNc DA neurons (Fig. 10, A and B). Consistent with our qRT-PCR data that compared with nigral DA neurons, SNr GABA neurons had more Nav1.6 and Navβ4 subunit expression; our nucleated patch-clamp recordings showed that INaR was larger in SNr GABA neurons than in nigral DA neurons (Fig. 10, P < 0.01, Table 3). Nav1.6 and Navβ4 subunit expression has been reported to be the main subunits responsible for large INaR in other types of neurons (Aman and Raman 2007; Bant and Raman 2010; Grieco et al. 2004; Mercer et al. 2007; Rush et al. 2005).
Fig. 10.
Resurgent Na current (INaR) is larger in SNr GABA neurons than in SNc DA neurons. A and B: representative traces of INaR in nucleated membrane patches from a SNr GABA neuron (A1) and a SNc DA neuron (B1). The boxed areas in A1 and B1 are expanded and displayed in A2 and B2, respectively. C: polled data from SNr GABA neurons (n = 5) and SNc DA neurons (n = 4). The difference was significant (P < 0.05). D: voltage waveform used to generate INaR. Holding potential between the pulses was −90 mV.
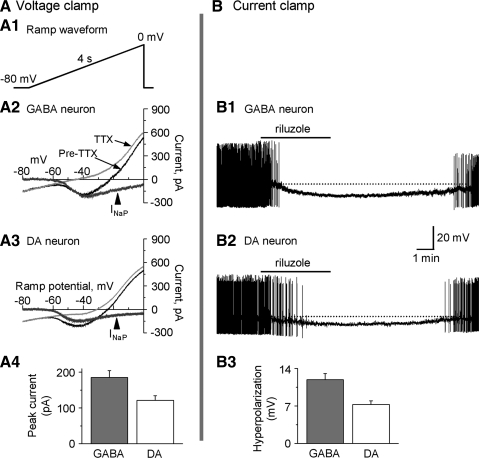
INaP is larger in SNr GABA neurons than in nigral DA neurons.
Separate studies have examined INaP in SNr GABA neurons (Atherton & Bevan 2005) and SNc DA neurons (Puopolo et al. 2007). But a direct, side-by-side comparison of INaP between the two types of neurons was lacking. So we set out to compare the amplitude of INaP and their possible roles in firing properties and membrane potentials between SNr GABA neurons and SN DA neurons. Since INaP was slow, conventional whole cell patch-clamp recording was suitable. We used the CsCl-based intracellular solution (see methods section) to block K+ channels. We also used a slow 4-s linear voltage ramp from −80 mV to 0 mV to inactivate the INaT (Fig. 11A) (Gorelova and Yang 2000; Koizumi and Smith 2008). Since INaP is known to be blocked by 1 μM TTX (Crill 1996; French et al. 1990; Gorelova and Yang 2000; Koizumi and Smith 2008), the slow TTX-sensitive current was taken as the INaP. After a stable baseline recording was obtained, 1 μM TTX was bath-applied. As shown in Fig. 11, a quite large TTX-sensitive, slow sodium current or INaP was recorded in both SNr GABA neurons and nigral DA neurons (Fig. 11, A2 and A3). INaP started to activate around −60 mV and peaked around −40 mV. The peak amplitude of INaP was 185.4 ± 17.5 pA in seven SNr GABA neurons and 121.3 ± 12.7 pA in six SNc SN DA neurons (P < 0.05, Fig. 11A4, Table 3). To explore the functional roles of INaP, we recorded the membrane potential and action potential firing in conventional whole cell mode with the 135 mM KCl-based intracellular solution and the normal extracellular solution (see methods). After obtaining a stable baseline, 10 μM riluzole, an established INaP blocker (Koizumi and Smith 2008; Urbani and Belluzzi 2000), was bath-applied. As shown in Fig. 11B, bath application of 10 μM riluzole induced a 11.9 ± 1.1 mV hyperpolarization from its baseline potential around −50 mV in SNr GABA neurons (n = 6) and a 7.3 ± 0.6 mV hyperpolarization from its baseline potential around −50 mV in nigral DA neurons (n = 5), leading to a cessation of spontaneous firing in both neuron types. Since the slow hyperpolarization is not likely due to a potential riluzole inhibition of INaT (Urbani and Belluzzi 2000), these results indicate that INaP was active at the baseline membrane potential around −50 mV in SNr GABA neurons and nigral DA neurons. The larger INaP in SNr GABA neurons may contribute to the faster pacemaking activity in SNr GABA neurons than in SN DA neurons.
Fig. 11.
Persistent Na current (INaP) is larger in SNr GABA neurons than in SNc DA neurons. A: voltage clamp data on INaP. A1: diagram showing the voltage ramp waveform consisting of a 4-s ramp from −80 mV to 0 mV. A2 and A3: representative current traces before (black line) and after (thin gray line) perfusion of 1 μM tetrodotoxin (TTX) evoked by the voltage ramp, in GABA neurons (A2) and DA neurons (A3). TTX-sensitive INaP (thick gray line) was obtained by subtraction traces before after perfusion of 1 μM TTX. A4: pooled data of peak INaP in SNr GABA (n = 7) and SNc DA (n = 6) neurons. B: current clamp data on INaP. B1 and B2: representative traces showing the effects of 10 μM riluzole in SNr GABA neurons (B1) and SNc DA neurons (B2). Bath perfusion of 10 μM riluzole induced a stronger hyperpolarization in GABA neurons than in DA neurons. Spike amplitudes were truncated due to slow resampling (1 kHz) for reducing the large data size of long recording segments. B3: pooled data on 10 μM riluzole-induced hyperpolarization in SNr GABA neurons (n = 6) and SNc DA neurons (n = 5). The difference was significant (P < 0.05).
INaT in SNr GABA neurons and nigral DA neurons at room temperature.
To verify that our conclusions based on recordings made at 30°C and filtered at 10 kHz were correct, additional experiments were performed at room temperature (25°C), low-pass filtered at 30 kHz, and sampled at 200 kHz. INaT activation, decay, and deactivation were reexamined because these parameters were fast and thus sensitive to recording temperature and signal filtering. As shown in Figs. 12 and 13 and Table 4, although slower than at 30°C, the kinetic differences seen at 30°C between these two cell types remained at room temperature, while the kinetic parameters that were similar at 30°C were still similar at room temperature. The only change in INaT kinetics that needs to be mentioned in particular is that the 10–90% decay time was prolonged more substantially than the 10–90% rise time. Although the mechanisms are not clear, differential temperature effects on the rise and the decay of INaT have been observed (Magatomo et al. 1998; Sah et al. 1988). The INaT density is similarly reduced in both cell types at room temperature compared with that at 30°C, consistent with literature data (Sah et al. 1988). These data indicate that the kinetic differences in INaT in nigral DA neurons and SNr GABA neurons observed at 30°C may be extrapolated to 37°C, the normal body temperature, and thus are physiologically important.
Table 4.
INaT current properties in substantia nigra pars reticulata GABA and nigral DA neurons at 25°C, filtered at 30 kHz, and sampled at 200 kHz
| Parameter | GABA Neurons | DA Neurons | Significance P |
|---|---|---|---|
| Activation midpoint, mV | −34.81 ± 1.2 (n = 9) | −29.90 ± 1.30 (n = 11) | <0.05 |
| Slope factor k | 7.04 ± 0.17 (n = 9) | 7.75 ± 0.13 (n = 11) | <0.05 |
| 10-90% Rise time (at 0 mV), ms | 0.10 ± 0.004 (n = 9) | 0.11 ± 0.004 (n = 11) | >0.05 |
| 90-10% Decay time (at 0 mV), ms | 0.72 ± 0.03 (n = 9) | 0.68 ± 0.04 (n = 11) | >0.05 |
| Deactivation τ (at −40 mV), ms | 0.16 ± 0.01 (n = 4) | 0.25 ± 0.02 (n = 5) | <0.05 |
Values are means ± SE; n, no. of neurons.
DISCUSSION
The main findings of this study are that, compared with slow-spiking nigral DA neurons, fast-spiking SNr GABA neurons had higher gene expression levels for NaVα1.1, NaVα1.6, NaVβ1, and Navβ4; and larger amplitudes of INaT, INaP, and INaR. INaT also had a steeper voltage-dependent activation, a faster deactivation, and a faster recovery from inactivation in SNr GABA neurons than in nigral DA neurons. These differences in NaV currents may contribute to the striking differences in spiking properties in these two cell types.
Different NaV mRNA expression in SNr GABA neurons and nigral DA neurons.
The strikingly different spike waveform and spiking pattern in SNr GABA neurons and nigral DA neurons suggest possible molecular and functional differences in NaV channels between the two neuron types. Our qualitative scRT-PCR analysis revealed similar types of NaV mRNAs in SNr GABA neurons and nigral DA neurons, indicating the two cell types probably express qualitatively similar but quantitatively different NaV subunits, leading to the formation of NaV channels with different subunit composition, particularly with different regulatory β-subunits. Indeed, using qRT-PCR on immunochemically identified SNr GABA and SNc DA neurons, we found that SNr GABA neurons had higher expression levels of NaV1.1, NaV1.6, NaVβ1, and NaVβ4 and lower expression levels of NaV1.2, NaVβ2, and NaVβ3 than SN DA neuron (Fig. 3). The high Navβ4 level in SNr GABA neurons compared with SNc DA neurons is also consistent with a histochemical study that detected high Navβ4 mRNA in SNr but not SNc (Yu et al. 2003). These differences in NaV channel expression levels may affect NaV channel expression density and also subunit composition and hence NaV channel properties and function. Specifically, the higher NaV1.1 and NaV1.6 mRNA expression, together with Navβ1 and Navβ4 mRNA expression, likely contributes to the larger amplitude or higher density of INaT, INaP, and INaR in SNr GABA neurons than in nigral DA neurons. NaVβ1 may enhance NaV channel cell surface expression and increase INaT (Isom et al. 1992, 1995; McEwen et al. 2004). NaVβ1 may also promote fast gating for NaV α-subunits (Goldin 2001). Recent studies indicate that Navβ4 may interact with NaV α-subunit and enhance INaP and INaR (Bant and Raman 2010; Grieco et al. 2005) and will be further discussed below. The significance of higher NaV1.2, NaVβ2, and NaVβ3 mRNA expression in DA neurons than in GABA neurons is not clear. One possibility is that our semiquantitative qRT-PCR only compared the relative abundance of NaV1.2 mRNA between the two cell types and did not compare the relative abundance of NaV1.1 mRNA, NaV1.2 mRNA, and NaV1.6 mRNA within each cell type. Thus it is possible that NaV1.1 and NaV1.6 are the dominant NaV subunits, such that the difference in NaV1.2 is not important. Alternatively, we speculate that, while NaV1.1 and NaV1.6 are the major α-subunits with NaVβ1 and NaVβ4 as the regulatory subunits in SNr GABA neurons, NaV1.2 and NaV1.6 may be the major α-subunits with NaVβ2 and NaVβ3 as the regulatory subunits in nigral DA neurons. Future molecular studies are needed to determine the absolute mRNA expression levels for these different NaV subunits and NaV channel protein subunit composition.
Different INaT density and kinetics in SNr GABA neuron and nigral DA neuron.
In the present study, we found that INaT and conductance density were considerably higher in fast-spiking SNr GABA neurons than in slow-spiking nigral DA neurons: 2,481.8 ± 296.2 pS/pF vs. 1,433.5 ± 234.2 pS/pF (Fig. 5, Table 3). This may be due to higher expression of NaV1.1 and NaV1.6 and also auxiliary Navβ1 and Navβ4. The higher INaT density may contribute to the faster rise and larger amplitude of the action potentials in SNr GABA neurons.
In addition to the difference in INaT density, we also detected differences in INaT kinetics between SNr GABA neurons and SNc DA neurons. To compare the very fast kinetics of INaT, we used the nucleated patch-clamp techniques that provide outstanding space and voltage clamp (Ding et al. 2011; Martina and Jonas 1997). We found that the activation and inactivation time courses of INaT were similar in SNr GABA neurons and nigral DA neurons (Fig. 6, Table 3). However, INaT had a steeper voltage-dependent activation and a faster deactivation in SNr GABA neurons than those in nigral DA neurons (Figs. 6 and 9, Table 3), potentially contributing to the fast rise and short duration of spikes in the fast-spiking SNr GABA neurons. INaT also recovered more quickly from inactivation in SNr GABA neurons than in SN DA neurons, leading to less cumulative inactivation in SNr GABA neurons. These results are consistent with the combination of our qPCR data that SNr GABA neurons express more NaV1.6 and less NaV1.2 than DA neurons (Fig. 3) and published results that NaV1.2 undergo more cumulative inactivation than NaV1.6 in peripheral neurons (Rush et al. 2005). The different rate of recovery from inactivation may contribute to the fact that spike firing was not or was only slightly adaptive in amplitude in SNr GABA neurons, whereas it was strongly adaptive in SNc DA neurons (Fig. 1, D and E).
The differences in INaT kinetics between fast-spiking SNr GABA neurons and slow-spiking DA were relatively modest (Table 3). This is consistent with published studies that different heterologously expressed Nav channel subunits or isoforms display only minor or subtle differences in their kinetic properties (Goldin 2001; Smith and Goldin 1998). However, subtle differences in INaT kinetics may be sufficient to affect spike initiation and waveform (Engel and Jonas 2005; Hu et al. 2009). Parts of our results on INaT (INaV density and inactivation time course) are also consistent with a recent study (Seutin and Engel 2010). However, some of our results on INaT kinetics are in contrast to Seutin and Engel (2010). For example, Seutin and Engel (2010) indicated a faster rise time for INaT in GABA neurons than in DA neurons, whereas our data indicated a similar rise time for INaT for both cell types. Seutin and Engel (2010) also indicated that there was no difference in activation, deactivation, and recovery from inactivation, whereas we found differences in these three parameters. Specifically, Seutin and Engel (2010) reported that the midpoint potentials of activation were −9.6 mV and −12.6 mV for DA and GABA neurons, respectively. These two values are over 10 mV more positive than the V1/2 of INaT activation reported for multiple types of neurons (Colbert and Pan 2002; Gittis and Lac 2008; Hu et al. 2009; Martina and Jonas 1997; Maurice et al. 2004; Raman et al. 2000). Furthermore, Seutin and Engel (2010) reported that the midpoint potential of steady-state inactivation was more positive (−49 mV) in DA neurons than in GABA neurons (−56 mV), indicating that NaV channels in DA neurons are less likely to inactivate, whereas we found there was no significant difference in this parameter: −63.3 mV for SNr GABA neurons and −61.3 mV for nigral DA neurons. INaT recovery from inactivation is important and needs to be discussed. Seutin and Engel (2010) also found no difference in time course of recovery from fast inactivation among the two cell types, while we found that INaT recovered more quickly from both fast and slow inactivation in SNr GABA neurons than in nigral DA neurons. We also found that there was less cumulative inactivation in INaT in SNr GABA neurons than in SNc DA neurons, indicating that more NaV channels become available after action potential firing and the refractory period is shorter. Another discrepancy between our present study and Seutin and Engel (2010) is the deactivation time course. Our data indicate a faster deactivation in SNr GABA neurons than in nigral DA neurons (Fig. 9), whereas Seutin and Engel (2010) reported no difference. We believe that this discrepancy can be explained by the fact that we used a different deactivation protocol. We first used the same protocol with 300 μs at 0-mV activation pulse used by Seutin and Engel (2010) and saw no apparent difference in deactivation (data not shown). However, we noticed that, at the end of 300 μs at 0 mV, INaT had inactivated significantly. Thus 300 μs at 0-mV activation pulse was too long for studying deactivation under our recording conditions. Based on our activation data, INaT was at its peak at 200 μs after stepping to 0 mV. Thus we shortened the activation part to 200 μs in the deactivation protocol and detected the faster deactivation in SNr GABA neurons. In our opinion, our data on INaT kinetics can better explain the differences in spiking properties in fast spiking SNr GABA neurons and slow spiking DA neurons. For example, NaV channels with a steeper activation slope, faster deactivation, and faster recovery from inactivation can support the fast spiking in SNr GABA neurons. Certainly, independent studies are required to resolve these discrepancies.
Larger INaR in SNr GABA neurons than in SN DA neurons.
The INaR is generated during membrane repolarization after depolarization and inactivation of INaT (Raman and Bean 1997). Particularly, NaVβ4 may act as an open channel blocker, binding to the open NaV channels upon depolarization. Upon repolarization, Navβ4 peptide unbinds and allows Na+ flow briefly, thereby producing INaR (Bant and Raman 2010; Grieco et al. 2005). INaR is much smaller but longer-lasting than INaT. Evidence indicates that NaV1.6 (α6) and NaVβ4 (β4) are critical for INaR generation (Bant and Raman 2010' Do and Bean 2004; Grieco and Raman 2004; Raman et al. 1997; Grieco et al. 2005). Since INaR is induced upon repolarization, it may promote repetitive firing (Khaliq et al. 2003).
Our qRT-PCR data indicated that Nav1.6 and NaVβ4 mRNA are expressed at higher levels in SNr GABA neurons than in nigral DA neurons (Fig. 3). In addition, our nucleated patch-clamp recordings showed that INaR was larger in SNr GABA neurons than in nigral DA neurons (Fig. 10). These results are consistent with reports suggesting that Nav1.6 and Navβ4 subunits are the key subunits responsible for generating INaR in other neuron types (Aman and Raman 2007; Bant and Raman 2010; Grieco et al. 2004; Raman et al. 1997; Mercer et al. 2007; Rush et al. 2005).
Larger INaP in SNr GABA neurons than in SN DA neurons.
Although the precise mechanisms generating INaP are not clear, studies indicate that INaP may be generated by the same NaV channels that generate the INaT, but via different gating mechanisms that allow a small fraction of NaV channels to remain open for long periods of time (Alzheimer et al. 1993; Bant and Raman 2010; Crill 1996; Patlak and Ortiz 1986; Taddese and Bean 2002). Evidence also indicates that NaVβ4 (β4) subunit, when coexpressed NaV1.1 (α1) subunit, promotes the generation of INaP and INaR (Aman et al. 2009; Bant and Raman 2010). Although the amplitude of INaP is only a fraction of INaT, its duration is much longer than that of INaT, such that it can enhance neuronal excitability (Crill 1996). Consequently, INaP is involved in pacemaking activity in many types of neurons (Do and Bean 2003; Jackson et al. 2004; Khaliq and Bean 2010; Koizumi and Smith 2008; Mercer et al. 2007; Puopolo et al. 2007; Swensen and Bean 2003; Taddese and Bean 2002). Previous studies also examined INaP in SNr GABA neurons and SNc DA neurons (Atherton and Bevan 2005; Chan et al. 2007; Puopolo et al. 2007), although a direct side-by-side comparison of INaP in these two neuron types was lacking. Based on our qRT-PCR results, we predicted larger INaP in SNr GABA neurons than SN DA neurons, and our experiment proved this hypothesis. We also found that INaP took part in maintaining the membrane potential at a relatively depolarized range, in addition to pacemaking. Blocking INaP with 10 μM riluzole caused a larger hyperpolarization in SNr GABA neurons than SN DA neurons. Those results indicated that INaP played an important role in shaping the different firing patterns between SNr GABA neurons and SN DA neurons.
Our electrophysiological results are consistent with our qRT-PCR data that SNr GABA neurons had higher expression of NaV1.1 and NaV1.6 than SN DA neurons, while SN DA neurons had higher expression of NaV1.2 than SNr GABA neurons. These results were also consistent with literature data that heterologously expressed NaV1.1 and NaV1.6 conduct larger INaP than NaV1.2 (Smith et al. 1998).
Functional implications of differences in NaV currents in fast-spiking SNr GABA neurons and slow-spiking nigral DA neurons.
According to our present study, INaT has a higher density and a steeper voltage-dependent activation in SNr GABA neurons than in nigral DA neurons. These two properties can contribute to the faster rise rate and the larger amplitude of action potentials in SNr GABA neurons than in nigral DA neurons (Fig. 1, C–F). Equally important, compared with nigral DA neurons, INaT in SNr GABA neurons recovered more quickly from inactivation and resisted cumulative inactivation, thus enabling or supporting the sustained high-frequency firing in SNr GABA neurons. The large INaP in SNr GABA neurons that activates at or below −60 mV may drive the membrane potential toward the INaT activation threshold potential around −50 mV, triggering action potentials. Our laboratory has previously indicated that SNr GABA neurons have constitutively active type 3 canonical transient receptor potential cation channels that can depolarize SNr GABA neurons, even when they are at very negative membrane potentials (Zhou et al. 2008). On the hand, DA neurons have a prominent Ih cation current that can depolarize the neurons to approximately −65 mV, the threshold for INaP activation (Fig. 1B; Fig. 10A). SNr GABA neurons also have a robust expression of Kv3-like channels that can quickly repolarize the membrane after each action potential (Ding et al. 2011). Combination of differential expression of these nonselective cation channels, Kv channels, and NaV channels can support the strikingly different spike waveforms and spiking patterns in SNr GABA neurons and nigral DA neurons.
GRANTS
This work was supported by National Institutes of Health grants R01NS058850 and R01DA021194 and an American Parkinson Disease Association grant to F.-M. Zhou.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D. and F.-M.Z. conception and design of research; S.D. and W.W. performed experiments; S.D. and F.-M.Z. analyzed data; S.D. and F.-M.Z. interpreted results of experiments; S.D. and F.-M.Z. prepared figures; S.D. and F.-M.Z. drafted manuscript; S.D. and F.-M.Z. edited and revised manuscript; S.D., W.W., and F.-M.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Angela Cantrell and Robert Foehring for comments on the manuscript.
REFERENCES
- Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci 13: 660–673, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman TK, Raman IM. Subunit dependence of Na channel slow inactivation and open channel block in cerebellar neurons. Biophys J 92: 1938–1951, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman TK, Grieco-Calub TM, Chen C, Rusconi R, Slat EA, Isom LL, Raman IM. Regulation of persistent Na current by interactions between beta subunits of voltage-gated Na channels. J Neurosci 29: 2027–2042, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JF, Bevan MD. Ionic mechanisms underlying autonomous action potential generation in the somata and dendrites of GABAergic substantia nigra pars reticulata neurons in vitro. J Neurosci 25: 8272–8281, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bant JS, Raman IM. Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons. Proc Natl Acad Sci U S A 107: 12357–12362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Jonas P. Action potential propagation into the presynaptic dendrites of rat mitral cells. J Physiol 504: 359–365, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blythe SN, Atherton JF, Bevan MD. Synaptic activation of dendritic AMPA and NMDA receptors generates transient high-frequency firing in substantia nigra dopamine neurons in vitro. J Neurophysiol 97: 2837–2850, 2007 [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat 196: 527–542, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbidge SA, Dale TJ, Powell AJ, Whitaker WR, Xie XM, Romanos MA, Clare JJ. Molecular cloning, distribution and functional analysis of the NAv1.6 voltage-gated sodium channel from human brain. Brain Res Mol Brain Res 103: 80–90, 2002 [DOI] [PubMed] [Google Scholar]
- Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26: 13–25, 2000 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev 57: 397–409, 2005 [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. “Rejuvenation” protects neurons in mouse models of Parkinson's disease. Nature 447: 1081–1086, 2007 [DOI] [PubMed] [Google Scholar]
- Chen YH, Dale TJ, Romanos MA, Whitaker WR, Xie XM, Clare JJ. Cloning, distribution and functional analysis of the type III sodium channel from human brain. Eur J Neurosci 12: 4281–4289, 2000 [PubMed] [Google Scholar]
- Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J Neurosci 16: 6676–6686, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci 5: 533–538, 2002 [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349–362, 1996 [DOI] [PubMed] [Google Scholar]
- Cummins TR, Xia Y, Haddad GG. Functional properties of rat and human neocortical voltage-sensitive sodium currents. J Neurophysiol 71: 1052–1064, 1994 [DOI] [PubMed] [Google Scholar]
- Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: a window to basal ganglia output. Prog Brain Res 160: 151–172, 2007 [DOI] [PubMed] [Google Scholar]
- Ding S, Zhou FM. Molecular and functional differences in voltage-activated Na+ channels between GABA neurons and dopamine neurons in substantia nigra (Abstract). Society for Neuroscience Abstracts 339.22151–172, 2010 [Google Scholar]
- Ding S, Matta SG, Zhou FM. Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. J Neurophysiol 105: 554–570, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, Bean BP. Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39: 109–120, 2003 [DOI] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron 45: 405–417, 2005 [DOI] [PubMed] [Google Scholar]
- French CR, Sah P, Buckett KJ, Gage PW. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol 95: 1139–1157, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuyama T, Morita Y, Inagaki S, Takagi H. Distribution of I, II and III subtypes of voltage-sensitive Na+ channel mRNA in the rat brain. Brain Res Mol Brain Res 17: 169–173, 1993 [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Similar properties of transient, persistent, and resurgent Na currents in GABAergic and non-GABAergic vestibular nucleus neurons. J Neurophysiol 99: 2060–2065, 2008 [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol 63: 871–894, 2001 [DOI] [PubMed] [Google Scholar]
- Gong B, Rhodes KJ, Bekele-Arcuri Z, Trimmer JS. Type I and type II Na(+) channel alpha-subunit polypeptides exhibit distinct spatial and temporal patterning, and association with auxiliary subunits in rat brain. J Comp Neurol 412: 342–352, 1999 [PubMed] [Google Scholar]
- González-Hernández T, Rodríguez M. Compartmental organization and chemical profile of dopaminergic and GABAergic neurons in the substantia nigra of the rat. J Comp Neurol 421: 107–135, 2000 [DOI] [PubMed] [Google Scholar]
- Gorelova NA, Yang CR. Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol 84: 75–87, 2000 [DOI] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron 45: 233–244, 2005 [DOI] [PubMed] [Google Scholar]
- Grieco TM, Raman IM. Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin. J Neurosci 24: 35–42, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron 15: 637–647, 1995 [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes (3rd Ed.). Sunderland, MA: Sinauer, 2001 [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Nav1.6 and Nav12 in action potential initiation and backpropagation. Nat Neurosci 12: 996–1002, 2009 [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience 114: 475–492, 2002 [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. Primary structure and functional expression of the beta1 subunit of the rat brain sodium channel. Science 256: 839–842, 1992 [DOI] [PubMed] [Google Scholar]
- Isom LL, Scheuer T, Brownstein AB, Ragsdale DS, Murphy BJ, Catterall WA. Functional co-expression of the beta1 and type IIA alpha subunits of sodium channels in a mammalian cell line. J Biol Chem 270: 3306–3312, 1995 [DOI] [PubMed] [Google Scholar]
- Jackson AC, Yao GL, Bean BP. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci 24: 7985–7998, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Bean BP. Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci 30: 7401–7413, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J Neurosci 23: 4899–4912, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Smith JC. Persistent Na+ and K+-dominated leak currents contribute to respiratory rhythm generation in the pre-Bötzinger complex in vitro. J Neurosci 28: 1773–1785, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. A calcium-activated nonselective cation conductance underlies the plateau potential in rat substantia nigra GABAergic neurons. J Neurosci 27: 6531–6541, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip31 transcription. EMBO J 20: 5715–5724, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu-The V, Paquet N, Calvo E, Cumps J. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. Biotechniques 38: 287–293, 2005 [DOI] [PubMed] [Google Scholar]
- Martina M, Jonas P. Functional differences in Na+ channel gating between fast-spiking interneurones and principal neurones of rat hippocampus. J Physiol 505: 593–603, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen DP, Meadows LS, Chen C, Thyagarajan V, Isom LL. Sodium channel beta1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin. J Biol Chem 279: 16044–16049, 2004 [DOI] [PubMed] [Google Scholar]
- Mercer JN, Chan CS, Tkatch T, Held J, Surmeier DJ. Nav1.6 sodium channels are critical to pacemaking and fast spiking in globus pallidus neurons. J Neurosci 27: 13552–13566, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP. Beta3: an additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci U S A 97: 2308–2313, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatomo T, Fan Z, Ye B, Tonkovich GS, January CT, Kyle JW, Makielski JC. Temperature dependence of early and late currents in human cardiac wild-type and long Q-T delta KPQ Na+ channels. Am J Physiol Heart Circ Physiol 275: H2016–H2024, 1998 [DOI] [PubMed] [Google Scholar]
- Nelson EL, Liang CL, Sinton CM, German DC. Midbrain dopaminergic neurons in the mouse: computer-assisted mapping. J Comp Neurol 369: 361–371, 1996 [DOI] [PubMed] [Google Scholar]
- Patlak JB, Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol 87: 305–326, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo M, Raviola E, Bean BP. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J Neurosci 27: 645–656, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci 17: 4517–4526, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron 19: 881–891, 1997 [DOI] [PubMed] [Google Scholar]
- Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci 20: 9004–9016, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience 80: 545–557, 1997 [DOI] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, NaV1.2 and NaV16, expressed in mouse spinal sensory neurones. J Physiol 564: 803–815, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Vogel W. Single voltage-activated Na+ and K+ channels in the somata of rat motoneurones. J Physiol 487: 91–106, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Gibb AJ, Gage PW. The sodium current underlying action potentials in guinea pig hippocampal CA1 neurons. J Gen Physiol 91: 373–398, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seutin V, Engel D. Differences in Na+ conductance density and Na+ channel functional properties between dopamine and GABA neurons of the rat substantia nigra. J Neurophysiol 103: 3099–3114, 2010 [DOI] [PubMed] [Google Scholar]
- Smith MR, Smith RD, Plummer NW, Meisler MH, Goldin AL. Functional analysis of the mouse Scn8a sodium channel. J Neurosci 18: 6093–6102, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Goldin AL. Functional analysis of the rat I sodium channel in xenopus oocytes. J Neurosci 18: 811–820, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci 16: 6579–6591, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J Neurosci 23: 9650–9663, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron 33: 587–600, 2002 [DOI] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci 15: 3092–3103, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci 12: 3567–3574, 2000 [DOI] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci 23: 7577–7585, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Jin Y, Matta SG, Xu M, Zhou FM. An ultra-short dopamine pathway regulates basal ganglia output. J Neurosci 29: 10424–10435, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Matta SG, Zhou FM. Constitutively active TRPC3 channels regulate basal ganglia output neurons. J Neurosci 28: 473–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Xu JJ, Zhao Y, LeDoux MS, Zhou FM. Opposite functions of histamine H1 and H2 receptors and H3 receptor in substantia nigra pars reticulata. J Neurophysiol 96: 1581–1591, 2006 [DOI] [PubMed] [Google Scholar]
- Zhou W, Goldin AL. Use-dependent potentiation of the Nav1.6 sodium channel. Biophys J 87: 3862–3872, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]