Abstract
Cervical cancer is caused by Human papillomavirus (HPV) in virtually all cases. These HPV-induced cancers express the viral oncogenes E6 and E7 and are therefore potentially recognized by the immune system. Despite the abundant presence of these foreign antigens, the immune system is unable to cope with the tumor. Due to the constant immunological pressure, cervical cancers can evolve different immune evasion strategies, which will be described in the current review. Several approaches for immunotherapy of cervical cancer are currently under development, which aim at inducing strong HPV-specific immunity. Besides the reinforcement of potent anti-tumor immune responses, immunotherapy could also enhance HPV-specific T regulatory cells. Supplementary strategies that neutralize an immunosuppressive milieu may have great potential. These strategies are discussed as well.
Keywords: Cervical Cancer, Human papilloma virus, Immune response, Immune evasion, T regulatory cell
Cervical Cancer and Human Papilloma Viruses
Cervical cancer is caused by HPV in virtually all cases and is the second most common cancer in women worldwide [1–3]. The most prevalent type is high-risk type HPV16, which accounts worldwide for over 50% of the cases of cervical cancer. The second most prevalent type in the Caucasian population is HPV18, which accounts for more than 15%. Other high-risk types of HPV, of which over 15 have been identified, contribute substantially to cervical cancer cases as well [4].
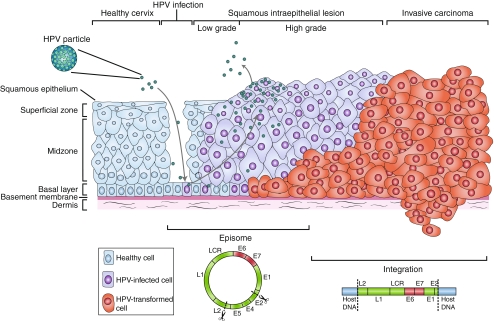
HPV is a small double stranded DNA virus (7–8 kb), which can infect the basal layers of the epidermis and mucosal epithelium. The viral life cycle is tightly regulated to the cycle of the host cell. In the basal layers the proliferation-inducing early genes (including E6 and E7) are expressed, resulting in lateral expansion of the infected cells. After entry into the suprabasal layers the viral genes responsible for viral replication, structural proteins and viral assembly are expressed. Subsequently, infectious particles are released (Reviewed in [5, 6] (Fig. 1).
Fig. 1.
Human papilloma virus (HPV)-induced malignant progression. Infection with HPV likely occurs in the basal layer of the cervical epithelium, which is exposed in a microlesion. During the productive lifecycle the early genes (E1, E2, E4, E5, E6 and E7) are expressed and viral DNA is replicated from episomal DNA. Hereafter, in the upper layers the late genes L1 and L2 are expressed and viral particles are assembled. Subsequently the virions are shed and new infection can be commenced. Low-grade squamous intraepithelial lesion (LSIL) support the production of viral particles. In a minority of infected women the lesion progresses into high-grade squamous intraepithelial lesion (HSIL). Progression towards microinvasive and invasive carcinoma is associated with the integration of the viral genome into host DNA and is frequently accompanied by loss of part of the viral genome, including disruption of the E2 gene. As a result, expression of the viral oncoproteins E6 and E7 are upregulated. LCR, long control region. Adapted by permission from Macmillan Publishers Ltd: [Nature Reviews Cancer] (Woodman et al. [130]), copyright (2007)
The properties of both E6 and E7 are essential for HPV-induced malignant transformation and are therefore known as viral oncogenes [7]. Both proteins interact with multiple host proteins to promote cell proliferation and inhibition of apoptosis. E6 is well known for its ability to promote p53 and BAK degradation, thereby inhibiting apoptosis [8, 9]. Additionally, E6 can also promote the activation of telomerase [10]. E7 on the other hand is able to interact with the retinoblastoma family members and thereby it enhances cell proliferation [11]. Moreover, E7 stimulates cyclin A and E as well, promoting G0/G1 progression [12].
The progression of HPV infection to cervical cancer is a slow process and can be divided in 4 stages (Fig. 1). The first stage comprises of infection with HPV, in most infected individuals the virus is cleared within 2 years. However, in approximately 10% of the infections the virus persists. The virus can persist for several years and is strongly linked to a higher risk for the diagnosis of low-grade squamous intraepithelial lesion (LSIL) (the second stage). This stage is characterized by mild dysplasia due to progression of persistently infected cells to precancer. This lesion can further progress into high-grade squamous intraepithelial lesion (HSIL), which is characterized by moderate dysplasia to in situ carcinoma (third stage). The HSIL can progress further into the last stage, invasive carcinoma (reviewed in [13]). During the first 2 stages, spontaneous regression and/or clearance are common. It has been estimated that less than 1% of the infected women develop cervical cancer [14, 15]. Little is known on the progression versus spontaneous regression rates in HSIL since surgical intervention therapies are used to treat HSIL. However, the general acceptance is that HSIL do not regress spontaneously [16]. Additionally, early studies suggested that less than 30% of HSIL progress further into invasive carcinoma within 10 years [17].
During malignant transformation, the DNA of HPV is able to integrate into the host genome at random positions [18, 19]. The integration of the viral DNA is associated with the transition into invasive carcinoma [20]. During insertion into the host DNA, the integrated DNA is either complete or there is partial loss of the viral genes. Loss of E1, E2, E4, E5 and L2 occurs frequently, increasing the immortalization potential of E6 and E7 [5, 21]. Moreover, E6 and E7 are essential in maintaining the malignant phenotype of the tumor cells and are therefore expressed in every tumor cell [22]. Consequently, HPV E6 and E7 represent potential good targets for the immune system.
Immune Responses in Cervical Cancer Patients
The role of the immune system in controlling HPV-infections is illustrated by the observation that strong proliferative HPV16 E2- and E6-specific T-cell memory responses are frequently detected in HPV-negative healthy women as witness of previous infection. These responses are accompanied by IFNγ and IL-5 production and low levels of IL-10 [23–25]. Similar findings have been described for HPV18 [26]. Occasional responses against E7 are observed as well [24, 27]. T-helper responses against the C-terminal domain of HPV16E2 frequently occur at the time of virus clearance [28]. A recent prospective study showed that presence of HPV16 E2 specific T-cell responses were correlated with the absence of progression in LSIL patients, indicating a protective effect of E2-specific immunity [29].
In contrast, in cervical cancer patients HPV-specific T-cell responses are detected only in half of the Cervical Cancer (CxCa) and HSIL patients. In these patients a weak proliferative response was observed. This response was not associated with production of the proinflammatory cytokines IL-5 and IFNγ, but the anti-inflammatory cytokine IL-10 was still detected in CxCa patients [25, 30]. Similar results were found for HPV18 as well [26]. Consistent with these results other studies report that HPV16-specific proliferative responses are occasionally observed whereas Th1 type responses, as defined by IL-2 production, are low or lacking in cervical cancer patients [31–34]. The presence of HPV16 E6-specific responses in CxCa patients are associated with invasion depth and are associated with disease free survival [35]. HPV16-specific CTL can only rarely be detected in the peripheral blood of HSIL and CxCa patients [36–39], whereas such responses are frequently detected in healthy donors [40, 41]. Since CD4 T cells are essential in the induction and maintenance of CD8 cytotoxic T-lymphocyte (CTL) immunity [42], the defective Th1 response in CxCa patients may explain the low levels of HPV-specific CTL. Furthermore, the CD4 T-cell response is accompanied by IL-10 production, indicating a role for active suppression.
HPV-specific T cells have been reported to infiltrate cervical neoplastic tissues and metastatic lymph nodes as well [43–47]. These infiltrating T cells are not specific for preferential regions within the E6 and E7 proteins. Remarkably, most of the CD4 restricted T-cell responses were restricted by HLA-DP [43]. This might be specific for HPV-induced tumors, but warrants further investigation. Within a single patient the HPV-specific T-cell response is broad as is indicated by the recognition of multiple E6 and E7 epitopes and multiple T-cell receptor Vβ usage [48].
On the other hand, Natural Killer (NK) cells seem to play only a limited role in the immune surveillance of the primary tumor in cervical cancer patients, as only low numbers of CD57 + CD3- cells, encompassing a subpopulation of NK cells, are infiltrating tumor tissue [49, 50]. Despite their absence at the tumor site they are present in vast numbers in the peripheral blood and in the lymph system, where they may kill metastasizing cells.
Despite the abundant presence of HPV-specific T cells in neoplastic tissue, the immune system is unable to eradicate the tumor. This suggests the existence of an immunosuppressive microenvironment in cervical cancer patients.
Immune Evasion Strategies Employed by Cervical Tumors
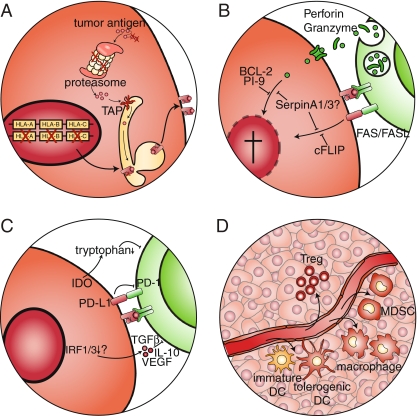
During malignant transformation, a continuous struggle exists between the tumor cells and the immune system. Because of continuous immunological pressure, the tumor develops several mechanisms to escape immunosurveillance. As the tumor persists it may accumulate such mechanisms, thereby evading control by the immune system. This is a slow process that can take years or even decades and is known as cancer immunoediting [51]. In many tumors the transformed cells have acquired several mechanisms to protect them from immune cell mediated killing. These mechanisms include (A) MHC class I downregulation and impaired antigen processing to prevent antigen presentation, (B) resistance to immune-mediated apoptosis, (C) the expression of immunosuppressive factors and (D) the attraction of immune cells that are able to inhibit the immune response (Fig. 2). The different mechanisms described in the literature and their role in cervical cancer will be discussed below.
Fig. 2.
Immune evasion mechanisms employed by tumors. In many tumors the transformed cells have acquired several mechanisms to protect them from immune cell mediated killing. These mechanisms include a downregulation of the antigen presentation machinery, b insensitivity to CTL-mediated cytotoxicity, c production of immunosuppressive cytokines and d attraction of immune cells with immunosuppressive properties
Direct Evasion of the Anti-Tumor Response
The occurrence of antigen loss has been well demonstrated in an immunogenic tumor mouse model [52, 53]. These studies collectively show that tumor cells are able to lose the expression of antigens as a result of immunological pressure. Occurrence of antigen loss has also been illustrated in melanoma patients. Antigens normally expressed by melanocytes are frequently lacking in tumor cell lines and tumor tissue from melanoma patients (reviewed in [54]). However, antigen loss is almost absent in cervical cancer patients, as HPV DNA can be detected in virtually all tumors and E6 and E7 RNA is present throughout malignant transformation in all cases [1, 55, 56]. The E6 and E7 proteins are essential in maintaining the malignant phenotype of the tumor cells, which may explain the absence of antigen loss in HPV-induced cervical cancer.
The two major pathways used by lymphocytes to induce apoptosis in target cells are the granule exocytosis pathway and the FAS/FASL pathway [57]. For these apoptotic pathways, tumor-escape variants have been described (reviewed in [58]). Examples of such escape-mechanisms are overexpression of the anti-apoptotic gene BCL-2 [59], expression of the FASL-inhibitors FLICE-inhibitory protein (cFLIP) [60, 61] and expression of the Granzyme B inhibitor PI-9 [62] in mouse models. cFLIP has been shown to be overexpressed in cervical tumor tissue compared to healthy cervix, but the impact on survival is still unclear [63]. Recently, SerpinA1 and SerpinA3 have been shown to be overexpressed in tumors of a subpopulation of cervical cancer patients. In this study overexpression of these proteins correlated with a poorer survival [64]. Since SerpinA1 and SerpinA3 both have been implicated in inhibition of apoptosis [65, 66], overexpression of these proteins may render the tumor cells insensitive for immune mediated apoptosis.
Many tumors downregulate MHC-class I to evade recognition by the immune system. Downregulation of the HLA class I genes can originate from multiple mechanisms (Reviewed in [67]). Mutations of the individual HLA alleles together with the deletion of the common β2 microglobulin genes are commonly observed in many types of cancer. A different immune escape mechanism employed by a number of tumors is defects in the antigen processing machinery. Defective antigen processing leads to impaired antigen presentation of tumor antigens, as a result viral and tumor associated antigens normally produced by the proteasome and transported through TAP cannot be presented on MHC class I [54]. Defects in the antigen machinery include decreased expression of proteasome subunits (eg. LMP2 and LMP7) and transporter subunits (TAP1 and TAP2). The frequencies of these defects differ between tumor types [68]. Since antigen presentation of the HPV16E6 protein depends on TAP and the proteasome [69], defects in these proteins result in decreased recognition of tumor cells by HPV-specific T cells. E7 of the low-risk HPV11 has been implicated in TAP inhibition in laryngeal papillomatosis [70, 71], but this effect has not been reported for other HPV types. On the other hand, E7 of HPV6, -16 and −18 has been shown to reduce the expression of MHC class I heavy chain, LMP2 and/or TAP1 [72]. HPV16 E5 has been shown to downregulate HLA-A and -B cell surface expression, but no decrease was found in total HLA class I expression [73]. However, the exact mechanism by which HPV16 E5 modulates Class I surface expression is not known.
Despite direct interactions of HPV proteins with TAP, MHC class I is rarely completely lost in LSIL or HSIL lesions [74]. Moreover, interference with TAP is detected in a subpopulation of the cervical cancer patients, indicating that the observed downregulation in the MHC class I pathway is not directly caused by HPV [75, 76]. Interference of HPV proteins with MHC class I presentation machinery is therefore not likely to have a dominant role in cervical cancer patients. Alternatively, MHC class I defects may develop during malignant transformation, due to immunological pressure on the tumor. In cervical cancer patients abnormalities in the MHC class I presentation machinery has been well documented [49, 74–82]. Alterations in MHC-class I presentation pathway has been observed in approximately 90% of the patients with cervical cancer [79]. However, in this study total loss of MHC class I has been observed in only 10% of the patients, indicating that 90% of the cervical cancer patients could benefit from T-cell mediated immunotherapy.
Indirect Evasion of T-Cell Mediated Killing
Many tumors express inhibitory coreceptors [83]. The inhibitory B7 family member B7-H1 (PD-L1) is expressed on a wide variety of tumors [84]. This molecule can interact with PD-1 and CD80 on T cells, thereby inducing apoptosis, anergy or exhaustion of effector T cells [85, 86]. In a variety of tumors, expression of PD-L1 is associated with poorer survival [87–94]. Unexpectedly, expression of cell-surface PD-L1 in cervical cancer patients was associated with improved survival [95]. This phenomenon could be explained by incapacitation of infiltrating PD1+ regulatory T cells (Tregs) through PD1:PD-L1 interactions. The recently identified B7-H3 and B7x have been found to be expressed on tumors and B7-H3 expression has been shown to be correlated to decreased survival in renal cell carcimoma [96, 97], but their role in cervical cancer is unknown.
Immunosuppressive factors produced by tumor cells can also contribute to the immunosuppressive microenvironment. These factors include indoleamine 2,3-dioxygenase (IDO), vascuar endotheial growth factor (VEGF), tumor growth factor β (TGFβ) and IL-10. The IDO pathway has also been implicated in indirect immune escape by tumors. The immune tolerant effect of IDO functions through the depletion of tryptophan and the generation of kynurenine metabolites, resulting in affected T-cell proliferation and survival [98]. A few studies showed that IDO is expressed by the tumor and the level of expression is an independent prognostic factor in colorectal cancer [99, 100]. IDO has been implicated to interfere with the initial immune response to tumor antigens, the cytolytic capacity of CTL and enhanced suppressive capacity of Tregs in several tumor types (reviewed in [101]). IDO has been shown to be present in HSIL and CxCa, but the functional consequence of IDO was not addressed [102]. Therefore, the exact role of IDO in cervical cancer remains unclear. VEGF, which is normally involved in vessel formation, also contributes to the immune suppressive environment by the attraction of immature dendritc cells (DCs) and macrophages, which will be discussed below [103]. TGFβ is expressed in many tumors and is known to inhibit immune responses at multiple levels [104, 105]. In cervical tumors, TGFβ mRNA is frequently detected but does not correlate with survival [106]. However, PAI-1 and αvβ6 integrin expression, which reflect the presence of active TGFβ, has a negative influence on survival [106, 107]. Next to immune regulation, TGFβ also modulates other processes, which include cell invasion and metastatic colonization. The impact of TGFβ on immune escape is therefore difficult to determine in cancer patients [104]. However, an inverse relation exists between TGFβ expression in tumors and tumor infiltrating lymphocytes, indicating that TGFβ may hamper the infiltration of lymphocytes in cervical cancer [108]. HPV has also been implicated to induce the production of immunosuppressive factors. The E6 and E7 proteins have been reported to inhibit Interferon regulatory factor (IRF3 and IRF1 respectively), which are transcription factors involved in immune pathways [109, 110]. Interference with these proteins results in an impaired IFN-pathway and thereby NFκB-stimulated genes. This results in lower levels of pro-inflammatory cytokines, which may be a direct mechanism by which HPV creates an immunosuppressive microenvironment [111].
Attraction of Innate Immune Cells with Immunosuppressive Properties
A third mechanism of immune evasion by tumors is the attraction of immune cells with immunosuppressive properties. These include both members from the innate and the adaptive immune system that are able to suppress anti-tumor responses. Macrophages are recruited by many types of tumors in high numbers, in these tumors they differentiate predominantly into a M2 phenotype [112, 113]. These tumor-associated macrophages (TAMs) have direct effects on tumor growth, vascularization and modulation of the tumor stroma. Moreover, TAMs also produce a wide array of cytokines and chemokines, resulting in immune evasion at multiple levels. These evasive mechanisms include alteration of DC phenotype and modulation of T-cell responses [113]. Tumor infiltrating CD68+ macrophages have been found to infiltrate cervical tumors and metastatic lymph nodes [80, 114, 115]. The TAMs reach numbers similar to infiltrating T cells in cervical tumors [80]. However, the type of macrophage and their impact on the immune system have not been addressed in these studies.
Dendritic cells (DCs) are the key players in orchestration and initiation of the immune response. DCs have been shown to infiltrate human tumors. However, they usually have an immature phenotype as they lack costimulatory molecules (Reviewed in [116]). These improperly polarized DCs induce rather T-cell deletion and anergy as opposed to induction of effector T cells which are able to eradicate the tumor [117]. In cervical cancer, similar numbers of immature DCs were found in tumor tissue as compared to healthy cervix [50, 115]. The number of mature DCs was increased in tumor tissue, which may indicate that DCs may become activated in the tumor, but have decreased capacity to migrate out of the tumor. Alternatively, the observed number of DCs reflects a snap-shot of a population of DCs, which are preparing to migrate out of the tumor. In tumor draining lymph nodes of cervical cancer patients, IDO expressing DCs have been found [102], indicating that they may play a role in immune escape.
Myeloid derived suppressor cells (MDSCs) represent a heterogeneous population of incompletely differentiated myeloid cells [83]. Their characterization is difficult due to the complicated phenotype. They are generally characterized as CD11b + CD14-, CD33 + HLA-DR- or CD14 + HLA-DR- [118, 119]. They are elevated in the peripheral blood of cancer patients (reviewed in [118]). Even though MDSCs in tumors has not been studied extensively, these cells have been shown to infiltrate hepatocellular- and head and neck carcinoma [120, 121]. MDSCs are able to directly inhibit T-cell responses via ROS and iNOS [122], and in mice these cells promote tumor progression [123]. Their impact on tumor progression in cervical cancer patients is, however, still unclear.
Even though the DC, TAM and MDSC populations are described as separate entities above, they all are of myeloid origin and therefore derived from the same precursors. As a result, there may be a spectrum between these populations in which single cells may have characteristics from multiple cell populations.
T Regulatory Cells in Cervical Cancer Patients
CD4+ Tregs have emerged as an arm of the adaptive immune response involved in counteracting the anti-tumor immune response. Early studies showed increased numbers of Treg, based on CD25 and CD152 expression in proximal tumor draining lymph nodes and these cells contained suppressive capacity [124]. Although Tregs can only be identified based on suppressive function, the transcription factor FOXP3 is currently the most widely used marker for Tregs [125]. The infiltration of tumors with FOXP3+ Tregs is unfavorable for patient survival in many types of cancer (reviewed in [126]). In cervical cancer patients the effect of FOXP3+ Treg was more pronounced when the ratio between infiltrating CD8+ T cells and FOXP3+ Tregs was calculated [49, 50]. The balance between infiltrating CD8+ T cells and Tregs was found to be an independent prognostic factor for patient survival [49]. Metastatic tumor cells in tumor draning lymph nodes were found to correlate with increased numbers of Treg in the respective lymph node [127].
As HPV-induced tumors express the viral antigens E6 and E7, the infiltrating Tregs potentially encompass HPV-specific Tregs. Indeed, Tregs specific for the E6 and E7 antigens have been detected in tumor- and HSIL infiltrating lymphocytes as well as tumor draining lymph nodes [30, 128]. Interestingly, these Tregs included both FOXP3+ and FOXP3- cells [126, 128]. This observation indicates that enumeration of Tregs on the basis of FOXP3 expression likely underestimates the total number of infiltrating Tregs in cervical tumors.
It is difficult to determine the true origin and role of HPV-specific Tregs during disease progression in cervical cancer patients. Possibly, Tregs are induced as part of the normal immune response against HPV, as acute infections such as influenza can mount virus-specific Tregs as well [129]. Generally, HPV infections are cleared quite slowly (median of 6 months) [130], while acute viral infections such as influenza are cleared within weeks. Therefore, the immune system seems to be inefficient in clearing HPV infections. This may be caused by early interactions of the host with the virus at multiple levels. Firstly, Langerhans cells, which are the professional antigen presenting cells in initiating mucosal immune responses, are improperly activated upon encounter of L2-containing virus like particles [131]. Secondly, HPV also interferes with the IFN pathway in infected keratinocytes, caused by the oncogenes E6 and E7 (Reviewed in [132]). This results in a stronger immunosuppressive microenvironment and may thereby promote the induction and expansion of HPV-specific Tregs. One or combinations of these interactions may result in enhanced induction of HPV-specific Tregs and as such induce a more immunosuppressive virus-specific immune response compared to acute viral infections. However, these observations do not explain why most people are able to clear persistent HPV infections, whereas a minority of the infected women are not able to cope with the virus and as a result develop cervical cancer. Both genetic and environmental factors have been implicated in HPV oncogenesis, however a clear picture is still missing [133].
Accumulating numbers of circulating Tregs (defined as CD4 + CD25+) have been detected in the peripheral blood of HSIL patients as witness of an immunosuppressive milieu in these patients [134, 135]. In line with these findings, a substantial number of infiltrating FOXP3+ Treg have been detected in HSIL patients [136, 137], but no significant differences were observed between HSIL and LSIL [137]. Moreover, HPV-specific Tregs were detected among cervical infiltrating lymphocytes in a patient with HSIL [30]. This is indicative of an immunosuppressive HPV-specific response in this patient. The immunosuppressive microenvironment in HSIL patients may subsequently favour the progression towards invasive carcinoma by evading immunosurveillance.
Additionally, HPV-induced tumor cells overexpress different self-antigens as well, including hTERT and p16 [138–141]. For this reason it is likely that Tregs specific for these antigens also infiltrate cervical tumors and contribute to the establishment of an immunosuppressive microenvironment in the tumor.
Immunotherapy of HPV-Induced Malignacies
Many different strategies have been developed for the immunotherapy of cancer [142]. Strategies against HPV-induced malignancies include synthetic long peptide vaccines, targeting the E6 and E7 proteins (reviewed in [42]). Although these therapeutic vaccines are designed to enhance CD4+ and CD8+ T-cell effector immunity, they may also activate pre-existing tumor antigen-specific FOXP3 + CD4+ Tregs present in the lymph nodes and tumors of both cervical cancer and melanoma patients [143, 144]. In mice, boosting of Tregs after therapeutic vaccination was associated with subsequent failure of the anti-tumor immune response [145]. A recent study in vulvar intraepithelial neoplasia patients showed both clinical and immunological responses after vaccination against HPV16 E6 and E7 [146]. In this study patients who did not display a complete clinical response, mounted both HPV-specific effector T cells and HPV-specific FOXP3+ Tregs following vaccination. In contrast, patients who displayed a complete clinical response mounted predominantly HPV16-specific T effector cells [147]. These data indicate that those patients in whom the current HPV-specific therapeutic approach is unsuccessful could benefit from an alternative therapy that includes the neutralization of Tregs (Fig. 3).
Fig. 3.
Strategies that could bypass vaccination-induced Treg expansion. Depletion of Treg before or during treatment with CD25-targeting compounds or with low dose cyclophosphamide decreases the initial numbers of Treg. Blockade of CTLA-4 signaling both dampens Treg as well as releases the brakes on effector cells. Blockade of PD-1:PD-L1 interaction results in enhancement of effector responses, but also can enhance Treg function. Several agents can be used to skew the antigen presenting compartment to an immunogenic phenotype. These approaches include maturation of DC, modulation of macrophage phenotype and targeting myeloid suppressor cells (MDSC)
Intervention Strategies to Bypass Vaccination-Induced Treg Expansion
Depletion of Treg Based on CD25 Expression
In several mouse models, treatment with an anti-CD25 depleting antibody enhanced the anti-tumor immune response (reviewed in [148]). For translation to the clinic a hybrid molecule has been used (ONTAK). This molecule contains full-length IL-2 for binding to CD25 and the translocation and toxic domains of diphtheria toxin to induce apoptosis [149]. In mice this molecule was able to deplete FOXP3+ Tregs in different compartments and was able to enhance vaccination-induced T-cell responses [150]. In combination with vaccination, ONTAK is able to deplete Tregs and thereby boosting the tumor-specific immune response in renal cell carcinoma, CEA-positive and melanoma patients [151–153]. In contrast, in one study ONTAK was unsuccessful in depleting Tregs in metastatic melanoma patients [154]. Together, these studies show that ONTAK as supplementary therapy in vaccination trials may be promising, however caution is needed as this therapy is not always successful.
LMB-2 is another immunotoxin, which targets CD25. LMB-2 is a hybrid molecule consisting of pseudomonas exotoxin A and the Fv chain of anti-CD25 [155]. In a small human trial, LMB-2 was able to partially deplete Tregs, but no effect was seen on vaccine-induced responses in patients with metastatic melanoma [156]. Further studies are required to determine a potential additive effect of LMB-2 treatment and HPV vaccination strategies.
Depletion of Tregs Based on Cytotoxic Chemotherapy
Low-dose cyclophosphamide, which is a cytotoxic alkylating compound, reduces both the number of Tregs as well as their function in mice [157]. A recent study showed enhanced Treg depletion in the tumor when cyclophosphamide was used in combination with an agonistic anti-OX40 antibody. This regime induced hyperactivation and cell death in the Treg compartment [158]. In animal models, low-dose cyclophosphamide was able to enhance vaccine-induced anti-tumor responses [159, 160]. In humans, cyclophosphamide used as a single agent was shown to inhibit the Treg compartment, while the effector compartment was not negatively influenced [161, 162]. In cervical cancer patients Treg numbers were decreased after preoperative low-dose chemoradiation therapy [127, 163]. Combinational therapy has not been studied in cervical cancer patients, but may prove to be an effective approach to enhance anti-tumor vaccination strategies.
CTLA-4 Blockade to Improve Anti-Tumor Immunity
CTLA-4 is an inhibitory co-receptor that is expressed both on activated T cells and constitutively on thymus derived Tregs. In mouse models, it has been shown that combination therapy of CTLA-4 blockade, especially together with CD25 depletion or GM-CSF secreting vaccine improves immunotherapy against established tumors [164–166]. CTLA-4 blockade both on the effector population as well as on the Treg compartment is important in the enhancement of anti-tumor responses [167]. Two monoclonal blocking antibodies (ipilimumab and tremelimumab) are currently being tested in clinical trials [168]. Since these antibodies affect all T cells regardless of specificity, side effects of these antibodies include mild to severe autoimmunity [168, 169]. Early promising clinical trials show enhanced anti-tumor T-cell responses upon treatment with anti-CTLA4 antibodies [170–172]. A recent phase III trial showed increased survival in melanoma patients after ipilimumab treatment [173]. However, in this study treatment was not improved by gp100 specific vaccination. These monoclonal antibodies may provide a window in which CTLA-4 blockade combined with vaccination against HPV16 E6 and E7 may improve the treatment of cervical cancer patients.
Blockade of the PD-L1-PD1 Axis
Blockade of PD1 or PD-L1 improves anti tumor-responses in several mouse models (Reviewed in [174]). A humanized blocking antibody to PD-1 has been tested in a phase I trial in patients with hematological malignancies and was found to be well tolerated in these patients [175]. As PDL-1 expression in cervical cancer affects patient survival differently compared to other types of cancer, treatment with PD-L1 blocking antibodies may have unexpected results in cervical cancer patients.
Modulating Antigen Presenting Cells
Different subsets of APC have the capacity to induce Tregs [118, 176, 177]. As these cell types are not affected using the strategies described above, depletion of Tregs does not exclude de novo induction of HPV-specific Tregs upon tumor-specific vaccination. Therefore, strategies to modulate these cells as well may prove to be a valuable supplementary therapy to enhance tumor-specific immune responses.
Several approaches have been proposed to skew the phenotype of DCs in cancer patients from a tolerogenic into a pro-inflammatory phenotype (reviewed in [117]). These approaches include activation of DCs by anti-CD40 antibodies, Toll-like receptor ligands, activation of the inflammasome and immunogenic cell death by chemotherapy and radiation therapy [178–180]. In cervical cancer patients, these properly activated DCs may in turn shift the balance from a Treg dominated response into a Th1/CTL dominated HPV-specific response, which is subsequently able to mount a full-blown attack against the tumor.
Tumor associated macrophages promote the immunosuppressive microenvironment. Targeting these cells may therefore augment vaccination protocols. Two recent studies described that skewing of the phenotype towards a proinflammatory M1 phenotype by inhibition of IKKβ results in improved tumoricidal activity [181, 182]. The M1 macrophages in turn may promote anti-tumor immune responses. Even though subversion of the phenotype of macrophages represents a promosing approach for anti-cancer therapy, agents are not yet available to promote M1 macrophage differentiation in the clinic. However, a recent study in a mouse model of HPV-induced tumors showed that depletion of TAM by clodronate-containing liposomes impaired tumor growth in mice [183]. These strategies are still in preclinical models, but hold potential to improve therapeutic HPV vaccination.
Several agents are currently tested in preclinical models to inhibit expansion and function of MDSCs (Reviewed in [118]). These cells are implicated in the expansion of Tregs and are present in the peripheral blood of cancer patients in relatively high numbers. Therefore, depletion of MDSCs may result in abrogation of the immunosuppressive milieu, enabling effective vaccination against HPV E6 and E7 without vigorous expansion of Tregs.
Final Remarks
The local presence of an immunosuppressive microenvironment provides a plausible explanation for the inability of the immune system of cervical cancer patients to cope with the tumor. Moreover, HPV-specific Tregs are boosted upon vaccination with HPV16 synthetic long peptides and negatively correlate with clinical outcome. Therefore, elimination/reduction of the Treg compartment either before or during vaccination, will likely shift the balance from a Treg dominated response to an effector T-cell dominated response. This will result in improved vaccination efficacy. Strategies that elicit potent anti-tumor immune responses may also lead to the induction of different escape mechanisms. These mechanisms could include antigen loss, loss of MHC-class I molecules and impaired antigen processing. These escape variants can subsequently be targeted by alternative approaches, such as vaccination against epitopes that are associated with impaired antigen processing [184].
Finally, the tumor microenvironment observed in cervical cancer patients has similar characteristics to other types of cancer. Knowledge gathered on inducing a potent anti-tumor immune therapy in these patients may therefore be translated to other types of cancer as well.
Acknowledgements
The author would like to thank dr. S.H. van der Burg for critically reading the manuscript. The author apologizes on behalf of all the excellent research that could not be cited because of space limitations. The author declares that he has no conflict of interest.
Glossary
- HPV
Human papilloma virus
- LSIL
Low-grade squamous intraepithelial lesion
- HSIL
High-grade squamous intraepithelial lesion
- IFNγ
Interferon γ
- IL-5
Interleukin 5
- CxCa
Cervical cancer
- CTL
Cytotoxic T lymphocyte
- NK cell
Natural killer cell
- cFLIP
FLICE-inhibitory protein
- IDO
Indoleamine 2,3-dioxygenase
- VEGF
Vascuar endotheial growth factor
- TGFβ
Tumor growth factor β
- Tregs
Regulatory T cells
- IRF
Interferon regulatory factor
- TAM
Tumor-associated macrophage
- DC
Dedritic cell
- MDSC
Myeloid derived suppressor cells
- PD1
Programmed death 1
- APC
Antigen presenting cell
References
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Burchell AN, Schiffman M, Giuliano AR, Sanjose S, Bruni L, Tortolero-Luna G, Kjaer SK, Munoz N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine. 2008;26(Suppl 10):K1–16. doi: 10.1016/j.vaccine.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomavirus infections–a major cause of human cancers. Biochim Biophys Acta. 1996;1288(2):F55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 6.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 7.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248(4951):76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 9.Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14(23):3065–3073. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75(9):4467–4472. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 12.Zerfass K, Schulze A, Spitkovsky D, Friedman V, Henglein B, Jansen-Durr P. Sequential activation of cyclin E and cyclin A gene expression by human papillomavirus type 16 E7 through sequences necessary for transformation. J Virol. 1995;69(10):6389–6399. doi: 10.1128/jvi.69.10.6389-6399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 14.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102(5A):3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 15.Evander M, Edlund K, Gustafsson A, Jonsson M, Karlsson R, Rylander E, Wadell G. Human papillomavirus infection is transient in young women: a population-based cohort study. J Infect Dis. 1995;171(4):1026–1030. doi: 10.1093/infdis/171.4.1026. [DOI] [PubMed] [Google Scholar]
- 16.Barron BA, Cahill MC, Richart RM. A statistical model of the natural history of cervical neoplastic disease: the duration of carcinoma in situ. Gynecol Oncol. 1978;6(2):196–205. doi: 10.1016/0090-8258(78)90022-7. [DOI] [PubMed] [Google Scholar]
- 17.Kinlen LJ, Spriggs AI. Women with positive cervical smears but without surgical intervention. A follow-up study. Lancet. 1978;2(8087):463–465. doi: 10.1016/s0140-6736(78)91457-5. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 19.Wentzensen N, Vinokurova S, Knebel DM. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004;64(11):3878–3884. doi: 10.1158/0008-5472.CAN-04-0009. [DOI] [PubMed] [Google Scholar]
- 20.Hopman AH, Smedts F, Dignef W, Ummelen M, Sonke G, Mravunac M, Vooijs GP, Speel EJ, Ramaekers FC. Transition of high-grade cervical intraepithelial neoplasia to micro-invasive carcinoma is characterized by integration of HPV 16/18 and numerical chromosome abnormalities. J Pathol. 2004;202(1):23–33. doi: 10.1002/path.1490. [DOI] [PubMed] [Google Scholar]
- 21.Chen CM, Shyu MP, Au LC, Chu HW, Cheng WT, Choo KB. Analysis of deletion of the integrated human papillomavirus 16 sequence in cervical cancer: a rapid multiplex polymerase chain reaction approach. J Med Virol. 1994;44(2):206–211. doi: 10.1002/jmv.1890440216. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways. Proc Natl Acad Sci USA. 2000;97(23):12513–12518. doi: 10.1073/pnas.97.23.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jong A, Burg SH, Kwappenberg KM, Hulst JM, Franken KL, Geluk A, Meijgaarden KE, Drijfhout JW, Kenter G, Vermeij P, Melief CJ, Offringa R. Frequent detection of human papillomavirus 16 E2-specific T-helper immunity in healthy subjects. Cancer Res. 2002;62(2):472–479. [PubMed] [Google Scholar]
- 24.Welters MJ, Jong A, Eeden SJ, Hulst JM, Kwappenberg KM, Hassane S, Franken KL, Drijfhout JW, Fleuren GJ, Kenter G, Melief CJ, Offringa R, Burg SH. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63(3):636–641. [PubMed] [Google Scholar]
- 25.Jong A, Poelgeest MI, Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, Kenter G, Offringa R, Burg SH. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64(15):5449–5455. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 26.Welters MJ, Logt P, Eeden SJ, Kwappenberg KM, Drijfhout JW, Fleuren GJ, Kenter GG, Melief CJ, Burg SH, Offringa R. Detection of human papillomavirus type 18 E6 and E7-specific CD4+ T-helper 1 immunity in relation to health versus disease. Int J Cancer. 2006;118(4):950–956. doi: 10.1002/ijc.21459. [DOI] [PubMed] [Google Scholar]
- 27.Burg SH, Ressing ME, Kwappenberg KM, Jong A, Straathof K, Jong J, Geluk A, Meijgaarden KE, Franken KL, Ottenhoff TH, Fleuren GJ, Kenter G, Melief CJ, Offringa R. Natural T-helper immunity against human papillomavirus type 16 (HPV16) E7-derived peptide epitopes in patients with HPV16-positive cervical lesions: identification of 3 human leukocyte antigen class II-restricted epitopes. Int J Cancer. 2001;91(5):612–618. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1119>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 28.Bontkes HJ, Gruijl TD, Bijl A, Verheijen RH, Meijer CJ, Scheper RJ, Stern PL, Burns JE, Maitland NJ, Walboomers JM. Human papillomavirus type 16 E2-specific T-helper lymphocyte responses in patients with cervical intraepithelial neoplasia. J Gen Virol. 1999;80(Pt 9):2453–2459. doi: 10.1099/0022-1317-80-9-2453. [DOI] [PubMed] [Google Scholar]
- 29.Woo YL, Hende M, Sterling JC, Coleman N, Crawford RA, Kwappenberg KM, Stanley MA, Burg SH. A prospective study on the natural course of low-grade squamous intraepithelial lesions and the presence of HPV16 E2-, E6- and E7-specific T-cell responses. Int J Cancer. 2010;126(1):133–141. doi: 10.1002/ijc.24804. [DOI] [PubMed] [Google Scholar]
- 30.De Vos van Steenwijk PJ, Piersma SJ, Welters MJ, Hulst JM, Fleuren G, Hellebrekers BW, Kenter GG, Burg SH. Surgery followed by persistence of high-grade squamous intraepithelial lesions is associated with the induction of a dysfunctional HPV16-specific T-cell response. Clin Cancer Res. 2008;14(22):7188–7195. doi: 10.1158/1078-0432.CCR-08-0994. [DOI] [PubMed] [Google Scholar]
- 31.Luxton JC, Nath R, Derias N, Herbert A, Shepherd PS. Human papillomavirus type 16-specific T cell responses and their association with recurrence of cervical disease following treatment. J Gen Virol. 2003;84(Pt 5):1063–1070. doi: 10.1099/vir.0.18931-0. [DOI] [PubMed] [Google Scholar]
- 32.Gruijl TD, Bontkes HJ, Stukart MJ, Walboomers JM, Remmink AJ, Verheijen RH, Helmerhorst TJ, Meijer CJ, Scheper RJ. T cell proliferative responses against human papillomavirus type 16 E7 oncoprotein are most prominent in cervical intraepithelial neoplasia patients with a persistent viral infection. J Gen Virol. 1996;77(Pt 9):2183–2191. doi: 10.1099/0022-1317-77-9-2183. [DOI] [PubMed] [Google Scholar]
- 33.Gruijl TD, Bontkes HJ, Walboomers JM, Stukart MJ, Doekhie FS, Remmink AJ, Helmerhorst TJ, Verheijen RH, Duggan-Keen MF, Stern PL, Meijer CJ, Scheper RJ. Differential T helper cell responses to human papillomavirus type 16 E7 related to viral clearance or persistence in patients with cervical neoplasia: a longitudinal study. Cancer Res. 1998;58(8):1700–1706. [PubMed] [Google Scholar]
- 34.Tsukui T, Hildesheim A, Schiffman MH, Lucci J, 3rd, Contois D, Lawler P, Rush BB, Lorincz AT, Corrigan A, Burk RD, Qu W, Marshall MA, Mann D, Carrington M, Clerici M, Shearer GM, Carbone DP, Scott DR, Houghten RA, Berzofsky JA. Interleukin 2 production in vitro by peripheral lymphocytes in response to human papillomavirus-derived peptides: correlation with cervical pathology. Cancer Res. 1996;56(17):3967–3974. [PubMed] [Google Scholar]
- 35.Heusinkveld M, Welters MJ, Poelgeest MI, Hulst JM, Melief CJ, Fleuren GJ, Kenter GG, Burg SH. The detection of circulating Human Papillomavirus (HPV)-specific T cells is associated with improved survival of patients with deeply infiltrating tumors. Int J Cancer. 2010 doi: 10.1002/ijc.25361. [DOI] [PubMed] [Google Scholar]
- 36.Ressing ME, Driel WJ, Celis E, Sette A, Brandt MP, Hartman M, Anholts JD, Schreuder GM, Harmsel WB, Fleuren GJ, Trimbos BJ, Kast WM, Melief CJ. Occasional memory cytotoxic T-cell responses of patients with human papillomavirus type 16-positive cervical lesions against a human leukocyte antigen-A *0201-restricted E7-encoded epitope. Cancer Res. 1996;56(3):582–588. [PubMed] [Google Scholar]
- 37.Bontkes HJ, Gruijl TD, Muysenberg AJ, Verheijen RH, Stukart MJ, Meijer CJ, Scheper RJ, Stacey SN, Duggan-Keen MF, Stern PL, Man S, Borysiewicz LK, Walboomers JM. Human papillomavirus type 16 E6/E7-specific cytotoxic T lymphocytes in women with cervical neoplasia. Int J Cancer. 2000;88(1):92–98. [PubMed] [Google Scholar]
- 38.Nimako M, Fiander AN, Wilkinson GW, Borysiewicz LK, Man S. Human papillomavirus-specific cytotoxic T lymphocytes in patients with cervical intraepithelial neoplasia grade III. Cancer Res. 1997;57(21):4855–4861. [PubMed] [Google Scholar]
- 39.Youde SJ, Dunbar PR, Evans EM, Fiander AN, Borysiewicz LK, Cerundolo V, Man S. Use of fluorogenic histocompatibility leukocyte antigen-A*0201/HPV 16 E7 peptide complexes to isolate rare human cytotoxic T-lymphocyte-recognizing endogenous human papillomavirus antigens. Cancer Res. 2000;60(2):365–371. [PubMed] [Google Scholar]
- 40.Nakagawa M, Stites DP, Farhat S, Sisler JR, Moss B, Kong F, Moscicki AB, Palefsky JM. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J Infect Dis. 1997;175(4):927–931. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa M, Stites DP, Palefsky JM, Kneass Z, Moscicki AB. CD4-positive and CD8-positive cytotoxic T lymphocytes contribute to human papillomavirus type 16 E6 and E7 responses. Clin Diagn Lab Immunol. 1999;6(4):494–498. doi: 10.1128/cdli.6.4.494-498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melief CJ, Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer. 2008;8(5):351–360. doi: 10.1038/nrc2373. [DOI] [PubMed] [Google Scholar]
- 43.Piersma SJ, Welters MJ, van der Hulst JM, Kloth JN, Kwappenberg KM, Trimbos BJ, Melief CJ, Hellebrekers BW, Fleuren GJ, Kenter GG, Offringa R, van der Burg SH (2007) Human papilloma virus specific T cells infiltrating cervical cancer and draining lymph nodes show remarkably frequent use of HLA-DQ and -DP as a restriction element. Int J Cancer [DOI] [PubMed]
- 44.Evans EM, Man S, Evans AS, Borysiewicz LK. Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T-lymphocytes. Cancer Res. 1997;57(14):2943–2950. [PubMed] [Google Scholar]
- 45.Oerke S, Hohn H, Zehbe I, Pilch H, Schicketanz KH, Hitzler WE, Neukirch C, Freitag K, Maeurer MJ. Naturally processed and HLA-B8-presented HPV16 E7 epitope recognized by T cells from patients with cervical cancer. Int J Cancer. 2005;114(5):766–778. doi: 10.1002/ijc.20794. [DOI] [PubMed] [Google Scholar]
- 46.Hohn H, Pilch H, Gunzel S, Neukirch C, Freitag K, Necker A, Maeurer MJ. Human papillomavirus type 33 E7 peptides presented by HLA-DR*0402 to tumor-infiltrating T cells in cervical cancer. J Virol. 2000;74(14):6632–6636. doi: 10.1128/jvi.74.14.6632-6636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hohn H, Pilch H, Gunzel S, Neukirch C, Hilmes C, Kaufmann A, Seliger B, Maeurer MJ. CD4+ tumor-infiltrating lymphocytes in cervical cancer recognize HLA-DR-restricted peptides provided by human papillomavirus-E7. J Immunol. 1999;163(10):5715–5722. [PubMed] [Google Scholar]
- 48.de Vos van Steenwijk PJ, Heusinkveld M, Ramwadhdoebe TH, Lowik MJ, van der Hulst JM, Goedemans R, Piersma SJ, Kenter GG, van der Burg SH (2011) An unexpectedly large polyclonal repertoire of HPV-specific T cells is poised for action in patients with cervical cancer. Cancer Res. 70(7):2707–2717. doi:10.1158/0008-5472.CAN-09-4299 [DOI] [PubMed]
- 49.Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, Burg SH, Fleuren GJ. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008;14(7):2028–2035. doi: 10.1158/1078-0432.CCR-07-4554. [DOI] [PubMed] [Google Scholar]
- 50.Piersma SJ, Jordanova ES, Poelgeest MI, Kwappenberg KM, Hulst JM, Drijfhout JW, Melief CJ, Kenter GG, Fleuren GJ, Offringa R, Burg SH. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007;67(1):354–361. doi: 10.1158/0008-5472.CAN-06-3388. [DOI] [PubMed] [Google Scholar]
- 51.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 52.Biddison WE, Palmer JC. Development of tumor cell resistance to syngeneic cell-mediated cytotoxicity during growth of ascitic mastocytoma P815Y. Proc Natl Acad Sci USA. 1977;74(1):329–333. doi: 10.1073/pnas.74.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uyttenhove C, Maryanski J, Boon T. Escape of mouse mastocytoma P815 after nearly complete rejection is due to antigen-loss variants rather than immunosuppression. J Exp Med. 1983;157(3):1040–1052. doi: 10.1084/jem.157.3.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 55.Stoler MH, Rhodes CR, Whitbeck A, Wolinsky SM, Chow LT, Broker TR. Human papillomavirus type 16 and 18 gene expression in cervical neoplasias. Hum Pathol. 1992;23(2):117–128. doi: 10.1016/0046-8177(92)90232-r. [DOI] [PubMed] [Google Scholar]
- 56.Brule AJ, Cromme FV, Snijders PJ, Smit L, Oudejans CB, Baak JP, Meijer CJ, Walboomers JM. Nonradioactive RNA in situ hybridization detection of human papillomavirus 16-E7 transcripts in squamous cell carcinomas of the uterine cervix using confocal laser scan microscopy. Am J Pathol. 1991;139(5):1037–1045. [PMC free article] [PubMed] [Google Scholar]
- 57.Shresta S, Pham CT, Thomas DA, Graubert TA, Ley TJ. How do cytotoxic lymphocytes kill their targets? Curr Opin Immunol. 1998;10(5):581–587. doi: 10.1016/s0952-7915(98)80227-6. [DOI] [PubMed] [Google Scholar]
- 58.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71(6):907–920. [PubMed] [Google Scholar]
- 59.Reed JC, Cuddy M, Slabiak T, Croce CM, Nowell PC. Oncogenic potential of bcl-2 demonstrated by gene transfer. Nature. 1988;336(6196):259–261. doi: 10.1038/336259a0. [DOI] [PubMed] [Google Scholar]
- 60.Djerbi M, Screpanti V, Catrina AI, Bogen B, Biberfeld P, Grandien A. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J Exp Med. 1999;190(7):1025–1032. doi: 10.1084/jem.190.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Medema JP, Jong J, Hall T, Melief CJ, Offringa R. Immune escape of tumors in vivo by expression of cellular FLICE-inhibitory protein. J Exp Med. 1999;190(7):1033–1038. doi: 10.1084/jem.190.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Medema JP, Jong J, Peltenburg LT, Verdegaal EM, Gorter A, Bres SA, Franken KL, Hahne M, Albar JP, Melief CJ, Offringa R. Blockade of the granzyme B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc Natl Acad Sci USA. 2001;98(20):11515–11520. doi: 10.1073/pnas.201398198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou JH, Chen HZ, Ye F, Lu WG, Xie X. Fas-mediated pathway and apoptosis in normal cervix, cervical intraepithelial neoplasia and cervical squamous cancer. Oncol Rep. 2006;16(2):307–311. [PubMed] [Google Scholar]
- 64.Kloth JN, Gorter A, Fleuren GJ, Oosting J, Uljee S, Haar N, Dreef EJ, Kenter GG, Jordanova ES. Elevated expression of SerpinA1 and SerpinA3 in HLA-positive cervical carcinoma. J Pathol. 2008;215(3):222–230. doi: 10.1002/path.2347. [DOI] [PubMed] [Google Scholar]
- 65.Emoto T, Nakamura K, Nagasaka Y, Numa F, Suminami Y, Kato H. Alpha 1-antichymotrypsin inhibits chymotrypsin-induced apoptosis in rat hepatoma cells. Apoptosis. 1998;3(3):155–160. doi: 10.1023/a:1009694621397. [DOI] [PubMed] [Google Scholar]
- 66.Poe M, Blake JT, Boulton DA, Gammon M, Sigal NH, Wu JK, Zweerink HJ. Human cytotoxic lymphocyte granzyme B. Its purification from granules and the characterization of substrate and inhibitor specificity. J Biol Chem. 1991;266(1):98–103. [PubMed] [Google Scholar]
- 67.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 68.Seliger B, Maeurer MJ, Ferrone S. TAP off–tumors on. Immunol Today. 1997;18(6):292–299. doi: 10.1016/s0167-5699(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 69.Evans M, Borysiewicz LK, Evans AS, Rowe M, Jones M, Gileadi U, Cerundolo V, Man S. Antigen processing defects in cervical carcinomas limit the presentation of a CTL epitope from human papillomavirus 16 E6. J Immunol. 2001;167(9):5420–5428. doi: 10.4049/jimmunol.167.9.5420. [DOI] [PubMed] [Google Scholar]
- 70.Vambutas A, DeVoti J, Pinn W, Steinberg BM, Bonagura VR. Interaction of human papillomavirus type 11 E7 protein with TAP-1 results in the reduction of ATP-dependent peptide transport. Clin Immunol. 2001;101(1):94–99. doi: 10.1006/clim.2001.5094. [DOI] [PubMed] [Google Scholar]
- 71.Vambutas A, Bonagura VR, Steinberg BM. Altered expression of TAP-1 and major histocompatibility complex class I in laryngeal papillomatosis: correlation of TAP-1 with disease. Clin Diagn Lab Immunol. 2000;7(1):79–85. doi: 10.1128/cdli.7.1.79-85.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Georgopoulos NT, Proffitt JL, Blair GE. Transcriptional regulation of the major histocompatibility complex (MHC) class I heavy chain, TAP1 and LMP2 genes by the human papillomavirus (HPV) type 6b, 16 and 18 E7 oncoproteins. Oncogene. 2000;19(42):4930–4935. doi: 10.1038/sj.onc.1203860. [DOI] [PubMed] [Google Scholar]
- 73.Ashrafi GH, Haghshenas MR, Marchetti B, O’Brien PM, Campo MS. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer. 2005;113(2):276–283. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- 74.Bontkes HJ, Walboomers JM, Meijer CJ, Helmerhorst TJ, Stern PL. Specific HLA class I down-regulation is an early event in cervical dysplasia associated with clinical progression. Lancet. 1998;351(9097):187–188. doi: 10.1016/S0140-6736(05)78209-X. [DOI] [PubMed] [Google Scholar]
- 75.Cromme FV, Airey J, Heemels MT, Ploegh HL, Keating PJ, Stern PL, Meijer CJ, Walboomers JM. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J Exp Med. 1994;179(1):335–340. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keating PJ, Cromme FV, Duggan-Keen M, Snijders PJ, Walboomers JM, Hunter RD, Dyer PA, Stern PL. Frequency of down-regulation of individual HLA-A and -B alleles in cervical carcinomas in relation to TAP-1 expression. Br J Cancer. 1995;72(2):405–411. doi: 10.1038/bjc.1995.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Connor ME, Stern PL. Loss of MHC class-I expression in cervical carcinomas. Int J Cancer. 1990;46(6):1029–1034. doi: 10.1002/ijc.2910460614. [DOI] [PubMed] [Google Scholar]
- 78.Cromme FV, Meijer CJ, Snijders PJ, Uyterlinde A, Kenemans P, Helmerhorst T, Stern PL, Brule AJ, Walboomers JM. Analysis of MHC class I and II expression in relation to presence of HPV genotypes in premalignant and malignant cervical lesions. Br J Cancer. 1993;67(6):1372–1380. doi: 10.1038/bjc.1993.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koopman LA, Corver WE, Slik AR, Giphart MJ, Fleuren GJ. Multiple genetic alterations cause frequent and heterogeneous human histocompatibility leukocyte antigen class I loss in cervical cancer. J Exp Med. 2000;191(6):961–976. doi: 10.1084/jem.191.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hilders CG, Munoz IM, Nooyen Y, Fleuren GJ. Altered HLA expression by metastatic cervical carcinoma cells as a factor in impaired immune surveillance. Gynecol Oncol. 1995;57(3):366–375. doi: 10.1006/gyno.1995.1156. [DOI] [PubMed] [Google Scholar]
- 81.Driel WJ, Tjiong MY, Hilders CG, Trimbos BJ, Fleuren GJ. Association of allele-specific HLA expression and histopathologic progression of cervical carcinoma. Gynecol Oncol. 1996;62(1):33–41. doi: 10.1006/gyno.1996.0186. [DOI] [PubMed] [Google Scholar]
- 82.Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57(2):197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 85.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 86.Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev. 2009;229(1):67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 87.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101(49):17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Chen L, Zincke H, Blute ML, Leibovich BC, Kwon ED. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer. 2005;104(10):2084–2091. doi: 10.1002/cncr.21470. [DOI] [PubMed] [Google Scholar]
- 90.Thompson RH, Kuntz SM, Leibovich BC, Dong HD, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 91.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56(8):1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 93.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1):19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 94.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 95.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15(20):6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 96.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13(18 Pt 1):5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 97.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich BC, Blute ML, Kwon ED. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14(16):5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24(5):242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 99.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G, Margreiter R, Konigsrainer A, Fuchs D, Amberger A. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12(4):1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 100.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 101.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, Saito S. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98(6):874–881. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 104.Massague J. TGFbeta in Cancer. Cell. 2008;134(2):215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 106.Hazelbag S, Kenter GG, Gorter A, Fleuren GJ. Prognostic relevance of TGF-beta1 and PAI-1 in cervical cancer. Int J Cancer. 2004;112(6):1020–1028. doi: 10.1002/ijc.20512. [DOI] [PubMed] [Google Scholar]
- 107.Hazelbag S, Kenter GG, Gorter A, Dreef EJ, Koopman LA, Violette SM, Weinreb PH, Fleuren GJ. Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol. 2007;212(3):316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 108.Hazelbag S, Gorter A, Kenter GG, Broek L, Fleuren G. Transforming growth factor-beta1 induces tumor stroma and reduces tumor infiltrate in cervical cancer. Hum Pathol. 2002;33(12):1193–1199. doi: 10.1053/hupa.2002.130109. [DOI] [PubMed] [Google Scholar]
- 109.Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998;12(13):2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Perea SE, Massimi P, Banks L. Human papillomavirus type 16 E7 impairs the activation of the interferon regulatory factor-1. Int J Mol Med. 2000;5(6):661–666. doi: 10.3892/ijmm.5.6.661. [DOI] [PubMed] [Google Scholar]
- 111.Nees M, Geoghegan JM, Hyman T, Frank S, Miller L, Woodworth CD. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J Virol. 2001;75(9):4283–4296. doi: 10.1128/JVI.75.9.4283-4296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 113.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 114.Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A. The absence of CCL2 expression in cervical carcinoma is associated with increased survival and loss of heterozygosity at 17q11.2. J Pathol. 2006;208(4):507–517. doi: 10.1002/path.1918. [DOI] [PubMed] [Google Scholar]
- 115.Zijlmans HJ, Fleuren GJ, Baelde HJ, Eilers PH, Kenter GG, Gorter A. Role of tumor-derived proinflammatory cytokines GM-CSF, TNF-alpha, and IL-12 in the migration and differentiation of antigen-presenting cells in cervical carcinoma. Cancer. 2007;109(3):556–565. doi: 10.1002/cncr.22428. [DOI] [PubMed] [Google Scholar]
- 116.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4(12):941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 117.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29(3):372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 118.Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol [DOI] [PMC free article] [PubMed]
- 119.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25(18):2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 120.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1(1):95–103. [PubMed] [Google Scholar]
- 121.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 122.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5(8):641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 123.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55(3):237–245. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fattorossi A, Battaglia A, Ferrandina G, Buzzonetti A, Legge F, Salutari V, Scambia G. Lymphocyte composition of tumor draining lymph nodes from cervical and endometrial cancer patients. Gynecol Oncol. 2004;92(1):106–115. doi: 10.1016/j.ygyno.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 125.Lu LF, Rudensky A. Molecular orchestration of differentiation and function of regulatory T cells. Genes Dev. 2009;23(11):1270–1282. doi: 10.1101/gad.1791009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Piersma SJ, Welters MJ, Burg SH. Tumor-specific regulatory T cells in cancer patients. Hum Immunol. 2008;69(4–5):241–249. doi: 10.1016/j.humimm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Battaglia A, Buzzonetti A, Baranello C, Ferrandina G, Martinelli E, Fanfani F, Scambia G, Fattorossi A. Metastatic tumour cells favour the generation of a tolerogenic milieu in tumour draining lymph node in patients with early cervical cancer. Cancer Immunol Immunother. 2009;58(9):1363–1373. doi: 10.1007/s00262-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van der Burg SH, Piersma SJ, de Jong A, van der Hulst JM, Kwappenberg KM, van den Hende M, Welters MJ, Van Rood JJ, Fleuren GJ, Melief CJ, Kenter GG, Offringa R (2007) Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A [DOI] [PMC free article] [PubMed]
- 129.Piersma SJ, Hulst JM, Kwappenberg KM, Goedemans R, Minne CE, Burg SH. Influenza matrix 1-specific human CD4(+) FOXP3(+) and FOXP3(−) regulatory T cells can be detected long after viral clearance. Eur J Immunol. 2010;40(11):3064–3074. doi: 10.1002/eji.200940177. [DOI] [PubMed] [Google Scholar]
- 130.Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007;7(1):11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 131.Fausch SC, Silva DM, Rudolf MP, Kast WM. Human papillomavirus virus-like particles do not activate Langerhans cells: a possible immune escape mechanism used by human papillomaviruses. J Immunol. 2002;169(6):3242–3249. doi: 10.4049/jimmunol.169.6.3242. [DOI] [PubMed] [Google Scholar]
- 132.Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets. 2007;7(1):79–89. doi: 10.2174/156800907780006869. [DOI] [PubMed] [Google Scholar]
- 133.Madkan VK, Cook-Norris RH, Steadman MC, Arora A, Mendoza N, Tyring SK. The oncogenic potential of human papillomaviruses: a review on the role of host genetics and environmental cofactors. Br J Dermatol. 2007;157(2):228–241. doi: 10.1111/j.1365-2133.2007.07961.x. [DOI] [PubMed] [Google Scholar]
- 134.Molling JW, Gruijl TD, Glim J, Moreno M, Rozendaal L, Meijer CJ, Eertwegh AJ, Scheper RJ, Blomberg ME, Bontkes HJ. CD4(+)CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int J Cancer. 2007;121(8):1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- 135.Visser J, Nijman HW, Hoogenboom BN, Jager P, Baarle D, Schuuring E, Abdulahad W, Miedema F, Zee AG, Daemen T. Frequencies and role of regulatory T cells in patients with (pre)malignant cervical neoplasia. Clin Exp Immunol. 2007;150(2):199–209. doi: 10.1111/j.1365-2249.2007.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scott ME, Ma Y, Kuzmich L, Moscicki AB. Diminished IFN-gamma and IL-10 and elevated Foxp3 mRNA expression in the cervix are associated with CIN 2 or 3. Int J Cancer. 2009;124(6):1379–1383. doi: 10.1002/ijc.24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Woo YL, Sterling J, Damay I, Coleman N, Crawford R, Burg SH, Stanley M. Characterising the local immune responses in cervical intraepithelial neoplasia: a cross-sectional and longitudinal analysis. BJOG. 2008;115(13):1616–1621. doi: 10.1111/j.1471-0528.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 138.Jarboe EA, Liaw KL, Thompson LC, Heinz DE, Baker PL, McGregor JA, Dunn T, Woods JE, Shroyer KR. Analysis of telomerase as a diagnostic biomarker of cervical dysplasia and carcinoma. Oncogene. 2002;21(4):664–673. doi: 10.1038/sj.onc.1205073. [DOI] [PubMed] [Google Scholar]
- 139.Frost M, Bobak JB, Gianani R, Kim N, Weinrich S, Spalding DC, Cass LG, Thompson LC, Enomoto T, Uribe-Lopez D, Shroyer KR. Localization of telomerase hTERT protein and hTR in benign mucosa, dysplasia, and squamous cell carcinoma of the cervix. Am J Clin Pathol. 2000;114(5):726–734. doi: 10.1309/XWFE-ARMN-HG2D-AJYV. [DOI] [PubMed] [Google Scholar]
- 140.Sano T, Oyama T, Kashiwabara K, Fukuda T, Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153(6):1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, Dallenbach-Hellweg G, Schmidt D, Knebel DM. Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92(2):276–284. doi: 10.1002/ijc.1174. [DOI] [PubMed] [Google Scholar]
- 142.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 143.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, Melief CJ, Burg SH. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14(1):178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 144.Francois V, Ottaviani S, Renkvist N, Stockis J, Schuler G, Thielemans K, Colau D, Marchand M, Boon T, Lucas S, Bruggen P. The CD4(+) T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Cancer Res. 2009;69(10):4335–4345. doi: 10.1158/0008-5472.CAN-08-3726. [DOI] [PubMed] [Google Scholar]
- 145.Zhou G, Drake CG, Levitsky HI. Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood. 2006;107(2):628–636. doi: 10.1182/blood-2005-07-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 147.Welters MJ, Kenter GG, De Vos van Steenwijk PJ, Lowik MJ, Meer DM Berends-van, Essahsah F, Stynenbosch LF, Vloon AP, Ramwadhdoebe TH, Piersma SJ, Hulst JM, Valentijn AR, Fathers LM, Drijfhout JW, Franken KL, Oostendorp J, Fleuren GJ, Melief CJ, Burg SH. Success or failure of vaccination for HPV16-positive vulvar lesions correlates with kinetics and phenotype of induced T-cell responses. Proc Natl Acad Sci USA. 2010;107(26):11895–11899. doi: 10.1073/pnas.1006500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 149.Foss FM. DAB(389)IL-2 (ONTAK): a novel fusion toxin therapy for lymphoma. Clin Lymphoma. 2000;1(2):110–116. doi: 10.3816/clm.2000.n.009. [DOI] [PubMed] [Google Scholar]
- 150.Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C. IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 2007;110(9):3192–3201. doi: 10.1182/blood-2007-06-094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115(12):3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morse MA, Hobeika AC, Osada T, Serra D, Niedzwiecki D, Lyerly HK, Clay TM. Depletion of human regulatory T cells specifically enhances antigen-specific immune responses to cancer vaccines. Blood. 2008;112(3):610–618. doi: 10.1182/blood-2008-01-135319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mahnke K, Schonfeld K, Fondel S, Ring S, Karakhanova S, Wiedemeyer K, Bedke T, Johnson TS, Storn V, Schallenberg S, Enk AH. Depletion of CD4 + CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120(12):2723–2733. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 154.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother. 2005;28(6):582–592. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kreitman RJ, Wilson WH, White JD, Stetler-Stevenson M, Jaffe ES, Giardina S, Waldmann TA, Pastan I. Phase I trial of recombinant immunotoxin anti-Tac(Fv)-PE38 (LMB-2) in patients with hematologic malignancies. J Clin Oncol. 2000;18(8):1622–1636. doi: 10.1200/JCO.2000.18.8.1622. [DOI] [PubMed] [Google Scholar]
- 156.Powell DJ, Jr, Felipe-Silva A, Merino MJ, Ahmadzadeh M, Allen T, Levy C, White DE, Mavroukakis S, Kreitman RJ, Rosenberg SA, Pastan I. Administration of a CD25-directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179(7):4919–4928. doi: 10.4049/jimmunol.179.7.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lutsiak ME, Semnani RT, Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 158.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206(5):1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4 + CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 160.Vierboom MP, Bos GM, Ooms M, Offringa R, Melief CJ. Cyclophosphamide enhances anti-tumor effect of wild-type p53-specific CTL. Int J Cancer. 2000;87(2):253–260. doi: 10.1002/1097-0215(20000715)87:2<253::aid-ijc17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 161.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4 + CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: reduction of T-suppressor function without depletion of the CD8+ subset. Cancer Res. 1987;47(12):3317–3321. [PubMed] [Google Scholar]
- 163.Battaglia A, Buzzonetti A, Martinelli E, Fanelli M, Petrillo M, Ferrandina G, Scambia G, Fattorossi A. Selective changes in the immune profile of tumor-draining lymph nodes after different neoadjuvant chemoradiation regimens for locally advanced cervical cancer. Int J Radiat Oncol Biol Phys. 2010;76(5):1546–1553. doi: 10.1016/j.ijrobp.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 164.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 165.Sutmuller RP, Duivenvoorde LM, Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194(6):823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009 doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 169.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Klein O, Ebert LM, Nicholaou T, Browning J, Russell SE, Zuber M, Jackson HM, Dimopoulos N, Tan BS, Hoos A, Luescher IF, Davis ID, Chen W, Cebon J. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res. 2009;15(7):2507–2513. doi: 10.1158/1078-0432.CCR-08-2424. [DOI] [PubMed] [Google Scholar]
- 171.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, Manukian G, Xu Y, Roman RA, Terzulli SL, Heywood M, Pogoriler E, Ritter G, Old LJ, Allison JP, Wolchok JD. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA. 2008;105(51):20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Giacomo AM, Danielli R, Guidoboni M, Calabro L, Carlucci D, Miracco C, Volterrani L, Mazzei MA, Biagioli M, Altomonte M, Maio M. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 2009;58(8):1297–1306. doi: 10.1007/s00262-008-0642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 176.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 177.Savage ND, Boer T, Walburg KV, Joosten SA, Meijgaarden K, Geluk A, Ottenhoff TH. Human anti-inflammatory macrophages induce Foxp3+ GITR + CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181(3):2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 178.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 179.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 180.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 181.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205(6):1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fong CH, Bebien M, Didierlaurent A, Nebauer R, Hussell T, Broide D, Karin M, Lawrence T. An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. J Exp Med. 2008;205(6):1269–1276. doi: 10.1084/jem.20080124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lepique AP, Daghastanli KR, Cuccovia IM, Villa LL. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res. 2009;15(13):4391–4400. doi: 10.1158/1078-0432.CCR-09-0489. [DOI] [PubMed] [Google Scholar]
- 184.Hall T, Wolpert EZ, Veelen P, Laban S, Veer M, Roseboom M, Bres S, Grufman P, Ru A, Meiring H, Jong A, Franken K, Teixeira A, Valentijn R, Drijfhout JW, Koning F, Camps M, Ossendorp F, Karre K, Ljunggren HG, Melief CJ, Offringa R. Selective cytotoxic T-lymphocyte targeting of tumor immune escape variants. Nat Med. 2006;12(4):417–424. doi: 10.1038/nm1381. [DOI] [PubMed] [Google Scholar]