Abstract
Emerging evidence points to the involvement of the brain extracellular matrix (ECM) in the pathophysiology of schizophrenia (SZ). Abnormalities affecting several ECM components, including Reelin and chondroitin sulfate proteoglycans (CSPGs), have been described in subjects with this disease. Solid evidence supports the involvement of Reelin, an ECM glycoprotein involved in corticogenesis, synaptic functions and glutamate NMDA receptor regulation, expressed prevalently in distinct populations of GABAergic neurons, which secrete it into the ECM. Marked changes of Reelin expression in SZ have typically been reported in association with GABA-related abnormalities in subjects with SZ and bipolar disorder. Recent findings from our group point to substantial abnormalities affecting CSPGs, a main ECM component, in the amygdala and entorhinal cortex of subjects with schizophrenia, but not bipolar disorder. Striking increases of glial cells expressing CSPGs were accompanied by reductions of perineuronal nets, CSPG- and Reelin-enriched ECM aggregates enveloping distinct neuronal populations. CSPGs developmental and adult functions, including neuronal migration, axon guidance, synaptic and neurotransmission regulation are highly relevant to the pathophysiology of SZ. Together with reports of anomalies affecting several other ECM components, these findings point to the ECM as a key component of the pathology of SZ. We propose that ECM abnormalities may contribute to several aspects of the pathophysiology of this disease, including disrupted connectivity and neuronal migration, synaptic anomalies and altered GABAergic, glutamatergic and dopaminergic neurotransmission.
Keywords: extracellular matrix, chondroitin sulphate proteoglycans, perineuronal nets, astrocytes, schizophrenia, Reelin
1. INTRODUCTION
Recent years have seen remarkable progress in the identification of neuronal and glial abnormalities in schizophrenia (SZ) (e.g. Reynolds et al., 1990, Benes, 2000, Boksha, 2004, Stewart and Davis, 2004, Heckers and Benes, 2005, Mitterauer, 2005, Carter, 2006, Harrison and Law, 2006, Beneyto et al., 2007, Berretta et al., 2007, Kondziella et al., 2007, Pantazopoulos et al., 2007, Tamminga et al., 2010, Volk and Lewis, 2010, Miyake et al., 2011, Takahashi et al., 2011b). Emerging evidence suggests a key role for a third, until recently unsuspected, player: the extracellular matrix (ECM) (Impagnatiello et al., 1998, Guidotti et al., 2000a, Eastwood et al., 2003, Zaharieva et al., 2008, Pantazopoulos et al., 2010, Takahashi et al., 2011a). Decreased expression of the ECM glycoprotein Reelin represents one of the most solid findings in SZ (Guidotti et al., 2000a, Guidotti et al., 2000b, Costa et al., 2004, Dong et al., 2005). Recent findings from our group point to substantial abnormalities affecting chondroitin sulfate proteoglycans (CSPGs), a key component of the brain ECM, in subjects with SZ but not bipolar disorder (Pantazopoulos et al., 2010). Marked increases in the number of glial cells expressing CSPGs, not accompanied by astrocytosis, suggest anomalous regulation of CSPG expression. Concurrent reductions of CSPG-positive perineuronal nets (PNNs) point to altered CSPG content in the ECM. Evidence for genetic vulnerability related to some ECM molecules and abnormalities affecting ECM and ECM-related factors, further supports ECM abnormalities in SZ (Vawter et al., 2000, Vawter et al., 2001, Eastwood et al., 2003, Buxbaum et al., 2008, Shifman et al., 2008, Zaharieva et al., 2008, Pisante et al., 2009, Liu et al., 2010, Dow et al., 2011, Kahler et al., 2011).
In this review, we summarize basic current knowledge on the composition and functions of the brain ECM, with particular emphasis on CSPGs and Reelin and their relevance to the pathophysiology of SZ, and discuss evidence for the involvement of ECM in SZ and its implications with respect to current knowledge on the pathophysiology of this disease (for more comprehensive reviews on ECM structure and functions, please see Tissir and Goffinet, 2003, Dityatev and Schachner, 2006, Viapiano and Matthews, 2006, Galtrey and Fawcett, 2007, Dityatev et al., 2010a, Faissner et al., 2010, Gundelfinger et al., 2010). We put forth that interactions between ECM, neuronal and glial cell populations may be disrupted in SZ, potentially affecting key developmental and adult brain functions (Oohira et al., 2000, Yamaguchi, 2000, Pizzorusso et al., 2002, Carulli et al., 2005, Viapiano and Matthews, 2006).
1.1 Distribution of the ECM in the central nervous system (CNS)
The ECM occupies approximately a volume fraction of 20% of the normal adult brain (Sykova and Nicholson, 2008). It wraps tightly around synapses and completely surrounds all brain cells, separating them from each other by gaps spanning approximately 38-64 nm in width (Chvatal et al., 1999, Thorne and Nicholson, 2006, Sykova and Nicholson, 2008). The ECM also fills the synaptic cleft, although its biochemical composition within this specialized space is different, and less well known, with respect to the rest of the brain ECM (Zuber et al., 2005). In addition to the loosely organized ECM surrounding all cells, highly organized pericellular ECM aggregates, i.e. PNNs, ensheath the cell body, dendrites and proximal portion of the axon of distinct neuronal subpopulations (Fig. 1) (Hartig et al., 1995, Celio et al., 1998, Hartig et al., 1999, Wegner et al., 2003, Alpar et al., 2006, John et al., 2006, Pantazopoulos et al., 2006). PNNs represent the most well studied form of neural ECM; described by Camillo Golgi as early 1893, their molecular composition and functions have only recently come to the forefront of neuroscience research (Golgi, 1893, 1898, Celio et al., 1998, Spreafico et al., 1999, Pizzorusso et al., 2002, Dityatev and Schachner, 2003, Hensch, 2005, Carulli et al., 2006, Fawcett, 2009, Gogolla et al., 2009, Pizzorusso, 2009).
Figure 1. PNNs predominantly surround neurons expressing parvalbumin.
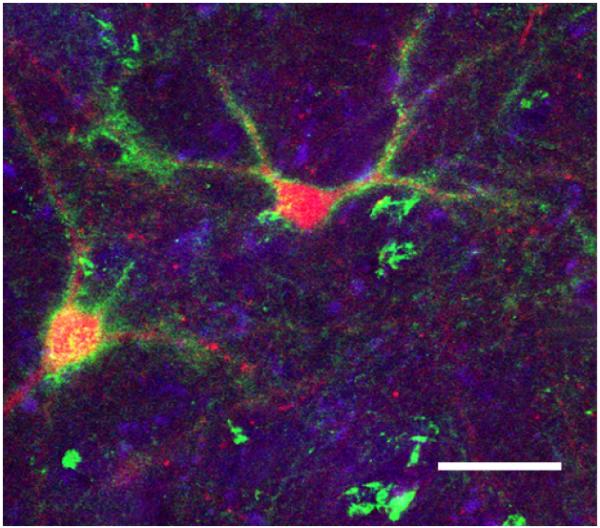
Confocal photomicrograph showing PNNs (green) enveloping PVB-positive neurons (red). Scale bar =100 μm. Modified from (Pantazopoulos et al., 2006).
1.2 ECM molecular composition and structure in CNS
The brain ECM has a unique molecular composition, based on aggregates of hyaluronan and CSPGs connected by glycoproteins (e.g. tenascins) (Fig. 2) (Bandtlow and Zimmermann, 2000, Yamaguchi, 2000, Rauch, 2004, Rauch et al., 2005, Viapiano and Matthews, 2006). Hyaluronan represents the backbone of the brain ECM, while CSPGs, in particular those belonging to the lectican family (aggrecan, versican, brevican and neurocan), are thought of as the ‘organizers of the brain ECM’ (Yamaguchi, 2000). A broad variety of other constituents, including the CSPG phosphacan, tenascins, heparan sulfate proteoglycans, Reelin, laminins, thrombospondins, hyaluronidases and proteases, add to this basic lattice and contribute to the structure and function of the ECM (Ruoslahti, 1996, Dityatev and Fellin, 2008, Frischknecht and Seidenbecher, 2008). ECM components are anchored to cell membranes through a variety of cell surface molecules, including the cell adhesion molecule (CAM) superfamily, membrane bound proteoglycans and hyaluronan synthase (Galtrey and Fawcett, 2007).
Figure 2. Simplified diagram depicting CSPG synthesis and their secretion into the ECM, and interaction with other ECM molecules.

CSPGs are formed by a core protein and a glycosaminoglycan component consisting of chondroitin sulfate chains (versican is represented here as an example). The core protein is synthesized in cytoplasmic ribosomes and then transferred to the Golgi apparatus for glycosylation. Several enzymes concur in adding repeating disaccharide units to form linear polysaccharide chains, which are then polymerized through the activity of chondroitin synthase and chondroitin polymerizing factor. Glycosaminglycan chains are modified by sulfation, packed into secretory vesicles, and released into the ECM, where they interact with a number of other components, such as hyaluronan and tenascins. Denser ECM around a neuron (yellow) represents a PNN.
The composition and structure of the ECM undergo substantial changes during CNS development. A juvenile form of ECM present in late embryonic life and early postnatal life does not organize into PNNs and supports neurogenesis and gliogenesis, cell migration, axonal outgrowth, synaptogenesis and synaptic rearrangement (Bandtlow and Zimmermann, 2000, Vitellaro-Zuccarello et al., 2001, Carulli et al., 2007, Zimmermann and Dours-Zimmermann, 2008, Maeda, 2010). In contrast, adult ECM is, in general, non-permissive for axonal outgrowth and regeneration (Rhodes and Fawcett, 2004, Galtrey and Fawcett, 2007, Fawcett, 2009); PNN maturation occurs late in postnatal development (Carulli et al., 2006, Gundelfinger et al., 2010). The functional specialization of these ECM structures is reflected in their distinct molecular composition, which includes link proteins, is enriched in particular CSPGs and is specific to distinct neuronal populations (Lander et al., 1997, Hagihara et al., 1999, Matthews et al., 2002, Wegner et al., 2003, Fawcett, 2009).
1.3 Cellular sources of ECM molecules
Several types of neurons and glial cells contribute to the synthesis of ECM molecules and their secretion into the extracellular space in a cell- and development stage- specific manner (Bandtlow and Zimmermann, 2000, Ogawa et al., 2001, Carulli et al., 2006, Viapiano and Matthews, 2006). Recent findings also suggest species differences. For instance, CSPG detection by histochemical Wisteria Floribunda agglutinin (WFA) labeling, a method widely used for CSPG detection, labels exclusively PNNs in rodents and non-human primates (Hartig et al., 1992, Bruckner et al., 1994, Celio et al., 1998, Pizzorusso et al., 2002, Murakami and Ohtsuka, 2003, Wegner et al., 2003, Baig et al., 2005, Carulli et al., 2006, Deepa et al., 2006, Viapiano and Matthews, 2006). In contrast, WFA-CSPG detection in the normal human amygdala, reveals not only PNNs but also intense intracellular CSPG labeling in a subpopulation of astrocytes (Pantazopoulos et al., 2008). The highly segregated distribution of CSPG-positive astrocytes within the human amygdala is consistent across subjects, suggesting association with specific neurochemical and/or anatomical features. These findings suggest an intriguing specie-specific role for astrocytes as CSPG sources in the human CNS.
Cell sources of Reelin also vary during development. During early stages, Reelin is synthesized and secreted by Cajal-Retzius cells, located in the marginal zone of the developing cortex. These cells almost completely disappear by the time migration of cortical neurons is completed (D’Arcangelo et al., 1995, Alcantara et al., 1998, Drakew et al., 1998, Forster et al., 2010, Meyer, 2010). Throughout adulthood, Reelin is expressed prevalently by GABAergic neurons not typically associated with PNNs, such as those expressing neuropeptide Y and somatostatin, and to a lesser extent by some calretinin- and calbindin-positive neurons (Pesold et al., 1999, Ramos-Moreno et al., 2006, Campo et al., 2009). Neurons expressing parvalbumin (PVB), typically associated with PNNs, do not contain Reelin (Pesold et al., 1999, Campo et al., 2009).
2. EVIDENCE FOR ECM ABNORMALITIES IN SZ
Growing evidence from postmortem investigations points to ECM abnormalities in SZ. Abnormal expression of ECM glycoprotein Reelin is one of the most solid findings in this disorder (Impagnatiello et al., 1998, Guidotti et al., 2000a, Costa et al., 2001). Marked increase of CSPG-positive glial cells and reduction of CSPG-positive PNNs were detected in the medial temporal lobe of subjects with SZ (Pantazopoulos et al., 2010). Current knowledge on the involvement of CSPGs and Reelin, and other ECM and ECM-related factors, in SZ is briefly reviewed below.
2.1 CSPG-glial abnormalities in SZ
Mounting evidence suggests that astrocytes play a key role in regulating ECM properties, synthesizing CSPGs, and building PNNs (Faissner et al., 2010, Gundelfinger et al., 2010). In the amygdala and entorhinal cortex (ECx) of subjects with SZ, glial cells expressing CSPGs were found to be massively increased (Pantazopoulos et al., 2010) (Fig. 3). These cells were morphologically identified as astrocytes, consistent with the exclusive co-localization of WFA-CSPG with glial fibrillary acidic protein (GFAP), a marker typically used to label astrocytes, in the normal human amygdala (Pantazopoulos et al., 2008). Expression of genes encoding for five major CSPGs in the brain, i.e. versican, aggrecan, neurocan, brevican and phosphacan, was tested in the basal nucleus of the amygdala and found to be increased by 1.3 to 2.03 folds (significant for versican; qRT-PCR) in subjects with SZ, supporting histochemical results. These changes were not accompanied by astrogliosis, as shown by normal numbers of cells expressing GFAP. Increased numbers of CSPG-positive glial cells in the absence of astrocytosis may reflect induction of CSPG expression in cells that do not express these molecules constitutively and/or increased expression in astrocytes normally containing them at low levels, perhaps as a consequence of overproduction, increased internalization and/or impaired secretion. We hypothesized that such dysregulation may in turn affect CSPG expression in the ECM. Indeed, significant PNN reductions were detected in lateral nucleus of the amygdala and layer II of lateral ECx. Notably, in healthy human subjects, these subregions contain by far the highest densities of PNNs (Bruckner et al., 1999, Pantazopoulos et al., 2006). Normal numbers of PVB-positive neuron numbers within these same regions in SZ (Pantazopoulos et al., 2007, Pantazopoulos et al., 2010) point to reduced CSPG levels in the ECM of SZ, rather than loss of PNN-associated neurons. No CSPG-related changes were detected in bipolar disorder, suggesting pathophysiological differences between this disease and SZ with regard to ECM abnormalities.
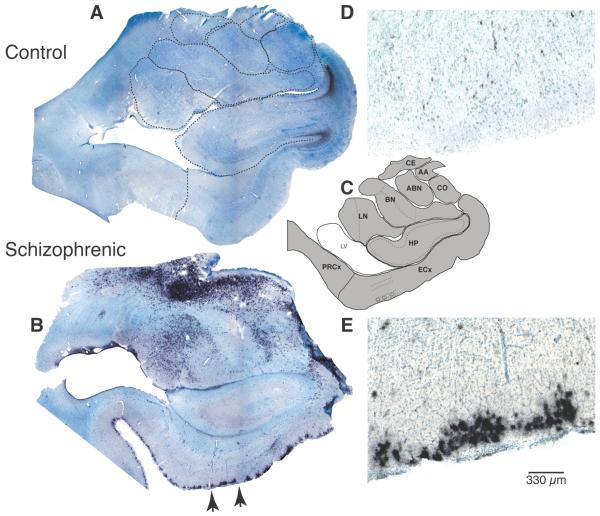
Figure 3. Marked increased of WFA-labeled elements in subjects with schizophrenia.
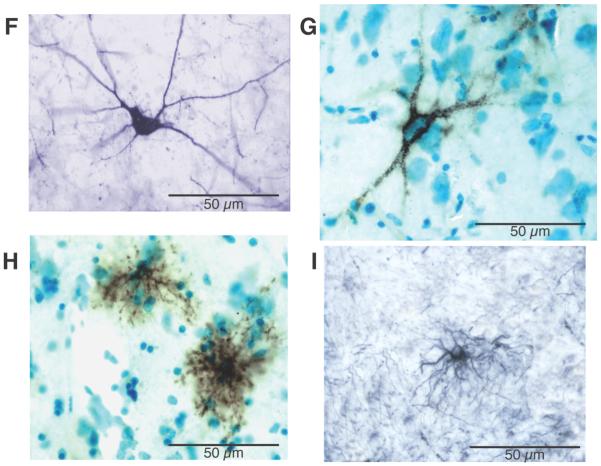
Photo-composites of sample coronal sections containing amygdala nuclei and ECx from a normal control (A) and a SZ subject (B) show marked WFA-labeling (black reaction product) in SZ. Note increased WFA labeling in subject with SZ. Dashed lines on A indicate the borders of each region (see C). Photomicrographs in D and E show a portion of the superficial layers of the ECx (segment shown in E corresponds to that marked by arrows in B). Photomicrographs in F-I show examples of PVB-IR neurons (F), PNNs (G), WFA-labeled glial cells (H), and GFAP-labeled cells (I). Photomicrographs in G and H are from sections labeled with WFA lectin histochemistry and Nissl-counterstained. PNN (G) net-like labeling is extracellular and surrounds the neurons soma and dendrites. WFA-labeled PNNs are easily distinguishable from WFA-labeled glial cells (H), characterized by intense intracellular labeling showing short “bushy” processes and small cell bodies. Their size and morphological characteristics are instead very similar to those of GFAP-IR astrocytes (I). Black reaction product in A, B, D-I corresponds to nickel-enhanced diaminobenzidine staining. Abbreviations: AA, anterior amygdaloid nucleus; CE, central nucleus; HP, hippocampus; PRCx, perirhinal cortex. Modified from (Pantazopoulos et al., 2010).
These results point to substantial anomalies affecting CSPG expression in glial cells and ECM perineuronal aggregates in SZ. Importantly, these changes were not affected by pharmacological treatment, such as exposure to antipsychotics (chlorpromazine-equivalent dose) or lithium salt throughout lifetime or during last six months’ of life, and other therapeutic drugs (e.g. serotonin-reuptake inhibitors and valproic acid), or other therapeutic intervention such as electroconvulsive therapy (for more details see Pantazopoulos et al., 2010). The only exception was a significant negative effect of lifetime exposure to antipsychotics on numbers of CSPG-positive glial cells in the basal nucleus of the amygdala in subjects with SZ, confirmed by negative correlation, indicating that antipsychotic exposure tend to decrease CSPG-positive glial cells in this nucleus. Given the direction of this effect, antipsychotics are unlikely to be responsible for the observed increase. Drugs of abuse, e.g. cocaine, marijuana, alcohol, nicotine, and a number of other potential confounds including age, gender, cause of death, brain weight, hemisphere etc., were not found to account for the abnormalities observed. Finally, these changes did not vary with duration of illness or age at onset of illness suggesting that they may be inherent to the disease (Pantazopoulos et al., 2010). If CSPG abnormalities in SZ were present during development, CSPG dysregulation may interfere with neuronal migration, axon outgrowth and synaptic maturation within the amygdala and ECx of subjects with SZ. Disruption of PNNs formation during later stages of development may lead to failure to stabilize successful sets of neural connections, complete maturation of selective neuronal populations, including those expressing PVB, and transition to an adult form of plasticity (see also 3.3.1). Such ECM/PNN defects in the amygdala may affect the ability to attribute appropriate emotional valence to sensory inputs and form stable, contextualized memories (Gloor, 1986, LeDoux, 1992, Gogolla et al., 2009, Pizzorusso, 2009). In the ECx, ECM/PNN abnormalities may impair the establishment and consolidation of long-term declarative memory and perceptual processing, that may be relevant to the impairment of learning, memory, and executive functions typically described in subjects with SZ (e.g.Kuperberg and Heckers, 2000, Riley et al., 2000). Overall, set in the context of medial temporal lobe role in emotion and perceptual processing, social behavior and affect regulation, a disruption of ECM functions has strong potential to contribute to the pathophysiological underpinnings of some of the main symptoms of SZ, such as flat affect, social and cognitive impairment, delusional thinking and anxiety. Preliminary results suggest that ECM abnormalities in SZ may not be unique to the medial temporal lobe, but instead involve other cortical and subcortical regions (Guillin et al., 2007, Murray et al., 2008). It is therefore possible that ECM changes affecting key regions in the pathophysiology of SZ may contribute to different aspects of functional impairment observed in this disease.
2.2 Reelin abnormalities in SZ
Reelin abnormalities have been extensively reported in SZ (seeFatemi et al., 2000, Guidotti et al., 2000a, Costa et al., 2001, Torrey et al., 2005, Eastwood and Harrison, 2006, Costa et al., 2007, Guidotti et al., 2010). In brief, downregulation of the reelin gene has been detected in several brain regions, including the hippocampus, prefrontal and temporal cortex, cerebellum, and caudate nucleus of subjects with SZ (Impagnatiello et al., 1998, Costa et al., 2001, Maloku et al., 2010). Aberrant epigenetic mechanisms as well as genetic abnormalities have been invoked to account for reelin downregulation. Epigenetic pathology is supported by findings of over-expression of DNA methyltransferases, enzymes that catalyze promoter methylation, in association with reelin downregulation, increased expression of methyl donor molecules, and increased methylation of reelin (Veldic et al., 2004, Abdolmaleky et al., 2005, Veldic et al., 2005, Costa et al., 2007, Guidotti et al., 2007, Guidotti et al., 2010). These findings may not exclude the possibility that polymorphisms of the reelin gene may represent a vulnerability factor for SZ, at least in specific subpopulations (Shifman et al., 2008, Wedenoja et al., 2008, Gregorio et al., 2009, Liu et al., 2010). Reelin downregulation is typically accompanied by decreased expression of glutamic acid decarboxylase 67 (GAD67; one of the two synthetic enzymes for GABA), suggesting a strong functional link between Reelin expression and GABAergic neurotransmission (Guidotti et al., 2000a, Veldic et al., 2004). Notably, Reelin downregulation was also detected in manic bipolar disorder subjects, leading to the suggestion that they may represent a shared molecular mechanism contributing to psychosis (Guidotti et al., 2000a, Costa et al., 2001). In light of the role of Reelin in corticogenesis, synaptic maturation and stabilization, and PNNs formation, it has been proposed that downregulation of reelin gene expression may contribute to neuropil hypoplasticity, decrease of dendritic spines and reduction of GAD67 expression (Costa et al., 2001). Similar Reelin-related findings in SZ and bipolar disorder with psychosis led to the suggestion that these mechanisms may represent a shared vulnerability to psychosis (Costa et al., 2001).
2.3 Other ECM molecules found to be involved in SZ
In addition to CSPGs and Reelin, several other ECM components, and ECM-interacting factors, have been found altered in SZ, further supporting the involvement of ECM in this disease. Recent results point to ADAMTSL3 gene (a disintegrin-like and metalloprotease domain with thrombospondin type I motifs-like-3) as a candidate gene for SZ (Need et al., 2009, Dow et al., 2011). This secreted enzyme is a member of the ADAMTS family, known for its role in cell-matrix interactions, ECM assembly and, specifically, CSPG proteolysis (Hall et al., 2003). Chondrex gene (YKL-40), which encodes for an extracellular matrix glycoprotein involved in cell growth and migration, was found to be significantly increased in the hippocampus of subjects with SZ (Chung et al., 2003). Semaphorin 3A, an ECM molecule with key developmental and adult functions (see 3.1) was reported to be increased in the cerebellum of subjects with SZ (Eastwood et al., 2003, Ben-David and Shifman, 2010, Zimmer et al., 2010, Kabayama et al., 2011, Nishiyama et al., 2011, Piaton et al., 2011). Notably, this increase was negatively correlated with Reelin expression and associated with downregulation of genes involved in synaptic formation and maintenance, suggesting that semaphorin 3A may contribute to the synaptic pathology of SZ. Further evidence comes from studies showing altered expression of neural cell adhesion molecules (N-CAM) in SZ (Vawter et al., 2000, Vawter et al., 2001). These molecules play key roles in neural development, signal transduction, synaptic stabilization establishing structural linkages to extracellular and intracellular proteins. Notably N-CAMs represent one of the main CSPG cell surface receptor, raising the possibility that abnormalities affecting these two families of molecules may be linked. Gene expression and genetic studies support the idea that neuregulin 1 contributes directly to SZ (Corfas et al., 2004, Hahn et al., 2006, Harrison and Law, 2006, Alaerts et al., 2009). This molecule was found to interact with ECM components and to share many of the CSPG functions (Corfas et al., 2004). Growth factors transforming growth factor beta (TGF-beta) and epidermal growth factor (EGF), shown to stimulate CSPGs synthesis in astrocytes (Smith and Strunz, 2005), are thought to play a role in the pathogenesis of SZ (Futamura et al., 2002, Kim et al., 2004, Benes et al., 2007, Ikeda et al., 2008, Zaharieva et al., 2008).
3. ECM FUNCTIONS BEAR DIRECT RELEVANCE TO THE PATHOPHYSIOLOGY OF SCHIZOPHRENIA
ECM functions during development and adulthood are highly reminiscent of those disrupted in SZ. Below, we focus in particular on those involving CSPGs and Reelin and place them in the context of known pathological findings in SZ.
3.1 Axonal pathfinding, neuronal differentiation and migration
Growing evidence supports the idea that neurodevelopmental processes such as neuronal differentiation and migration, and neuronal network formation may be disrupted in SZ (Akbarian et al., 1993a, Arnold and Trojanowski, 1996, Reif et al., 2006, Benes et al., 2009, Connor et al., 2010, Deutsch et al., 2010). Convergent findings point to abnormal distribution of interstitial white matter neurons (IWMNs) within the frontal, parietal and temporal lobes (Akbarian et al., 1993a, Akbarian et al., 1993b, Akbarian et al., 1996, Anderson et al., 1996, Kirkpatrick et al., 1999, Eastwood and Harrison, 2003). The majority of these results show an increase of IWMNs within the superficial white matter, interpreted as failure of these neurons to migrate into the cortical white matter. Interestingly, reelin mRNA expression in IWMNs was also decreased (Eastwood and Harrison, 2003), consistent with its potential contribution to a failure of neuronal migration from the white matter into the cortex. Aberrant IWMNs may originate from the cortical subplate and/or from the ganglionic eminence. A contribution from the latter is suggested by the fact that at least a portion of IWMNs increased in SZ is represented by interneurons expressing Reelin and/or somatostatin-neuropeptide Y (Eastwood and Harrison, 2003, Yang et al., 2011). Yang et al. have recently reported that an increase of IWMNs in the superficial layer of the prefrontal cortex in SZ was accompanied by a decrease of somatostatin mRNA expression, a peptide typically expressed in interneurons, in the gray matter, and an increase of somatostatin-positive neurons in the superficial white matter (Yang et al., 2011). Together, these findings suggest that migration of interneurons arising from the ganglionic eminence and traveling through the white matter to reach the cortex may be disrupted in SZ (Yang et al., 2011).
Within the ECx of SZ subjects, abnormal cytoarchitecture and heterotopic displacement of the cell clusters in layer II have been reported by several groups and attributed to anomalous neuronal migration (Jakob and Beckmann, 1986, Arnold et al., 1991, Jakob and Beckmann, 1994, Falkai et al., 2000, Kovalenko et al., 2003). Increases of CSPG-positive glial cells in the ECx were highly selective for layer II cell clusters (Pantazopoulos et al., 2010)(Fig. 2), matching the distribution of cytoarchitectonic abnormalities and raising the possibility that abnormal CSPG expression during development may have contributed to neuronal migration disturbances. Furthermore, increases of small caliber vertical axons in these layers (Longson et al., 1996) are consistent with a disruption of axonal guidance, another key CSPG function. These fibers were proposed to originate from the amygdala (Longson et al., 1996, Benes and Berretta, 2000), a possibility consistent with the idea that concurrent CSPG abnormalities in the amygdala and ECx may lead to miswiring of their reciprocal connections.
The ECM plays a key role in the regulation of cell differentiation and migration and axonal outgrowth and guidance (Bandtlow and Zimmermann, 2000). The structure of CSPGs changes throughout development, modulating chemical properties required for traction or repulsion of migrating cells and growing axons, and dictating which growth factors, chemokines, axon guidance molecules and other ECM factors they bind to (Maeda, 2010). During development, CSPGs are found to concentrate within areas of active cell proliferation such as the ventricular zone of the ganglionic eminence and dorsal telencephalon and ependymal layer of the spinal cord (Bandtlow and Zimmermann, 2000, Zimmer et al., 2010). Phosphacan, and its membrane-bound splice variant receptor-type tyrosine phosphatase-β (RPTPβ), localize along radial glial fibers and migrating neurons in the developing cortex (Maeda et al., 1995, Ishii and Maeda, 2008). A compelling example of the role played by CSPGs in neuronal migration is reported in a study by Zimmer et al. (Zimmer et al., 2010). These authors showed that CSPGs, linked to semaphorin 3A, guide cortical interneurons migrating from the ganglionic eminence to the cortex, preventing them from entering the striatal mantle zone. Experimental disruption of this combined CSPG-semaphorin 3A effect led to migrating ganglionic eminence cortical interneurons to inappropriately invade the striatal cortical mantle (Zimmer et al., 2010).
Reelin regulation of cerebral cortex neuronal migration is well documented. It affects both somal translocation during early corticogenesis and, later, radial glia-guided locomotion. It provides a complex set of instructions, as it attracts migrating neurons toward the pial surface and acts as a positional cue, causing neurons to detach from their guiding glial fibers and terminate migration (for reviews see Tissir and Goffinet, 2003, Forster et al., 2010, Zhao and Frotscher, 2010). Similar functions are postulated in several other brain regions, including the hippocampus, cerebellum, olfactory bulb and thalamus (for review see Tissir and Goffinet, 2003, Forster et al., 2006).
3.2 Axon myelination
Myelin abnormalities in SZ, first suggested in 1992 (Hyde et al., 1992), include reduction of myelin compactness and increased density of concentric lamellar bodies, altered expression of key myelin components such as sphingomyelin, galactocerebrosides, myelin-associated glycoprotein and reduction of oligodendrocytes and clusters of oligodendrocyte-related genes (Hakak et al., 2001, Uranova et al., 2001, Hof et al., 2002, Flynn et al., 2003, Hof et al., 2003, Tkachev et al., 2003, Schmitt et al., 2004, Uranova et al., 2004, Dwork et al., 2007, Le-Niculescu et al., 2007, Sokolov, 2007, Konrad and Winterer, 2008, Dean et al., 2009, English et al., 2009, Martins-de-Souza et al., 2009, Chan et al., 2010, Martins-de-Souza, 2010). Abnormalities affecting the nodes of Ranvier have been recently reported (Haroutunian et al., 2010).
ECM molecules, and CSPG in particular, are involved in several aspect of the myelination process. During early postnatal life, the CSPG brevican is expressed by immature oligodendrocytes in coincidence with the phase in which these cells extend membrane processes to ensheath axon fibers (Ogawa et al., 2001). During adulthood, brevican, tenascin-R and phosphacan are present at the nodes of Ranvier on myelinated axons with a particularly large diameter in the CNS (Bekku et al., 2009). Brevican deficiency was found to cause a reorganization of the ECM composition around the nodes, suggesting that this CSPG dictates the specialization of the hyaluronan-binding nodal matrix assemblies in large diameter nodes (Bekku et al., 2009). Together, these considerations support the hypothesis that a disruption of ECM composition in SZ may contribute to myelin defects reported in this disease.
3.3 Synaptic functions
Synaptic defects are arguably one of the most well studied abnormality in SZ. Synaptic structural and ultrastructural changes, altered expression of presynaptic proteins, reduction of dendritic spine density, fewer synaptic vesicles and shrinkage of axon boutons have been reported in several brain regions (Eastwood and Harrison, 1995, Perrone Bizzozero et al., 1996, Uranova et al., 1996, Glantz and Lewis, 1997, Garey et al., 1998, Harrison, 1999, Glantz and Lewis, 2000, Mirnics et al., 2000, Rosoklija et al., 2000, Prabakaran et al., 2004, Clark et al., 2006, Camargo et al., 2007, Le-Niculescu et al., 2007, Sweet et al., 2007, Pennington et al., 2008, Smalla et al., 2008, Sweet et al., 2009, Chan et al., 2010, Chu and Liu, 2010, English et al., 2011). Importantly, synaptic abnormalities have been detected in the entorhinal and perirhinal cortices, regions where CSPG changes were also found (Eastwood and Harrison, 1995, Arnold, 1997, Harrison, 1999, Hemby et al., 2002, Law et al., 2004, Beneyto et al., 2007, Pantazopoulos et al., 2010).
Synaptic regulation is a prominent ECM function. Several ECM molecules, such as Reelin, CSPGs, pentraxins, thrombospondins, tenascins and laminins, together with the main group of ECM receptors, integrins, act in concert to regulate synaptogenesis, synaptic stabilization, maintenance, plasticity and homeostasis (Dityatev and Schachner, 2006, Dityatev et al., 2010a, Faissner et al., 2010). Interactions between ECM, glial and neuronal elements are key to these functions, leading to the suggestion of a ‘quadripartite’ synapse, with the addition of ECM to the ‘tripartite’ constituted by pre- and postsynaptic elements and astrocytes (Sykova, 2004b, Dityatev and Schachner, 2006, Halassa et al., 2007, Frischknecht et al., 2009, Dityatev et al., 2010a, Faissner et al., 2010). Interactions between ECM, astrocytes and glutamatergic transmission, and their effects on synaptogenesis and synaptic stabilization/maturation, are highlighted here as particularly relevant to the pathophysiology of SZ.
3.3.1 Synaptogenesis, synaptic stabilization/maturation
Direct involvement of astrocyte-derived CSPG and other ECM molecules in synaptogenesis is broadly supported by cell-insert co-culture system experiments showing that perturbations of CSPGs synthesis or integrity significantly alter the number of newly-formed synapses (Faissner et al., 2010, Pyka et al., 2011). Indirect mechanisms, through ECM regulation of glutamatergic transmission vary across development. In early developmental stages, the extracellular concentration of glutamate regulates the motility of dendritic spine (elongation/retraction) and axonal filopodia, and thus their chances of finding a synaptic partner (Tashiro et al., 2003, Segal et al., 2010). Once a synapse is formed, its stabilization and maturation depends on glutamate concentration within the synaptic cleft, as well as on the availability of glutamate receptors to individual synapses (Hashimoto and Kano, 2003, Frischknecht et al., 2009, Dityatev, 2010). CSPG\hyaluronan-based ECM affects these parameters in several ways. First, the degree of ECM viscosity and the interactions between the negatively charged glycosaminoglycan proteoglycan chains and glutamate, which is itself negatively charged, control glutamate diffusion in the extracellular space (Sykova, 2004b, a, Sykova and Nicholson, 2008, Hrabetova et al., 2009). Second, later in postnatal development, hyaluronan\CSPG-based ECM in the form of PNNs, compartimentalizes the neuronal surface, restricting the surface mobility of integral membrane proteins, including glutamate AMPA receptors (Frischknecht et al., 2009, Dityatev et al., 2010b). This function defines the reservoir of receptors available to each synapse, an important determinant for the capability of high frequency firing (Frischknecht et al., 2009). Synergistic interactions between AMPA and NMDA receptors are needed to maintain overall integrity of glutamate synapses. In this regard, it is noteworthy that Reelin contributes to the regulation of NMDA receptors and thus strongly affects synaptic maturation and stabilization. Reelin postsynaptic membrane binding sites mediate its interactions with NMDA receptors, which have been shown to affect LTP induction (reviewed in Dityatev et al., 2010a). Reelin has also been shown to mediate a switch in the subunit composition of NMDA receptors (from accumulation to lack of the NR2B subunit) considered to be the hallmark of synaptic maturation (Sinagra et al., 2005). Thus, pathologies affecting ECM composition may affect several key aspects of glutamate transmission and, consequently, synaptic functions. Together, these considerations indicate that ECM abnormalities related to disease states may profoundly affect synaptogenesis and synaptic stabilization/maturation both directly and through glutamate-mediated mechanisms.
3.3.2 PNNs, PVB-positive neurons and adult synaptic plasticity
PNNs develop late in postnatal life, as they gradually mature into stable, dense ECM perisynaptic aggregates enveloping somata, dendrites and the initial axonal segment, and surrounding successful synaptic contacts, thus stabilizing successful neuronal connections and restricting further plasticity (Pizzorusso et al., 2002, Galtrey and Fawcett, 2007). This process occurs in an experience-driven manner, regulated by both excitatory glutamatergic inputs and inhibition mediated by GABA-A receptors, and coincides with the closure of critical developmental periods, and PVB-positive neurons maturation (Koppe et al., 1997, Vitellaro-Zuccarello et al., 2001, Pizzorusso et al., 2002, Dityatev and Schachner, 2003, Hensch, 2005, Dityatev and Schachner, 2006, Pizzorusso et al., 2006, Dityatev et al., 2007, Sugiyama et al., 2008, Balmer et al., 2009, Gogolla et al., 2009, Dityatev et al., 2010a). For instance, in the visual cortex maturation of PNNs around PVB-positive neurons is a key determinant for the establishment of ocular dominance, culminating in the closure of the critical period (Saghatelyan et al., 2001, Pizzorusso et al., 2002, Sugiyama et al., 2008). Similar findings in other brain regions suggest that this PNN role may not be specific to the visual cortex (Balmer et al., 2009, Gogolla et al., 2009). Particularly relevant to mental illnesses, a study by Gogolla et al. shows a similar mechanism in the amygdala (Gogolla et al., 2009). This region plays a key role in emotion processing and is necessary for fear memory acquisition and extinction (LeDoux et al., 1989, LeDoux, 2003, Akirav and Maroun, 2007, Myers and Davis, 2007). Gogolla et al. show that formation of PNNs in the amygdala marks the closure of a developmental period during which fear memories can be fully erased by fear extinction training (i.e. repeated exposure to the conditioned stimulus in absence of unconditioned reinforcement). Once this period is closed, the ability of creating erasure-resilient, contextualized, fear memories is instated (Bouton, 2004, Kim and Richardson, 2008, Gogolla et al., 2009). Thus, PNN maturation in the amygdala may be necessary for the transition to an adult form of emotion-related learning that allows the formation of erasure-resistant fear memories. Notably, abnormal fear conditioning and extinction has been observed in subjects with SZ (Holt et al., 2009). Together with our findings of decreased PNNs in the amygdala of subjects with SZ, these results suggest that, in this disease, ECM abnormalities may cause failure of key emotion-related circuitry to mature during late postnatal developmental stages. It is important to note that although the association between PNNs and PVB-positive neurons has been detected consistently (Fig. 1) (Celio, 1993, Hartig et al., 1994, Pantazopoulos et al., 2006), PNNs have been shown to be associated with a number of neuronal populations, including excitatory projection neurons and inhibitory interneurons, several of which express the voltage gated K+ channel Kv3.1b subunit, critical for high-frequency firing (Perney et al., 1992, Lenz et al., 1994, Weiser et al., 1995, Gan and Kaczmarek, 1998, Hartig et al., 1999, Murakami and Ohtsuka, 2003, Wegner et al., 2003, Bruckner et al., 2004, McDonald and Mascagni, 2006, Vitellaro-Zuccarello et al., 2007). It is therefore plausible to postulate that ECM abnormalities in SZ may affect the development and functions of both excitatory and inhibitory neuronal populations.
3.4 Neurotransmission and growth factor regulation
Abnormalities affecting growth factors and main neurotransmitters have been detected consistently in SZ. Cytokines/growth factors thought to be involved in SZ include brain derived neurotrophic factor (BDNF), transforming growth factor β1 (TGFβ1), neuregulin1 (NRG1), epidermal growth factor (EGF) (Takahashi et al., 2000, Futamura et al., 2002, Hahn et al., 2006, Harrison and Law, 2006, Dean et al., 2009, Jaaro-Peled et al., 2009, Kalkman, 2009, Sun et al., 2010, Watanabe et al., 2010). The role of TGFβ1 is particularly intriguing in the context of ECM abnormalities. On the one hand, growing evidence supports the involvement of TGFβ1 in SZ. Increases of TGFβ1 expression have been detected in the serum and several brain regions, together with anomalies of its signaling pathways (Kim et al., 2004, Benes et al., 2007, Kalkman, 2009, Mauney et al., 2011). Emerging evidence for TGFβ1 as a potential candidate gene for SZ suggests that these abnormalities may be, at least in part, related to genetic vulnerability (Zaharieva et al., 2008, Pantazopoulos et al., 2011). On the other hand, TGFβ1 powerfully stimulates CSPG synthesis in astrocytes and, in general, plays a key role in regulating ECM formation and astrocytic functions (Baghdassarian et al., 1993, Lawrence, 1996, Asher et al., 2000, Sometani et al., 2001, Hyytiainen et al., 2004, Gomes et al., 2005, Smith and Strunz, 2005, Vivien and Ali, 2006, Wilkins-Port and Higgins, 2007). Taken together, these observations suggest that increased TGF-β1 expression may contribute to CSPG upregulation in astrocytes from subjects with SZ. In turn, the ECM controls the access of growth factors/cytokines to their cellular targets. CSPGs concentrate a range of active molecules, including BDNF, in PNNs and present them to membrane receptors (Galtrey and Fawcett, 2007, Fawcett, 2009). A reduction of CSPG-positive PNNs in SZ may thus further disrupt BDNF, and other cytokine/growth factor, signaling in SZ (Takahashi et al., 2000, Futamura et al., 2002, Hahn et al., 2006, Harrison and Law, 2006, Jaaro-Peled et al., 2009, Kalkman, 2009, Pantazopoulos et al., 2010, Watanabe et al., 2010).
Strong evidence supports abnormalities affecting glutamate, GABA and dopamine in SZ (Olney, 1990, Coyle, 1996, Laruelle, 1998, Benes and Berretta, 2001, Goff and Coyle, 2001, Prabakaran et al., 2004, Lewis et al., 2005, Carlsson and Carlsson, 2006, Clark et al., 2006, Novikova et al., 2006, Yu et al., 2006, Le-Niculescu et al., 2007, Lo et al., 2007, Dean et al., 2009, Gupta et al., 2009, English et al., 2011). Abnormalities affecting glutamate ionotropic and metabotropic receptors have been reported in several brain regions (Meador-Woodruff and Healy, 2000, Harrison et al., 2008, Ghose et al., 2009, Javitt, 2010). Altered expression of GAD67, and several presynaptic and postsynaptic GABA-related factors are thought to reflect dysfunction of distinct subpopulations of GABAergic interneurons, including PNN-associated PVB-expressing neurons and somatostatin/NPY-expressing neurons, which synthesize and secrete Reelin (Woo et al., 1997, Guidotti et al., 2000a, Zhang and Reynolds, 2002, Woo et al., 2004, Lewis et al., 2005, Torrey et al., 2005, Benes et al., 2007, Huang and Akbarian, 2007, Hashimoto et al., 2008, Morris et al., 2008, Fung et al., 2010). In particular, abnormalities affecting neurons expressing PVB have been detected in several brain regions in subjects with SZ, and are thought to represent a key element of this disease (Lewis et al., 2004, Lewis et al., 2005, Pantazopoulos et al., 2007, Fung et al., 2010, Woo et al., 2010). Current views on a disruption of dopaminergic functions in SZ postulate combinations of factors affecting its release, uptake, receptor expression and responsivity and intracellular signal transduction, that may lead to altered dopaminergic tone (Grumet et al., 1996, Laruelle and Abi-Dargham, 1999, Laruelle et al., 1999, Svensson, 2000, Syed et al., 2011).
Several aspects of the complex interactions between ECM regulation of glutamate, GABA and dopamine neurotransmission have surfaced in the previous paragraphs. As discussed, the ECM controls the reservoir of AMPA receptors available to individual synapses, and modulates NMDA receptor subunit composition leading to maturation of excitatory synapses (Sinagra et al., 2005, Pizzorusso et al., 2006, Frischknecht et al., 2009). ECM abnormalities causing dysregulation of glutamate receptor availability at the synaptic site may compound the effects of altered expression of these receptors in SZ (Meador-Woodruff and Healy, 2000, Huerta et al., 2006, Beneyto et al., 2007, Hammond et al., 2010). As mentioned, PNNs are predominantly associated with neurons expressing GABAergic inhibitory interneurons expressing PVB in cortical and subcortical brain regions (Hartig et al., 1992, Seeger et al., 1996, Celio et al., 1998, Pizzorusso et al., 2002, Pantazopoulos et al., 2006, Pizzorusso et al., 2006, Sugiyama et al., 2008). PNNs abnormalities may impair the maturation and normal functions of PVB-expressing neurons, in turn affecting adult synaptic plasticity and gamma oscillatory activity (McRae et al., 2007, Gogolla et al., 2009, Woo et al., 2010). Furthermore, CSPGs have been shown to control the development of dopaminergic innervation (Gates et al., 1996, Charvet et al., 1998, Moon et al., 2002, Li et al., 2007). ECM abnormalities may disrupt dopaminergic innervation of key regions such as the striatum and amygdala.
In addition, the ECM plays a key role in modulating volume transmission, i.e. cell communication mediated by signal diffusion within the extracellular space (Agnati et al., 2006). Physical signals, e.g. electrotonic currents, as well as a broad variety of chemical signals, such as neurotransmitters (extrasynaptic release or synaptic spill-over and cross-talk), growth factors, ions, gases and enzymes, are transmitted through the ECM. Variations of ECM composition affect its viscosity and electrical charge, and thus signal diffusion (Sykova and Nicholson, 2008). For instance, the high content of negatively charged CSPGs is likely to affect local ion concentrations, as demonstrated by increased diffusion coefficient of Ca++ following enzymatic chondroitin sulfate chain removal, as well as diffusion of charged neurotransmitters such as glutamate (Hrabetova et al., 2009, Gundelfinger et al., 2010). Thus, pathological CSPG expression variations may profoundly affect volume transmission, altering a broad range of signals linking cellular networks (Sykova, 2004a, Agnati et al., 2006). In the amygdala, were reduced dopamine reuptake is suggested by findings of increased dopamine concentrations and decreased dopamine transporter expression in the amygdala of subjects with SZ (Reynolds, 1983, Berretta et al., manuscript in preparation), altered ECM composition may lead to excessive diffusion of dopamine in the extracellular space, with overstimulation of extrasynaptic dopamine D2 receptors (Agnati et al., 2006, Carlsson and Carlsson, 2006).
4. PHARMACOLOGICAL MANIPULATION OF ECM COMPONENTS
Investigations on pharmacological manipulation of proteoglycans have focused mostly on the treatment of brain injury, neurodegenerative disorders and cancer (Viapiano and Matthews, 2006). CSPGs are enriched in glial scars, such as those that develop following brain injury, and have strong inhibitory effects on axonal re-growth (Davies et al., 1997, Fawcett and Asher, 1999, Morgenstern et al., 2002, Fawcett, 2009). Proteoglycans, and CSPGs in particular, are also upregulated in advanced stages of at least some neurodegenerative diseases, such as amyloidopathies, and are thought to contribute to the pathophysiology of these diseases (DeWitt and Silver, 1996, Castillo et al., 1999, McLaurin and Fraser, 2000, Sobel and Ahmed, 2001, van Horssen et al., 2003, Rhodes and Fawcett, 2004). Malignant gliomas and other cancers alter the normal ECM composition and rely on overexpression of CSPGs to promote tumor growth, vascularization and invasiveness (Paulus et al., 1996, Nutt et al., 2001, Viapiano and Matthews, 2006, Cattaruzza et al., 2008). Pharmacological tools to address these effects have been developed in recent years, and include use of the enzyme chondroitinase ABC, which removes chondrotin sulfate chains from the core proteins, and ECM regulatory factors, in some cases secreted by cell transplants (Raisman, 2004, Chiu et al., 2009, Garcia-Alias et al., 2009, Hyatt et al., 2010, Lindsay et al., 2010, Jefferson et al., 2011, Mackay-Sim and St John, 2011, Tan et al., 2011, Wang et al., 2011). Interestingly, lithium inhibits factors impairing axon outgrowth, and was found to induce marked axon sprouting following brain injury (Dill et al., 2008). Furthermore, chlorpromazine affects ECM availability of byglican and decorin, leucin-rich small proteoglycans known to transduce signals through several growth factor receptors and to affect CSPG synthesis (Stichel et al., 1995, Oohira et al., 2000, Gotte et al., 2004, Feugaing et al., 2007, Galtrey and Fawcett, 2007).
Promising advances have been made toward pharmacological correction of Reelin abnormalities in SZ (Guidotti et al., 2009, Guidotti et al., 2011). As discussed, strong evidence points to epigenetic mechanisms as a causal factor in Reelin reduction in this disease (Veldic et al., 2004, Abdolmaleky et al., 2005, Veldic et al., 2005, Costa et al., 2007, Guidotti et al., 2007, Guidotti et al., 2010). In an elegant series of studies, Costa, Guidotti and co-workers have shown that co-administration of valproate and atypical antipsychotics facilitates chromatin remodeling, which in turn might contribute to reelin- and GAD(67)-promoter demethylation. By counteracting altered epigenetic mechanisms in SZ, this pharmacological strategy may contribute to bringing Reelin expression toward normal levels (Guidotti et al., 2009, Guidotti et al., 2011).
5. CONCLUSIONS
On the basis of the considerations discussed above, we put forward that ECM/glial abnormalities may represent a unifying factor contributing to disturbances of neuronal migration, synaptic connectivity and plasticity, and GABAergic, glutamatergic and dopaminergic neurotransmission in SZ. Interestingly, at least four genes potentially involved in ECM-glial-neuronal interactions, i.e. PTPRZ1, the gene encoding for phosphacan, reelin, ADAMTSL3, and TGF-β1 have been recently identified as potential SZ susceptibility genes (Buxbaum et al., 2008, Shifman et al., 2008, Wedenoja et al., 2008, Zaharieva et al., 2008, Gregorio et al., 2009, Liu et al., 2010){Dow, 2011 #11264}(Need et al., 2009), suggesting that ECM abnormalities in this disease may be, at least in part, linked to genetic factors and present during development. In the long term, knowledge of the pathophysiological mechanisms involved in ECM abnormalities in SZ, and their consequences at the cellular, neural circuit and functional levels, may allow our field to benefit from advanced therapeutic approaches targeting ECM components, and CSPGs in particular, in the context of research on brain injury, Alzheimer’s disease and cancer (DeWitt and Silver, 1996, Davies et al., 1997, Viapiano and Matthews, 2006, Cattaruzza et al., 2008, Kwok et al., 2008, Li et al., 2008, Yang and Schnaar, 2008).
Highlights.
The brain extracellular matrix may play a key role in schizophrenia.
Solid evidence supports the involvement of extracellular matrix glycoprotein Reelin Emerging findings point to chondroitin sulfate proteoglycans/glial interactions Extracellular matrix functions are relevant to the pathophysiology of schizophrenia
Abbreviations
- CSPGs
chondroitin sulfate proteoglycans
- EGF
epidermal growth factor
- ECM
extracellular matrix
- ECx
entorhinal cortex
- GAD67
67 kDa isoform of glutamic acid decarboxylase
- GFAP
glial fibrillary acid protein
- PNN
perineuronal net
- PVB
parvalbumin
- SZ
schizophrenia
- TGF-beta
transforming growth factor beta
- WFA
wisteria floribunda agglutinin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:60–66. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- Agnati LF, Leo G, Zanardi A, Genedani S, Rivera A, Fuxe K, Guidolin D. Volume transmission and wiring transmission from cellular to molecular networks: history and perspectives. Acta Physiol (Oxf) 2006;187:329–344. doi: 10.1111/j.1748-1716.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Bunney WE, Jr., Potkin SG, Wigal SB, Hagman JO, Sandman CA, Jones EG. Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry. 1993a;50:169–177. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr., Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- Akbarian S, ViÒuela A, Kim JJ, Potkin SG, Bunney WE, Jr., Jones EG. Distorted distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase neurons in temporal lobe of schizophrenics implies anomalous cortical development. Arch Gen Psychiatry. 1993b;50:178–187. doi: 10.1001/archpsyc.1993.01820150016002. [DOI] [PubMed] [Google Scholar]
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts M, Ceulemans S, Forero D, Moens LN, De Zutter S, Heyrman L, Lenaerts AS, Norrback KF, De Rijk P, Nilsson LG, Goossens D, Adolfsson R, Del-Favero J. Support for NRG1 as a susceptibility factor for schizophrenia in a northern Swedish isolated population. Arch Gen Psychiatry. 2009;66:828–837. doi: 10.1001/archgenpsychiatry.2009.82. [DOI] [PubMed] [Google Scholar]
- Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpar A, Gartner U, Hartig W, Bruckner G. Distribution of pyramidal cells associated with perineuronal nets in the neocortex of rat. Brain Res. 2006;1120:13–22. doi: 10.1016/j.brainres.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Volk DW, Lewis DA. Increased density of microtubule associated protein 2-immunoreactive neurons in the prefrontal white matter of schizophrenic subjects. Schizophr Res. 1996;19:111–119. doi: 10.1016/0920-9964(96)88521-5. [DOI] [PubMed] [Google Scholar]
- Arnold SE. The medial temporal lobe in schizophrenia. J Neuropsychiatry Clin Neurosci. 1997;9:460–470. doi: 10.1176/jnp.9.3.460. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Trojanowski JQ. Recent advances in defining the neuropathology of schizophrenia. Acta Neuropathol. 1996;92:217–231. doi: 10.1007/s004010050512. [DOI] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghdassarian D, Toru-Delbauffe D, Gavaret JM, Pierre M. Effects of transforming growth factor-beta 1 on the extracellular matrix and cytoskeleton of cultured astrocytes. Glia. 1993;7:193–202. doi: 10.1002/glia.440070302. [DOI] [PubMed] [Google Scholar]
- Baig S, Wilcock GK, Love S. Loss of perineuronal net N-acetylgalactosamine in Alzheimer’s disease. Acta Neuropathol (Berl) 2005;110:393–401. doi: 10.1007/s00401-005-1060-2. [DOI] [PubMed] [Google Scholar]
- Balmer TS, Carels VM, Frisch JL, Nick TA. Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci. 2009;29:12878–12885. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Bekku Y, Rauch U, Ninomiya Y, Oohashi T. Brevican distinctively assembles extracellular components at the large diameter nodes of Ranvier in the CNS. J Neurochem. 2009;108:1266–1276. doi: 10.1111/j.1471-4159.2009.05873.x. [DOI] [PubMed] [Google Scholar]
- Ben-David E, Shifman S. Further investigation of the association between rs7341475 and rs17746501 and schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1244–1247. doi: 10.1002/ajmg.b.31093. [DOI] [PubMed] [Google Scholar]
- Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Amygdalo-entorhinal inputs to the hippocampal formation in relation to schizophrenia. Ann N Y Acad Sci. 2000;911:293–304. doi: 10.1111/j.1749-6632.2000.tb06733.x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Gabaergic interneurons. Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Subburaju S. Site-specific regulation of cell cycle and DNA repair in post-mitotic GABA cells in schizophrenic versus bipolars. Proc Natl Acad Sci U S A. 2009;106:11731–11736. doi: 10.1073/pnas.0903066106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal Glutamate Receptor Expression in the Medial Temporal Lobe in Schizophrenia and Mood Disorders. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Lange N. Neuron Numbers and Volume of the Amygdala in Subjects Diagnosed with Bipolar Disorder or Schizophrenia. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Berretta S, Sin J, Pantazopolous H, Liu Y. (manuscript in preparation) Dopamine Transporter Abnormalities in the Amygdala of Subjects Diagnosed with Schizophrenia or Bipolar Disorder. [Google Scholar]
- Boksha IS. Coupling between Neuronal and Glial Cells via Glutamate Metabolism in Brain of Healthy Persons and Patients with Mental Disorders. Biochemistry (Mosc) 2004;69:705–719. doi: 10.1023/b:biry.0000040194.97348.e7. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bruckner G, Hausen D, Hartig W, Drlicek M, Arendt T, Brauer K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience. 1999;92:791–805. doi: 10.1016/s0306-4522(99)00071-8. [DOI] [PubMed] [Google Scholar]
- Bruckner G, Kacza J, Grosche J. Perineuronal nets characterized by vital labelling, confocal and electron microscopy in organotypic slice cultures of rat parietal cortex and hippocampus. J Mol Histol. 2004;35:115–122. doi: 10.1023/b:hijo.0000023374.22298.50. [DOI] [PubMed] [Google Scholar]
- Bruckner G, Seeger G, Brauer K, Hartig W, Kacza J, Bigl V. Cortical areas are revealed by distribution patterns of proteoglycan components and parvalbumin in the Mongolian gerbil and rat. Brain Res. 1994;658:67–86. doi: 10.1016/s0006-8993(09)90012-9. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Georgieva L, Young JJ, Plescia C, Kajiwara Y, Jiang Y, Moskvina V, Norton N, Peirce T, Williams H, Craddock NJ, Carroll L, Corfas G, Davis KL, Owen MJ, Harroch S, Sakurai T, O’Donovan MC. Molecular dissection of NRG1-ERBB4 signaling implicates PTPRZ1 as a potential schizophrenia susceptibility gene. Mol Psychiatry. 2008;13:162–172. doi: 10.1038/sj.mp.4001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in Schizophrenia 1 Interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. doi: 10.1038/sj.mp.4001880. [DOI] [PubMed] [Google Scholar]
- Campo CG, Sinagra M, Verrier D, Manzoni OJ, Chavis P. Reelin secreted by GABAergic neurons regulates glutamate receptor homeostasis. PLoS ONE. 2009;4:e5505. doi: 10.1371/journal.pone.0005505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A, Carlsson ML. A dopaminergic deficit hypothesis of schizophrenia: the path to discovery. Dialogues Clin Neurosci. 2006;8:137–142. doi: 10.31887/DCNS.2006.8.1/acarlsson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CJ. Schizophrenia susceptibility genes converge on interlinked pathways related to glutamatergic transmission and long-term potentiation, oxidative stress and oligodendrocyte viability. Schizophr Res. 2006;86:1–14. doi: 10.1016/j.schres.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Fawcett JW. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol. 2007;501:83–94. doi: 10.1002/cne.21231. [DOI] [PubMed] [Google Scholar]
- Castillo GM, Lukito W, Wight TN, Snow AD. The sulfate moieties of glycosaminoglycans are critical for the enhancement of beta-amyloid protein fibril formation. J Neurochem. 1999;72:1681–1687. doi: 10.1046/j.1471-4159.1999.721681.x. [DOI] [PubMed] [Google Scholar]
- Cattaruzza S, Nicolosi PA, Perris R. Proteoglycans in the control of tumor growth and metastasis formation. Connect Tissue Res. 2008;49:225–229. doi: 10.1080/03008200802143448. [DOI] [PubMed] [Google Scholar]
- Celio MR. Perineuronal nets of extracellular matrix around parvalbumin-containing neurons of the hippocampus. Hippocampus. 1993;3:55–60. [PubMed] [Google Scholar]
- Celio MR, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Perineuronal nets: past and present. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- Chan MK, Tsang TM, Harris LW, Guest PC, Holmes E, Bahn S. Evidence for disease and antipsychotic medication effects in post-mortem brain from schizophrenia patients. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.100. [DOI] [PubMed] [Google Scholar]
- Charvet I, Hemming FJ, Feuerstein C, Saxod R. Mosaic distribution of chondroitin and keratan sulphate in the developing rat striatum: possible involvement of proteoglycans in the organization of the nigrostriatal system. Brain Res Dev Brain Res. 1998;109:229–244. doi: 10.1016/s0165-3806(98)00088-1. [DOI] [PubMed] [Google Scholar]
- Chiu SC, Hung HS, Lin SZ, Chiang E, Liu DD. Therapeutic potential of olfactory ensheathing cells in neurodegenerative diseases. J Mol Med (Berl) 2009;87:1179–1189. doi: 10.1007/s00109-009-0528-2. [DOI] [PubMed] [Google Scholar]
- Chu TT, Liu Y. An integrated genomic analysis of gene-function correlation on schizophrenia susceptibility genes. J Hum Genet. 2010;55:285–292. doi: 10.1038/jhg.2010.24. [DOI] [PubMed] [Google Scholar]
- Chung C, Tallerico T, Seeman P. Schizophrenia hippocampus has elevated expression of chondrex glycoprotein gene. Synapse. 2003;50:29–34. doi: 10.1002/syn.10228. [DOI] [PubMed] [Google Scholar]
- Chvatal A, Anderova M, Ziak D, Sykova E. Glial depolarization evokes a larger potassium accumulation around oligodendrocytes than around astrocytes in gray matter of rat spinal cord slices. J Neurosci Res. 1999;56:493–505. doi: 10.1002/(SICI)1097-4547(19990601)56:5<493::AID-JNR5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Clark D, Dedova I, Cordwell S, Matsumoto I. A proteome analysis of the anterior cingulate cortex gray matter in schizophrenia. Mol Psychiatry. 2006;11:459–470. 423. doi: 10.1038/sj.mp.4001806. [DOI] [PubMed] [Google Scholar]
- Connor CM, Crawford BC, Akbarian S. White matter neuron alterations in schizophrenia and related disorders. Int J Dev Neurosci. 2010 doi: 10.1016/j.ijdevneu.2010.07.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis J, Grayson DR, Guidotti A, Pappas GD, Pesold C. Dendritic spine hypoplasticity and downregulation of reelin and gabaergic tone in schizophrenia vulnerability. Neurobiol Dis. 2001;8:723–742. doi: 10.1006/nbdi.2001.0436. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis JM, Dong E, Grayson DR, Guidotti A, Tremolizzo L, Veldic M. A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol. 2004;16:1–23. doi: 10.1615/critrevneurobiol.v16.i12.10. [DOI] [PubMed] [Google Scholar]
- Costa E, Dong E, Grayson DR, Guidotti A, Ruzicka W, Veldic M. Reviewing the role of DNA (cytosine-5) methyltransferase overexpression in the cortical GABAergic dysfunction associated with psychosis vulnerability. Epigenetics. 2007;2:29–36. doi: 10.4161/epi.2.1.4063. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harvard Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- Dean B, Boer S, Gibbons A, Money T, Scarr E. Recent advances in postmortem pathology and neurochemistry in schizophrenia. Curr Opin Psychiatry. 2009;22:154–160. doi: 10.1097/YCO.0b013e328323d52e. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Burket JA, Katz E. Does subtle disturbance of neuronal migration contribute to schizophrenia and other neurodevelopmental disorders? Potential genetic mechanisms with possible treatment implications. Eur Neuropsychopharmacol. 2010;20:281–287. doi: 10.1016/j.euroneuro.2010.02.005. [DOI] [PubMed] [Google Scholar]
- DeWitt DA, Silver J. Regenerative failure: a potential mechanism for neuritic dystrophy in Alzheimer’s disease. Exp Neurol. 1996;142:103–110. doi: 10.1006/exnr.1996.0182. [DOI] [PubMed] [Google Scholar]
- Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A. Remodeling of extracellular matrix and epileptogenesis. Epilepsia. 2010;51(Suppl 3):61–65. doi: 10.1111/j.1528-1167.2010.02612.x. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M. Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol. 2007;67:570–588. doi: 10.1002/dneu.20361. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Fellin T. Extracellular matrix in plasticity and epileptogenesis. Neuron Glia Biol. 2008;4:235–247. doi: 10.1017/S1740925X09000118. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci. 2003;4:456–468. doi: 10.1038/nrn1115. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. The extracellular matrix and synapses. Cell Tissue Res. 2006 doi: 10.1007/s00441-006-0217-1. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010a;11:735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010b;33:503–512. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc Natl Acad Sci U S A. 2005;102:12578–12583. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow DJ, Huxley-Jones J, Hall JM, Francks C, Maycox PR, Kew JN, Gloger IS, Mehta NA, Kelly FM, Muglia P, Breen G, Jugurnauth S, Pederoso I, St Clair D, Rujescu D, Barnes MR. ADAMTSL3 as a candidate gene for schizophrenia: gene sequencing and ultra-high density association analysis by imputation. Schizophr Res. 2011;127:28–34. doi: 10.1016/j.schres.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Drakew A, Frotscher M, Deller T, Ogawa M, Heimrich B. Developmental distribution of a reeler gene-related antigen in the rat hippocampal formation visualized by CR-50 immunocytochemistry. Neuroscience. 1998;82:1079–1086. doi: 10.1016/s0306-4522(97)00326-6. [DOI] [PubMed] [Google Scholar]
- Dwork AJ, Mancevski B, Rosoklija G. White matter and cognitive function in schizophrenia. Int J Neuropsychopharmacol. 2007;10:513–536. doi: 10.1017/S1461145707007638. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Decreased synaptophysin in the medial temporal lobe in schizophrenia demonstrated using immunoautoradiography. Neuroscience. 1995;69:339–343. doi: 10.1016/0306-4522(95)00324-c. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol Psychiatry. 2003;8:821–831. doi: 10.1038/sj.mp.4001399. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Cellular basis of reduced cortical reelin expression in schizophrenia. Am J Psychiatry. 2006;163:540–542. doi: 10.1176/appi.ajp.163.3.540. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;8:148–155. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- English JA, Dicker P, Focking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics. 2009;9:3368–3382. doi: 10.1002/pmic.200900015. [DOI] [PubMed] [Google Scholar]
- English JA, Pennington K, Dunn MJ, Cotter DR. The neuroproteomics of schizophrenia. Biol Psychiatry. 2011;69:163–172. doi: 10.1016/j.biopsych.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C. Contributions of astrocytes to synapse formation and maturation - Potential functions of the perisynaptic extracellular matrix. Brain Res Rev. 2010;63:26–38. doi: 10.1016/j.brainresrev.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Falkai P, Schneider-Axmann T, Honer WG. Entorhinal cortex pre-alpha cell clusters in schizophrenia: quantitative evidence of a developmental abnormality. Biol Psychiatry. 2000;47:937–943. doi: 10.1016/s0006-3223(99)00250-4. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5:654–663. 571. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- Fawcett J. Molecular control of brain plasticity and repair. Prog Brain Res. 2009;175:501–509. doi: 10.1016/S0079-6123(09)17534-9. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Feugaing DD, Tammi R, Echtermeyer FG, Stenmark H, Kresse H, Smollich M, Schonherr E, Kiesel L, Gotte M. Endocytosis of the dermatan sulfate proteoglycan decorin utilizes multiple pathways and is modulated by epidermal growth factor receptor signaling. Biochimie. 2007;89:637–657. doi: 10.1016/j.biochi.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- Forster E, Bock HH, Herz J, Chai X, Frotscher M, Zhao S. Emerging topics in Reelin function. Eur J Neurosci. 2010;31:1511–1518. doi: 10.1111/j.1460-9568.2010.07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster E, Zhao S, Frotscher M. Laminating the hippocampus. Nat Rev Neurosci. 2006;7:259–267. doi: 10.1038/nrn1882. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009 doi: 10.1038/nn.2338. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Seidenbecher CI. The crosstalk of hyaluronan-based extracellular matrix and synapses. Neuron Glia Biol. 2008;4:249–257. doi: 10.1017/S1740925X09990226. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of Interneuron Markers in the Dorsolateral Prefrontal Cortex of the Developing Human and in Schizophrenia. Am J Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Futamura T, Toyooka K, Iritani S, Niizato K, Nakamura R, Tsuchiya K, Someya T, Kakita A, Takahashi H, Nawa H. Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol Psychiatry. 2002;7:673–682. doi: 10.1038/sj.mp.4001081. [DOI] [PubMed] [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Gan L, Kaczmarek LK. When, where, and how much? Expression of the Kv3.1 potassium channel in high-frequency firing neurons. J Neurobiol. 1998;37:69–79. doi: 10.1002/(sici)1097-4695(199810)37:1<69::aid-neu6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia [see comments] J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates MA, Fillmore H, Steindler DA. Chondroitin sulfate proteoglycan and tenascin in the wounded adult mouse neostriatum in vitro: dopamine neuron attachment and process outgrowth. J Neurosci. 1996;16:8005–8018. doi: 10.1523/JNEUROSCI.16-24-08005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose S, Gleason KA, Potts BW, Lewis-Amezcua K, Tamminga CA. Differential expression of metabotropic glutamate receptors 2 and 3 in schizophrenia: a mechanism for antipsychotic drug action? Am J Psychiatry. 2009;166:812–820. doi: 10.1176/appi.ajp.2009.08091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:660–669. doi: 10.1001/archpsyc.1997.01830190088009. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gloor P. Role of the human limbic system in perception, memory and affect. In: Doane BK, Livingston KE, editors. The Limbic System. Raven Press; New York: 1986. pp. 159–169. [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Golgi C. Intorno all’origine del quarto nervo cerebrale e una questione isto-fisiologica che a questo argomento si collega. Rend R Acc Lincei. 1893;2:379–389. [Google Scholar]
- Golgi C. Intorno alla struttura delle cellule nervose. Boll Soc Med-Chir Pavia. 1898;1:1–14. [Google Scholar]
- Gomes FC, Sousa Vde O, Romao L. Emerging roles for TGF-beta1 in nervous system development. Int J Dev Neurosci. 2005;23:413–424. doi: 10.1016/j.ijdevneu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Gotte M, Sofeu Feugaing DD, Kresse H. Biglycan is internalized via a chlorpromazine-sensitive route. Cell Mol Biol Lett. 2004;9:475–481. [PubMed] [Google Scholar]
- Gregorio SP, Sallet PC, Do KA, Lin E, Gattaz WF, Dias-Neto E. Polymorphisms in genes involved in neurodevelopment may be associated with altered brain morphology in schizophrenia: preliminary evidence. Psychiatry Res. 2009;165:1–9. doi: 10.1016/j.psychres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Sakurai T. Functions of brain chondroitin sulfate proteoglycans during developments: interactions with adhesion molecules. Perspect Dev Neurobiol. 1996;3:319–330. [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, Grayson DR, Matrisciano F, Pinna G, Satta R, Sharma RP, Tremolizzo L, Tueting P. Epigenetic GABAergic targets in schizophrenia and bipolar disorder. Neuropharmacology. 2011;60:1007–1016. doi: 10.1016/j.neuropharm.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, Uzunov D, Costa E. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000a;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Dong E, Kundakovic M, Satta R, Grayson DR, Costa E. Characterization of the action of antipsychotic subtypes on valproate-induced chromatin remodeling. Trends Pharmacol Sci. 2009;30:55–60. doi: 10.1016/j.tips.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Pesold C, Costa E. New neurochemical markers for psychosis: a working hypothesis of their operation [In Process Citation] Neurochem Res. 2000b;25:1207–1218. doi: 10.1023/a:1007635927069. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Ruzicka W, Grayson DR, Veldic M, Pinna G, Davis JM, Costa E. S-adenosyl methionine and DNA methyltransferase-1 mRNA overexpression in psychosis. Neuroreport. 2007;18:57–60. doi: 10.1097/WNR.0b013e32800fefd7. [DOI] [PubMed] [Google Scholar]
- Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- Gundelfinger ED, Frischknecht R, Choquet D, Heine M. Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur J Neurosci. 2010;31:2156–2165. doi: 10.1111/j.1460-9568.2010.07253.x. [DOI] [PubMed] [Google Scholar]
- Gupta M, Chauhan C, Bhatnagar P, Gupta S, Grover S, Singh PK, Purushottam M, Mukherjee O, Jain S, Brahmachari SK, Kukreti R. Genetic susceptibility to schizophrenia: role of dopaminergic pathway gene polymorphisms. Pharmacogenomics. 2009;10:277–291. doi: 10.2217/14622416.10.2.277. [DOI] [PubMed] [Google Scholar]
- Hagihara K, Miura R, Kosaki R, Berglund E, Ranscht B, Yamaguchi Y. Immunohistochemical evidence for the brevican-tenascin-R interaction: colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J Comp Neurol. 1999;410:256–264. [PubMed] [Google Scholar]
- Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Hall NG, Klenotic P, Anand-Apte B, Apte SS. ADAMTSL-3/punctin-2, a novel glycoprotein in extracellular matrix related to the ADAMTS family of metalloproteases. Matrix Biol. 2003;22:501–510. doi: 10.1016/s0945-053x(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. 2010;35:2110–2119. doi: 10.1038/npp.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutunian V, Roussos P, Katsel P, Siever L, Davis K. American College of Neuropsychopharmacology. Miami Beach, Florida: 2010. The Node of Ranvier in Schizophrenia Postmortem Brain Tissue. [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol. 2008;22:308–322. doi: 10.1177/0269881108089818. [DOI] [PubMed] [Google Scholar]
- Hartig W, Brauer K, Bigl V, Bruckner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res. 1994;635:307–311. doi: 10.1016/0006-8993(94)91452-4. [DOI] [PubMed] [Google Scholar]
- Hartig W, Brauer K, Bruckner G. Wisteria floribunda agglutinin-labelled nets surround parvalbumin-containing neurons. Neuroreport. 1992;3:869–872. doi: 10.1097/00001756-199210000-00012. [DOI] [PubMed] [Google Scholar]
- Hartig W, Bruckner G, Brauer K, Schmidt C, Bigl V. Allocation of perineuronal nets and parvalbumin-, calbindin- D28k- and glutamic acid decarboxylase-immunoreactivity in the amygdala of the rhesus monkey. Brain Res. 1995;698:265–269. doi: 10.1016/0006-8993(95)01016-o. [DOI] [PubMed] [Google Scholar]
- Hartig W, Derouiche A, Welt K, Brauer K, Grosche J, Mader M, Reichenbach A, Bruckner G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–796. doi: 10.1016/s0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Benes F. Hippocampus, III: GABA-containing cell bodies and GAD mRNA. Am J Psychiatry. 2005;162:450. doi: 10.1176/appi.ajp.162.3.450. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Ginsberg SD, Brunk B, Arnold SE, Trojanowski JQ, Eberwine JH. Gene expression profile for schizophrenia: discrete neuron transcription patterns in the entorhinal cortex. Arch Gen Psychiatry. 2002;59:631–640. doi: 10.1001/archpsyc.59.7.631. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Copland C, Davis KL, Buxbaum JD. Molecular and cellular evidence for an oligodendrocyte abnormality in schizophrenia. Neurochem Res. 2002;27:1193–1200. doi: 10.1023/a:1020981510759. [DOI] [PubMed] [Google Scholar]
- Hof PR, Haroutunian V, Friedrich VL, Jr., Byne W, Buitron C, Perl DP, Davis KL. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova S, Masri D, Tao L, Xiao F, Nicholson C. Calcium diffusion enhanced after cleavage of negatively charged components of brain extracellular matrix by chondroitinase ABC. J Physiol. 2009;587:4029–4049. doi: 10.1113/jphysiol.2009.170092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Akbarian S. GAD1 mRNA expression and DNA methylation in prefrontal cortex of subjects with schizophrenia. PLoS One. 2007;2:e809. doi: 10.1371/journal.pone.0000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta I, McCullumsmith RE, Haroutunian V, Gimenez-Amaya JM, Meador-Woodruff JH. Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. Synapse. 2006;59:394–402. doi: 10.1002/syn.20250. [DOI] [PubMed] [Google Scholar]
- Hyatt AJ, Wang D, Kwok JC, Fawcett JW, Martin KR. Controlled release of chondroitinase ABC from fibrin gel reduces the level of inhibitory glycosaminoglycan chains in lesioned spinal cord. J Control Release. 2010;147:24–29. doi: 10.1016/j.jconrel.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol. 1992;49:401–406. doi: 10.1001/archneur.1992.00530280095028. [DOI] [PubMed] [Google Scholar]
- Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Yahata N, Ito I, Nagano M, Toyota T, Yoshikawa T, Okubo Y, Suzuki H. Low serum levels of brain-derived neurotrophic factor and epidermal growth factor in patients with chronic schizophrenia. Schizophr Res. 2008;101:58–66. doi: 10.1016/j.schres.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Impagnatiello F, Guidotti AR, Pesold C, Dwivedi Y, Caruncho H, Pisu MG, Uzunov DP, Smalheiser NR, Davis JM, Pandey GN, Pappas GD, Tueting P, Sharma RP, Costa E. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Maeda N. Oversulfated chondroitin sulfate plays critical roles in the neuronal migration in the cerebral cortex. J Biol Chem. 2008;283:32610–32620. doi: 10.1074/jbc.M806331200. [DOI] [PubMed] [Google Scholar]
- Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- Jakob H, Beckmann H. Circumscribed malformation and nerve cell alterations in the entorhinal cortex of schizophrenics. Pathogenetic and clinical aspects. J Neural Transm Gen Sect. 1994;98:83–106. doi: 10.1007/BF01277013. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 2010;47:4–16. [PubMed] [Google Scholar]
- Jefferson SC, Tester NJ, Howland DR. Chondroitinase ABC promotes recovery of adaptive limb movements and enhances axonal growth caudal to a spinal hemisection. J Neurosci. 2011;31:5710–5720. doi: 10.1523/JNEUROSCI.4459-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John N, Krugel H, Frischknecht R, Smalla KH, Schultz C, Kreutz MR, Gundelfinger ED, Seidenbecher CI. Brevican-containing perineuronal nets of extracellular matrix in dissociated hippocampal primary cultures. Mol Cell Neurosci. 2006;31:774–784. doi: 10.1016/j.mcn.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Kabayama H, Takeuchi M, Taniguchi M, Tokushige N, Kozaki S, Mizutani A, Nakamura T, Mikoshiba K. Syntaxin 1B Suppresses Macropinocytosis and Semaphorin 3A- Induced Growth Cone Collapse. J Neurosci. 2011;31:7357–7364. doi: 10.1523/JNEUROSCI.2718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler AK, Djurovic S, Rimol LM, Brown AA, Athanasiu L, Jonsson EG, Hansen T, Gustafsson O, Hall H, Giegling I, Muglia P, Cichon S, Rietschel M, Pietilainen OP, Peltonen L, Bramon E, Collier D, St Clair D, Sigurdsson E, Petursson H, Rujescu D, Melle I, Werge T, Steen VM, Dale AM, Matthews RT, Agartz I, Andreassen OA. Candidate gene analysis of the human natural killer-1 carbohydrate pathway and perineuronal nets in schizophrenia: B3GAT2 is associated with disease risk and cortical surface area. Biol Psychiatry. 2011;69:90–96. doi: 10.1016/j.biopsych.2010.07.035. [DOI] [PubMed] [Google Scholar]
- Kalkman HO. Altered growth factor signaling pathways as the basis of aberrant stem cell maturation in schizophrenia. Pharmacol Ther. 2009;121:115–122. doi: 10.1016/j.pharmthera.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J Neurosci. 2008;28:1282–1290. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ, Leonard BE. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1129–1134. doi: 10.1016/j.pnpbp.2004.05.047. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Conley RC, Kakoyannis A, Reep RL, Roberts RC. Interstitial cells of the white matter in the inferior parietal cortex in schizophrenia: An unbiased cell-counting study. Synapse. 1999;34:95–102. doi: 10.1002/(SICI)1098-2396(199911)34:2<95::AID-SYN2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Brenner E, Eyjolfsson EM, Sonnewald U. How do glial-neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia? Neurochem Int. 2007;50:291–301. doi: 10.1016/j.neuint.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Konrad A, Winterer G. Disturbed structural connectivity in schizophrenia primary factor in pathology or epiphenomenon? Schizophr Bull. 2008;34:72–92. doi: 10.1093/schbul/sbm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppe G, Bruckner G, Brauer K, Hartig W, Bigl V. Developmental patterns of proteoglycan-containing extracellular matrix in perineuronal nets and neuropil of the postnatal rat brain. Cell Tissue Res. 1997;288:33–41. doi: 10.1007/s004410050790. [DOI] [PubMed] [Google Scholar]
- Kovalenko S, Bergmann A, Schneider-Axmann T, Ovary I, Majtenyi K, Havas L, Honer WG, Bogerts B, Falkai P. Regio entorhinalis in schizophrenia: more evidence for migrational disturbances and suggestions for a new biological hypothesis. Pharmacopsychiatry. 2003;36(Suppl 3):S158–161. doi: 10.1055/s-2003-45124. [DOI] [PubMed] [Google Scholar]
- Kuperberg G, Heckers S. Schizophrenia and cognitive function. Curr Opin Neurobiol. 2000;10:205–210. doi: 10.1016/s0959-4388(00)00068-4. [DOI] [PubMed] [Google Scholar]
- Kwok JC, Afshari F, Garcia-Alias G, Fawcett JW. Proteoglycans in the central nervous system: plasticity, regeneration and their stimulation with chondroitinase ABC. Restor Neurol Neurosci. 2008;26:131–145. [PubMed] [Google Scholar]
- Lander C, Kind P, Maleski M, Hockfield S. A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J Neurosci. 1997;17:1928–1939. doi: 10.1523/JNEUROSCI.17-06-01928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Hyde TM, Kleinman JE, Harrison PJ. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am J Psychiatry. 2004;161:1848–1855. doi: 10.1176/ajp.161.10.1848. [DOI] [PubMed] [Google Scholar]
- Lawrence DA. Transforming growth factor-beta: a general review. Eur Cytokine Netw. 1996;7:363–374. [PubMed] [Google Scholar]
- Le-Niculescu H, Balaraman Y, Patel S, Tan J, Sidhu K, Jerome RE, Edenberg HJ, Kuczenski R, Geyer MA, Nurnberger JI, Jr., Faraone SV, Tsuang MT, Niculescu AB. Towards understanding the schizophrenia code: an expanded convergent functional genomics approach. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:129–158. doi: 10.1002/ajmg.b.30481. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, A X, L R. Indelebility of subcortical emotional memories. J Cognit Neurosci. 1989;1:238–243. doi: 10.1162/jocn.1989.1.3.238. [DOI] [PubMed] [Google Scholar]
- Lenz S, Perney TM, Qin Y, Robbins E, Chesselet MF. GABA-ergic interneurons of the striatum express the Shaw-like potassium channel Kv3.1. Synapse. 1994;18:55–66. doi: 10.1002/syn.890180108. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- Li HP, Homma A, Sango K, Kawamura K, Raisman G, Kawano H. Regeneration of nigrostriatal dopaminergic axons by degradation of chondroitin sulfate is accompanied by elimination of the fibrotic scar and glia limitans in the lesion site. J Neurosci Res. 2007;85:536–547. doi: 10.1002/jnr.21141. [DOI] [PubMed] [Google Scholar]
- Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112:159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay SL, Riddell JS, Barnett SC. Olfactory mucosa for transplant-mediated repair: a complex tissue for a complex injury? Glia. 2010;58:125–134. doi: 10.1002/glia.20917. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen PL, McGrath J, Wolyniec P, Fallin D, Nestadt G, Liang KY, Pulver A, Valle D, Avramopoulos D. Replication of an association of a common variant in the Reelin gene (RELN) with schizophrenia in Ashkenazi Jewish women. Psychiatr Genet. 2010;20:184–186. doi: 10.1097/YPG.0b013e32833a220b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WS, Harano M, Gawlik M, Yu Z, Chen J, Pun FW, Tong KL, Zhao C, Ng SK, Tsang SY, Uchimura N, Stober G, Xue H. GABRB2 association with schizophrenia: commonalities and differences between ethnic groups and clinical subtypes. Biol Psychiatry. 2007;61:653–660. doi: 10.1016/j.biopsych.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Longson D, Deakin JF, Benes FM. Increased density of entorhinal glutamate-immunoreactive vertical fibers in schizophrenia. J Neural Transm. 1996;103:503–507. doi: 10.1007/BF01276423. [DOI] [PubMed] [Google Scholar]
- Mackay-Sim A, St John JA. Olfactory ensheathing cells from the nose: clinical application in human spinal cord injuries. Exp Neurol. 2011;229:174–180. doi: 10.1016/j.expneurol.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Maeda N. Structural variation of chondroitin sulfate and its roles in the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10:22–31. doi: 10.2174/187152410790780136. [DOI] [PubMed] [Google Scholar]
- Maeda N, Hamanaka H, Oohira A, Noda M. Purification, characterization and developmental expression of a brain-specific chondroitin sulfate proteoglycan, 6B4 proteoglycan/phosphacan. Neuroscience. 1995;67:23–35. doi: 10.1016/0306-4522(94)00069-h. [DOI] [PubMed] [Google Scholar]
- Maloku E, Covelo IR, Hanbauer I, Guidotti A, Kadriu B, Hu Q, Davis JM, Costa E. Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci U S A. 2010;107:4407–4411. doi: 10.1073/pnas.0914483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-de-Souza D. Proteome and transcriptome analysis suggests oligodendrocyte dysfunction in schizophrenia. J Psychiatr Res. 2010;44:149–156. doi: 10.1016/j.jpsychires.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Martins-de-Souza D, Gattaz WF, Schmitt A, Maccarrone G, Hunyadi-Gulyas E, Eberlin MN, Souza GH, Marangoni S, Novello JC, Turck CW, Dias-Neto E. Proteomic analysis of dorsolateral prefrontal cortex indicates the involvement of cytoskeleton, oligodendrocyte, energy metabolism and new potential markers in schizophrenia. J Psychiatr Res. 2009;43:978–986. doi: 10.1016/j.jpsychires.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Kelly GM, Zerillo CA, Gray G, Tiemeyer M, Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauney S, Pantazopoulos H, Kim S, Berretta S, Woo T-UW. Society for Neuroscience. Washington D.C.: 2011. Perineuronal Net Abnormalities in the Prefrontal Cortex in Schizophrenia. [Google Scholar]
- McDonald AJ, Mascagni F. Differential expression of Kv3.1b and Kv3.2 potassium channel subunits in interneurons of the basolateral amygdala. Neuroscience. 2006;138:537–547. doi: 10.1016/j.neuroscience.2005.11.047. [DOI] [PubMed] [Google Scholar]
- McLaurin J, Fraser PE. Effect of amino-acid substitutions on Alzheimer’s amyloid-beta peptide-glycosaminoglycan interactions. Eur J Biochem. 2000;267:6353–6361. doi: 10.1046/j.1432-1327.2000.01725.x. [DOI] [PubMed] [Google Scholar]
- McRae PA, Rocco MM, Kelly G, Brumberg JC, Matthews RT. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J Neurosci. 2007;27:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Healy DJ. Glutamate receptor expression in schizophrenic brain. Brain Res Brain Res Rev. 2000;31:288–294. doi: 10.1016/s0165-0173(99)00044-2. [DOI] [PubMed] [Google Scholar]
- Meyer G. Building a human cortex: the evolutionary differentiation of Cajal-Retzius cells and the cortical hem. J Anat. 2010 doi: 10.1111/j.1469-7580.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Mitterauer B. Nonfunctional glial proteins in tripartite synapses: a pathophysiological model of schizophrenia. Neuroscientist. 2005;11:192–198. doi: 10.1177/1073858404265745. [DOI] [PubMed] [Google Scholar]
- Miyake N, Thompson J, Skinbjerg M, Abi-Dargham A. Presynaptic dopamine in schizophrenia. CNS Neurosci Ther. 2011;17:104–109. doi: 10.1111/j.1755-5949.2010.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Relationship between sprouting axons, proteoglycans and glial cells following unilateral nigrostriatal axotomy in the adult rat. Neuroscience. 2002;109:101–117. doi: 10.1016/s0306-4522(01)00457-2. [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Ohtsuka A. Perisynaptic barrier of proteoglycans in the mature brain and spinal cord. Arch Histol Cytol. 2003;66:195–207. doi: 10.1679/aohc.66.195. [DOI] [PubMed] [Google Scholar]
- Murray RM, Lappin J, Di Forti M. Schizophrenia: from developmental deviance to dopamine dysregulation. Eur Neuropsychopharmacol. 2008;18(Suppl 3):S129–134. doi: 10.1016/j.euroneuro.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Need AC, Ge D, Weale ME, Maia J, Feng S, Heinzen EL, Shianna KV, Yoon W, Kasperaviciute D, Gennarelli M, Strittmatter WJ, Bonvicini C, Rossi G, Jayathilake K, Cola PA, McEvoy JP, Keefe RS, Fisher EM, St Jean PL, Giegling I, Hartmann AM, Moller HJ, Ruppert A, Fraser G, Crombie C, Middleton LT, St Clair D, Roses AD, Muglia P, Francks C, Rujescu D, Meltzer HY, Goldstein DB. A genome-wide investigation of SNPs and CNVs in schizophrenia. PLoS Genet. 2009;5:e1000373. doi: 10.1371/journal.pgen.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, Togashi K, von Schimmelmann MJ, Lim CS, Maeda S, Yamashita N, Goshima Y, Ishii S, Hong K. Semaphorin 3A induces Ca(V)2.3 channel-dependent conversion of axons to dendrites. Nat Cell Biol. 2011;13:677–686. doi: 10.1038/ncb2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova SI, He F, Cutrufello NJ, Lidow MS. Identification of protein biomarkers for schizophrenia and bipolar disorder in the postmortem prefrontal cortex using SELDI-TOF-MS ProteinChip profiling combined with MALDI-TOF-PSD-MS analysis. Neurobiol Dis. 2006;23:61–76. doi: 10.1016/j.nbd.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Nutt CL, Matthews RT, Hockfield S. Glial tumor invasion: a role for the upregulation and cleavage of BEHAB/brevican. Neuroscientist. 2001;7:113–122. doi: 10.1177/107385840100700206. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Hagihara K, Suzuki M, Yamaguchi Y. Brevican in the developing hippocampal fimbria: differential expression in myelinating oligodendrocytes and adult astrocytes suggests a dual role for brevican in central nervous system fiber tract development. J Comp Neurol. 2001;432:285–295. doi: 10.1002/cne.1103. [DOI] [PubMed] [Google Scholar]
- Olney JW. Exitotoxic amino acids and neuropsychiatric disorders. Annu Rev Pharmacol Toxicol. 1990;30:47–71. doi: 10.1146/annurev.pa.30.040190.000403. [DOI] [PubMed] [Google Scholar]
- Oohira A, Matsui F, Tokita Y, Yamauchi S, Aono S. Molecular interactions of neural chondroitin sulfate proteoglycans in the brain development. Arch Biochem Biophys. 2000;374:24–34. doi: 10.1006/abbi.1999.1598. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Arnold SE, Berretta S. Society for Neuroscience. Washington D.C.: 2011. Extracellular matrix abnormalities in olfactory epithelium tissue from schizophrenic subjects. [Google Scholar]
- Pantazopoulos H, Lange N, Baldessarini RJ, Berretta S. Parvalbumin neurons in the entorhinal cortex of subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;61:640–652. doi: 10.1016/j.biopsych.2006.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Lange N, Hassinger L, Berretta S. Subpopulations of neurons expressing parvalbumin in the human amygdala. J Comp Neurol. 2006;496:706–722. doi: 10.1002/cne.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Murray EA, Berretta S. Total number, distribution, and phenotype of cells expressing chondroitin sulfate proteoglycans in the normal human amygdala. Brain Res. 2008;1207:84–95. doi: 10.1016/j.brainres.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Woo T-UW, Lim MP, Lange N, Berretta S. Extracellular Matrix-Glial Abnormalities in the Amygdala and Entorhinal Cortex of Subjects Diagnosed With Schizophrenia. Arch Gen Psychiatry. 2010;67:155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W, Baur I, Dours-Zimmermann MT, Zimmermann DR. Differential expression of versican isoforms in brain tumors. J Neuropathol Exp Neurol. 1996;55:528–533. doi: 10.1097/00005072-199605000-00005. [DOI] [PubMed] [Google Scholar]
- Pennington K, Dicker P, Dunn MJ, Cotter DR. Proteomic analysis reveals protein changes within layer 2 of the insular cortex in schizophrenia. Proteomics. 2008;8:5097–5107. doi: 10.1002/pmic.200800415. [DOI] [PubMed] [Google Scholar]
- Perney TM, Marshall J, Martin KA, Hockfield S, Kaczmarek LK. Expression of the mRNAs for the Kv3.1 potassium channel gene in the adult and developing rat brain. J Neurophysiol. 1992;68:756–766. doi: 10.1152/jn.1992.68.3.756. [DOI] [PubMed] [Google Scholar]
- Perrone Bizzozero NI, Sower AC, Bird ED, Benowitz LI, Ivins KJ, Neve RL. Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci U S A. 1996;93:14182–14187. doi: 10.1073/pnas.93.24.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesold C, Liu WS, Guidotti A, Costa E, Caruncho HJ. Cortical bitufted, horizontal, and Martinotti cells preferentially express and secrete reelin into perineuronal nets, nonsynaptically modulating gene expression. Proc Natl Acad Sci U S A. 1999;96:3217–3222. doi: 10.1073/pnas.96.6.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaton G, Aigrot MS, Williams A, Moyon S, Tepavcevic V, Moutkine I, Gras J, Matho KS, Schmitt A, Soellner H, Huber AB, Ravassard P, Lubetzki C. Class 3 semaphorins influence oligodendrocyte precursor recruitment and remyelination in adult central nervous system. Brain. 2011;134:1156–1167. doi: 10.1093/brain/awr022. [DOI] [PubMed] [Google Scholar]
- Pisante A, Bronstein M, Yakir B, Darvasi A. A variant in the reelin gene increases the risk of schizophrenia and schizoaffective disorder but not bipolar disorder. Psychiatr Genet. 2009;19:212. doi: 10.1097/YPG.0b013e32832cebe6. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T. Neuroscience. Erasing fear memories. Science. 2009;325:1214–1215. doi: 10.1126/science.1179697. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, Karp NA, Hester S, Tkachev D, Mimmack ML, Yolken RH, Webster MJ, Torrey EF, Bahn S. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697. 643. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Pyka M, Busse C, Seidenbecher C, Gundelfinger ED, Faissner A. Astrocytes are crucial for survival and maturation of embryonic hippocampal neurons in a neuron-glia cell-insert coculture assay. Synapse. 2011;65:41–53. doi: 10.1002/syn.20816. [DOI] [PubMed] [Google Scholar]
- Raisman G. Olfactory ensheathing cells and repair of brain and spinal cord injuries. Cloning Stem Cells. 2004;6:364–368. doi: 10.1089/clo.2004.6.364. [DOI] [PubMed] [Google Scholar]
- Ramos-Moreno T, Galazo MJ, Porrero C, Martinez-Cerdeno V, Clasca F. Extracellular matrix molecules and synaptic plasticity: immunomapping of intracellular and secreted Reelin in the adult rat brain. Eur J Neurosci. 2006;23:401–422. doi: 10.1111/j.1460-9568.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- Rauch U. Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci. 2004;61:2031–2045. doi: 10.1007/s00018-004-4043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch U, Zhou XH, Roos G. Extracellular matrix alterations in brains lacking four of its components. Biochem Biophys Res Commun. 2005;328:608–617. doi: 10.1016/j.bbrc.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch KP. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- Reynolds GP. Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature. 1983;305:527–529. doi: 10.1038/305527a0. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Czudek C, Andrews HB. Deficit and hemispheric asymmetry of GABA uptake sites in the hippocampus in schizophrenia. Biol Psychiatry. 1990;27:1038–1044. doi: 10.1016/0006-3223(90)90039-5. [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat. 2004;204:33–48. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EM, McGovern D, Mockler D, Doku VC, S OC, Fannon DG, Tennakoon L, Santamaria M, Soni W, Morris RG, Sharma T. Neuropsychological functioning in first-episode psychosis--evidence of specific deficits. Schizophr Res. 2000;43:47–55. doi: 10.1016/s0920-9964(99)00177-2. [DOI] [PubMed] [Google Scholar]
- Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ. Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry. 2000;57:349–356. doi: 10.1001/archpsyc.57.4.349. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–492. doi: 10.1093/glycob/6.5.489. [DOI] [PubMed] [Google Scholar]
- Saghatelyan AK, Dityatev A, Schmidt S, Schuster T, Bartsch U, Schachner M. Reduced perisomatic inhibition, increased excitatory transmission, and impaired long-term potentiation in mice deficient for the extracellular matrix glycoprotein tenascin-R. Mol Cell Neurosci. 2001;17:226–240. doi: 10.1006/mcne.2000.0922. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Wilczek K, Blennow K, Maras A, Jatzko A, Petroianu G, Braus DF, Gattaz WF. Altered thalamic membrane phospholipids in schizophrenia: a postmortem study. Biol Psychiatry. 2004;56:41–45. doi: 10.1016/j.biopsych.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Seeger G, Luth HJ, Winkelmann E, Brauer K. Distribution patterns of Wisteria floribunda agglutinin binding sites and parvalbumin-immunoreactive neurons in the human visual cortex: a double-labelling study. J Hirnforsch. 1996;37:351–366. [PubMed] [Google Scholar]
- Segal M, Vlachos A, Korkotian E. The spine apparatus, synaptopodin, and dendritic spine plasticity. Neuroscientist. 2010;16:125–131. doi: 10.1177/1073858409355829. [DOI] [PubMed] [Google Scholar]
- Shifman S, Johannesson M, Bronstein M, Chen SX, Collier DA, Craddock NJ, Kendler KS, Li T, O’Donovan M, O’Neill FA, Owen MJ, Walsh D, Weinberger DR, Sun C, Flint J, Darvasi A. Genome-wide association identifies a common variant in the reelin gene that increases the risk of schizophrenia only in women. PLoS Genet. 2008;4:e28. doi: 10.1371/journal.pgen.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinagra M, Verrier D, Frankova D, Korwek KM, Blahos J, Weeber EJ, Manzoni OJ, Chavis P. Reelin, very-low-density lipoprotein receptor, and apolipoprotein E receptor 2 control somatic NMDA receptor composition during hippocampal maturation in vitro. J Neurosci. 2005;25:6127–6136. doi: 10.1523/JNEUROSCI.1757-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalla KH, Mikhaylova M, Sahin J, Bernstein HG, Bogerts B, Schmitt A, van der Schors R, Smit AB, Li KW, Gundelfinger ED, Kreutz MR. A comparison of the synaptic proteome in human chronic schizophrenia and rat ketamine psychosis suggest that prohibitin is involved in the synaptic pathology of schizophrenia. Mol Psychiatry. 2008;13:878–896. doi: 10.1038/mp.2008.60. [DOI] [PubMed] [Google Scholar]
- Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- Sobel RA, Ahmed AS. White matter extracellular matrix chondroitin sulfate/dermatan sulfate proteoglycans in multiple sclerosis. J Neuropathol Exp Neurol. 2001;60:1198–1207. doi: 10.1093/jnen/60.12.1198. [DOI] [PubMed] [Google Scholar]
- Sokolov BP. Oligodendroglial abnormalities in schizophrenia, mood disorders and substance abuse. Comorbidity, shared traits, or molecular phenocopies? Int J Neuropsychopharmacol. 2007;10:547–555. doi: 10.1017/S1461145706007322. [DOI] [PubMed] [Google Scholar]
- Sometani A, Kataoka H, Nitta A, Fukumitsu H, Nomoto H, Furukawa S. Transforming growth factor-beta1 enhances expression of brain-derived neurotrophic factor and its receptor, TrkB, in neurons cultured from rat cerebral cortex. J Neurosci Res. 2001;66:369–376. doi: 10.1002/jnr.1229. [DOI] [PubMed] [Google Scholar]
- Spreafico R, De Biasi S, Vitellaro-Zuccarello L. The perineuronal net: a weapon for a challenge. J Hist Neurosci. 1999;8:179–185. doi: 10.1076/jhin.8.2.179.1834. [DOI] [PubMed] [Google Scholar]
- Stewart DG, Davis KL. Possible contributions of myelin and oligodendrocyte dysfunction to schizophrenia. Int Rev Neurobiol. 2004;59:381–424. doi: 10.1016/S0074-7742(04)59015-3. [DOI] [PubMed] [Google Scholar]
- Stichel CC, Kappler J, Junghans U, Koops A, Kresse H, Mu®ller HW. Differential expression of the small chondroitin/dermatan sulfate proteoglycans decorin and biglycan after injury of the adult rat brain. Brain Research. 1995;704:263–274. doi: 10.1016/0006-8993(95)01131-5. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Sun J, Jia P, Fanous AH, van den Oord E, Chen X, Riley BP, Amdur RL, Kendler KS, Zhao Z. Schizophrenia gene networks and pathways and their applications for novel candidate gene selection. PLoS One. 2010;5:e11351. doi: 10.1371/journal.pone.0011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson TH. Dysfunctional brain dopamine systems induced by psychotomimetic NMDA-receptor antagonists and the effects of antipsychotic drugs. Brain Res Brain Res Rev. 2000;31:320–329. doi: 10.1016/s0165-0173(99)00048-x. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed YA, Hand E, Mobius W, Zhao C, Hofer M, Nave KA, Kotter MR. Inhibition of CNS remyelination by the presence of semaphorin 3A. J Neurosci. 2011;31:3719–3728. doi: 10.1523/JNEUROSCI.4930-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykova E. Diffusion properties of the brain in health and disease. Neurochem Int. 2004a;45:453–466. doi: 10.1016/j.neuint.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Sykova E. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience. 2004b;129:861–876. doi: 10.1016/j.neuroscience.2004.06.077. [DOI] [PubMed] [Google Scholar]
- Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K, Koizumi S, Wakabayashi K, Takahashi H, Someya T, Nawa H. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry. 2000;5:293–300. doi: 10.1038/sj.mp.4000718. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T, Bozdagi-Gunal O, Dorr NP, Moy J, Krug L, Gama-Sosa M, Elder GA, Koch RJ, Walker RH, Hof PR, Davis KL, Buxbaum JD. Increased expression of receptor phosphotyrosine phosphatase-[beta]/[zeta] is associated with molecular, cellular, behavioral and cognitive schizophrenia phenotypes. Transl Psychiatry. 2011a;1:e8. doi: 10.1038/tp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011b;93:13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011;31:6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Dunaevsky A, Blazeski R, Mason CA, Yuste R. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors: a two-step model of synaptogenesis. Neuron. 2003;38:773–784. doi: 10.1016/s0896-6273(03)00299-x. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Goffinet AM. Reelin and brain development. Nat Rev Neurosci. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Uranova N, Orlovskaya D, Vikhreva O, Zimina I, Kolomeets N, Vostrikov V, Rachmanova V. Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull. 2001;55:597–610. doi: 10.1016/s0361-9230(01)00528-7. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Casanova MF, DeVaughn NM, Orlovskaya DD, Denisov DV. Ultrastructural alterations of synaptic contacts and astrocytes in postmortem caudate nucleus of schizophrenic patients [letter] Schizophr Res. 1996;22:81–83. doi: 10.1016/0920-9964(96)00059-x. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Wesseling P, van den Heuvel LP, de Waal RM, Verbeek MM. Heparan sulphate proteoglycans in Alzheimer’s disease and amyloid-related disorders. Lancet Neurol. 2003;2:482–492. doi: 10.1016/s1474-4422(03)00484-8. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Frye MA, Hemperly JJ, VanderPutten DM, Usen N, Doherty P, Saffell JL, Issa F, Post RM, Wyatt RJ, Freed WJ. Elevated concentration of N-CAM VASE isoforms in schizophrenia. J Psychiatr Res. 2000;34:25–34. doi: 10.1016/s0022-3956(99)00026-6. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Usen N, Thatcher L, Ladenheim B, Zhang P, VanderPutten DM, Conant K, Herman MM, van Kammen DP, Sedvall G, Garver DL, Freed WJ. Characterization of human cleaved N-CAM and association with schizophrenia. Exp Neurol. 2001;172:29–46. doi: 10.1006/exnr.2001.7790. [DOI] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viapiano MS, Matthews RT. From barriers to bridges: chondroitin sulfate proteoglycans in neuropathology. Trends Mol Med. 2006;12:488–496. doi: 10.1016/j.molmed.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Vitellaro-Zuccarello L, Bosisio P, Mazzetti S, Monti C, De Biasi S. Differential expression of several molecules of the extracellular matrix in functionally and developmentally distinct regions of rat spinal cord. Cell Tissue Res. 2007;327:433–447. doi: 10.1007/s00441-006-0289-y. [DOI] [PubMed] [Google Scholar]
- Vitellaro-Zuccarello L, Meroni A, Amadeo A, De Biasi S. Chondroitin sulfate proteoglycans in the rat thalamus: expression during postnatal development and correlation with calcium-binding proteins in adults. Cell Tissue Res. 2001;306:15–26. doi: 10.1007/s004410100425. [DOI] [PubMed] [Google Scholar]
- Vivien D, Ali C. Transforming growth factor-beta signalling in brain disorders. Cytokine Growth Factor Rev. 2006;17:121–128. doi: 10.1016/j.cytogfr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508. doi: 10.1007/7854_2010_44. [DOI] [PubMed] [Google Scholar]
- Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci. 2011;31:9332–9344. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Someya T, Nawa H. Cytokine hypothesis of schizophrenia pathogenesis: evidence from human studies and animal models. Psychiatry Clin Neurosci. 2010;64:217–230. doi: 10.1111/j.1440-1819.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- Wedenoja J, Loukola A, Tuulio-Henriksson A, Paunio T, Ekelund J, Silander K, Varilo T, Heikkila K, Suvisaari J, Partonen T, Lonnqvist J, Peltonen L. Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families. Mol Psychiatry. 2008;13:673–684. doi: 10.1038/sj.mp.4002047. [DOI] [PubMed] [Google Scholar]
- Wegner F, Hartig W, Bringmann A, Grosche J, Wohlfarth K, Zuschratter W, Bruckner G. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABA(A) receptor alpha1 subunit form a unique entity in rat cerebral cortex. Exp Neurol. 2003;184:705–714. doi: 10.1016/S0014-4886(03)00313-3. [DOI] [PubMed] [Google Scholar]
- Weiser M, Bueno E, Sekirnjak C, Martone ME, Baker H, Hillman D, Chen S, Thornhill W, Ellisman M, Rudy B. The potassium channel subunit KV3.1b is localized to somatic and axonal membranes of specific populations of CNS neurons. J Neurosci. 1995;15:4298–4314. doi: 10.1523/JNEUROSCI.15-06-04298.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins-Port CE, Higgins PJ. Regulation of extracellular matrix remodeling following transforming growth factor-beta1/epidermal growth factor-stimulated epithelial-mesenchymal transition in human premalignant keratinocytes. Cells Tissues Organs. 2007;185:116–122. doi: 10.1159/000101312. [DOI] [PubMed] [Google Scholar]
- Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18:173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LJ, Schnaar RL. Axon regeneration inhibitors. Neurol Res. 2008;30:1047–1052. doi: 10.1179/174313208X362523. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fung SJ, Rothwell A, Tianmei S, Weickert CS. Increased interstitial white matter neuron density in the dorsolateral prefrontal cortex of people with schizophrenia. Biol Psychiatry. 2011;69:63–70. doi: 10.1016/j.biopsych.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Chen J, Shi H, Stoeber G, Tsang SY, Xue H. Analysis of GABRB2 association with schizophrenia in German population with DNA sequencing and one-label extension method for SNP genotyping. Clin Biochem. 2006;39:210–218. doi: 10.1016/j.clinbiochem.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zaharieva I, Georgieva L, Nikolov I, Kirov G, Owen MJ, O’Donovan MC, Toncheva D. Association study in the 5q31-32 linkage region for schizophrenia using pooled DNA genotyping. BMC Psychiatry. 2008;8:11. doi: 10.1186/1471-244X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- Zhao S, Frotscher M. Go or stop? Divergent roles of Reelin in radial neuronal migration. Neuroscientist. 2010;16:421–434. doi: 10.1177/1073858410367521. [DOI] [PubMed] [Google Scholar]
- Zimmer G, Schanuel SM, Burger S, Weth F, Steinecke A, Bolz J, Lent R. Chondroitin sulfate acts in concert with semaphorin 3A to guide tangential migration of cortical interneurons in the ventral telencephalon. Cereb Cortex. 2010;20:2411–2422. doi: 10.1093/cercor/bhp309. [DOI] [PubMed] [Google Scholar]
- Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]
- Zuber B, Nikonenko I, Klauser P, Muller D, Dubochet J. The mammalian central nervous synaptic cleft contains a high density of periodically organized complexes. Proc Natl Acad Sci U S A. 2005;102:19192–19197. doi: 10.1073/pnas.0509527102. [DOI] [PMC free article] [PubMed] [Google Scholar]