Abstract
During angiogenesis, αv integrins are overexpressed on the endothelial cell surface to facilitate the growth and survival of newly forming vessels. Accordingly, blocking αv integrin function by disrupting ligand binding can produce an antiangiogenic effect. Although the integrin ectodomain regulates ligand binding specificity, the short cytoplasmic tail facilitates intracellular signaling pathways through the recruitment and activation of specific kinases and signaling intermediates. This in turn controls endothelial cell adhesion, morphology, migration, invasion, proliferation, and survival. These same integrin-mediated signaling pathways are exploited in cancer to promote the invasiveness and survival of tumor cells and to manipulate the host microenvironment to provide ample blood vessel and stromal resources to support tumor growth and metastatic spread. Because expression of αv integrins on distinct cell types contributes to cancer growth, αv integrin antagonists have the potential to disrupt multiple aspects of disease progression.
Vessel formation in tumors is mediated by αv integrins on the endothelial cell surface. αv integrin antagonists may disrupt cancer progression, especially when used with chemotherapy or radiotherapy.
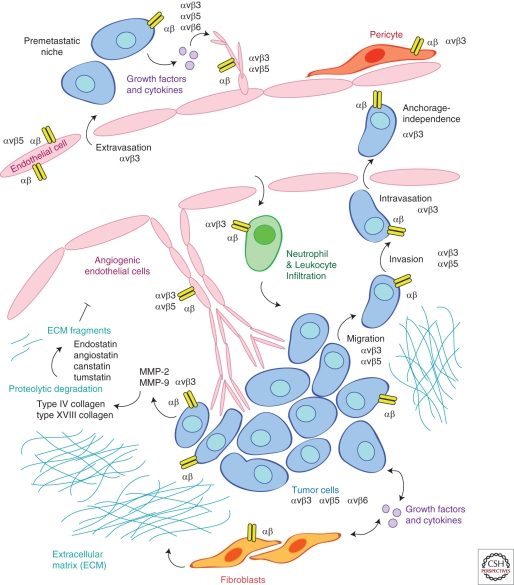
Integrins are transmembrane receptors that bind extracellular matrix proteins or other adhesion receptors on neighboring cells. Heterodimeric pairing of integrin α and β subunits confers specificity of binding to one or more substrates (see Humphries et al. 2006 for review). In particular, the αv subunit pairs with β1, β3, β5, β6, and β8 (Luo et al. 2007). Whereas some pairings preferentially bind a single ligand (αvβ5 for vitronectin), others recognize a number of ligands (αvβ3 binds vitronectin, fibronectin, vWF, tenascin, osteopontin, fibrillin, fibrinogen, and thrombospondin). Because the integrins expressed on the surface of a cell will determine whether it can adhere to and survive in a particular microenvironment, the matching of integrins and ligands plays a key role in the regulation of the sprouting ability of endothelial cells during angiogenesis, localization of inflammatory cells recruited to sites of repair, or the invasive potential of tumor cells. The αv integrins appear to be particularly important during the tissue remodeling associated with wound repair, angiogenesis, and cancer. Therefore, this article will focus on how the expression and function of αv integrins impacts tumor-associated angiogenesis and the growth and progression of tumors (Fig. 1).
Figure 1.
αv integrins expressed on multiple cell types contribute to angiogenesis and tumor progression. Sprouting endothelial cells express a unique profile of integrins that can be targeted to suppress vascular proliferation. Pericyte coverage of maturing blood vessels is influenced by integrin adhesion to extracellular matrix proteins present within the remodeling tissue. Tumor cells change integrin expression profiles to enhance their ability to migrate, invade, metastasize, and survive in hostile environments. Integrin signaling in fibroblasts directs synthesis of extracellular matrix proteins and growth factors that flood the tumor stroma. Proteolytic degradation of extracellular matrix proteins creates fragments that can bind to and inhibit the function of integrins expressed by angiogenic endothelial cells. Integrins on inflammatory cells participate in recruitment to sites of angiogenesis and remodeling, and can establish a premetastatic niche to promote metastatic spread to distant sites. In summary, integrin expression profiles of normal cells are distinct to those within remodeling tissues. Because integrin signaling pathways can influence the behavior of multiple cell types involved in angiogenesis and cancer, selective targeting of integrin-mediated adhesion and signaling represents an attractive therapeutic strategy.
INTEGRINS IN ANGIOGENESIS
αv Integrins Represent a Family of RGD-Binding Integrins
Thirty years ago, the arginine-glycine-aspartic acid (RGD) sequence present on certain matrix proteins (such as fibronectin, vitronectin, osteopontin, collagens, thrombospondin, fibrinogen, and von Willebrand factor) was identified as a ligand for integrins such as αvβ3, αvβ5, and α5β1 (Pierschbacher and Ruoslahti 1984a,b; Pytela et al. 1985a,b; Argraves et al. 1986; Suzuki et al. 1986). Because integrin-ligand binding can be mimicked with synthetic peptides containing the RGD sequence, exposing cells to a soluble cyclic RGD peptide competitively disrupts this binding and perturbs integrin-mediated signaling pathways. Considering that integrin-mediated attachment to substrate provides critical cues for cellular signaling pathways, RGD peptides have shown application as tools to both investigate integrin function and to regulate function therapeutically. However, many integrins do not recognize their ligand in the context of the RGD sequence but nevertheless have been shown to play a significant role in angiogenesis and cancer.
Expression of αvβ3 on Angiogenic Blood Vessels
Whereas many integrins are ubiquitously expressed in adult tissues, integrin αvβ3 is most abundantly expressed on angiogenic endothelial cells in remodeling and pathological tissues (Brooks et al. 1995b), and its expression is mediated by a transcriptional activator, Hox D3 (Boudreau et al. 1997). Discovery of this unique expression pattern has led to the development of multiple strategies for imaging, detecting, and treating angiogenesis-related diseases. Because αvβ3 is expressed by angiogenic endothelial cells (but not normal quiescent endothelial cells), treatment with either a cyclic RGD peptide or an αvβ3-specific monoclonal antibody such as LM609 can disrupt the invasive and proliferative program of sprouting endothelial cells and suppress angiogenesis (Brooks et al. 1994a,b, 1995a; Drake et al. 1995). In addition to targeting angiogenesis directly, many strategies have used the selective expression of αvβ3 to deliver imaging or therapeutic agents to angiogenic vascular beds. RGD-targeted nanoparticles accumulate within tumor-associated blood vessels, but show little binding to other vascular beds (Murphy et al. 2008). Thus, intravenously injected RGD-targeted nanoparticles are capable of delivering a payload of antiangiogenic or antitumor agents to a tumor while sparing normal tissues. Delivery of antiangiogenic agents to tumor-associated blood vessels can induce a reduction or even regression of solid tumor growth (Hood et al. 2002). Targeted delivery of chemotherapeutics can significantly reduce the dose required to achieve a desirable anticancer effect, dramatically limiting the toxic side effects of these drugs (Murphy et al. 2008). The utility of αvβ3 as a marker of angiogenic endothelial cells is not exclusive to cancer, because αvβ3 expression is a general feature of endothelial cell activation. Thus, active endothelial cells are sensitive to αvβ3 inhibition during such processes as wound repair (Clark et al. 1996), arthritis (Storgard et al. 1999), and proliferative diabetic retinopathy (Friedlander et al. 1996).
Integrins αvβ3 and αvβ5 Promote Distinct Pathways of Angiogenesis
Although αvβ3 expression is observed on all angiogenic endothelial cells, the involvement of αvβ3 with angiogenic signaling pathways is not necessarily generic. The development of integrin function blocking antibodies and antagonists revealed that these agents show preferential effects in certain pathological models. These experiments led to the observation that two cytokine-dependent pathways of angiogenesis exist defined by their requirement for the function of distinct αv integrins. Angiogenesis induced by basic fibroblast growth factor (bFGF) or tumor necrosis factor α (TNF-α) requires the function of integrin αvβ3, whereas angiogenesis induced by vascular endothelial growth factor (VEGF) or transforming growth factor α (TGF-α) requires the function of integrin αvβ5 (Friedlander et al. 1995). This distinction is a function of the differential ability of β3 and β5 integrins to activate the Ras/Raf/MEK/Erk pathway in blood vessels (Hood et al. 2003). Specifically, the αvβ5 integrin pathway downstream of VEGF leads to activation of focal adhesion kinase (FAK) and Src kinase, whereas the αvβ3 pathway involves p21-activated kinase (PAK) (Hood et al. 2003). Furthermore, the αvβ5 pathway results in activation of Raf on serines 338/339 which leads to Raf-1 mitochondrial translocation and endothelial cell protection from the intrinsic pathway of apoptosis (induced by stress or DNA damaging agents), independent of MEK1 (Alavi et al. 2003). In contrast, αvβ3 signaling activates Raf on tyrosines 340/341 and MEK1-dependent protection from extrinsic-mediated apoptosis (induced by receptor binding to proapoptotic death ligands such as TNF-α and Fas) (Alavi et al. 2003). The differences between the αvβ3 and αvβ5 signaling pathways exemplify how the local expression of integrins and extracellular matrix ligands can confer specificity and drive distinct cellular behaviors.
β3 Integrin Knockout and Knock-in Mouse Models
Genetic ablation of integrin β3 produces the expected bleeding phenotype because of loss of αIIbβ3 required for platelet clotting (Hodivala-Dilke et al. 1999). However, in contrast to β3 antagonist studies, β3 knockout mice show the unexpected phenotype of enhanced angiogenesis and tumor growth (Reynolds et al. 2002; Robinson et al. 2004), as well as elevated atherosclerosis (Weng et al. 2003) and wound healing (Reynolds et al. 2005) responses. Instead of reflecting the true role of β3 in vivo, these observations were determine to be a consequence of compensatory increased expression and activity of VEGFR2 (Reynolds et al. 2004). During vascular development, β3 expression is lost as the vasculature matures. In the heart for example, β3 expression decreases significantly during the first weeks of life after the cardiac blood vessels remodel to support the demands of the growing heart (Weis et al. 2007). In mice lacking β3, the capillaries in the heart fail to mature and retain the luminal protrusions and vacuoles characteristic of immature or activated endothelium (Weis et al. 2007). As expected, this phenotype was a function of VEGF hypersensitivity, as VEGF receptor antagonism normalized the vascular aberrations while application of exogenous VEGF induced similar features in capillaries in the hearts of wild-type mice (Weis et al. 2007).
Mice expressing a mutant β3 unable to undergo tyrosine phosphorylation (DiYF knock-in mice) do not show compensatory changes in VEGF signaling (Mahabeleshwar et al. 2006), and thus show a suppression of angiogenesis consistent with the β3 antagonism studies. Importantly, a molecular complex between VEGFR2 and β3 is observed in angiogenic blood vessels in vivo, and requires activation of both VEGFR2 and β3 (Mahabeleshwar et al. 2008). Consistent with the idea that bone marrow-derived cells drive angiogenesis (Ziegelhoeffer et al. 2004; Grunewald et al. 2006), the angiogenic defect in the DiYF mice can be rescued by bone marrow transplantation (Feng et al. 2008). In particular, β3 integrin expression on bone marrow-derived cells is required for their recruitment and retention at sites of angiogenic remodeling, but not for their initial release from the bone marrow niche (Feng et al. 2008). These studies suggest that the antiangiogenic capacity of β3 inhibitors is likely a function of targeting β3 on a number of cell types involved in vascular remodeling.
Most recently, “floxed” mice with the conditional deletion of β3 integrin in either platelets or myeloid cells have been generated (Morgan et al. 2009). As expected, β3 knockout in platelets leads to a bleeding phenotype but no effect on tumor growth and angiogenesis, whereas β3 knockout in myeloid cells produced osteopetrosis similar to that observed in the global β3 knockout mice (Morgan et al. 2009). Future work with this new mouse model will allow testing the function of β3 in additional cell types relevant for angiogenesis and the tumor host response.
Integrins and Neurovascular Patterning
Blood vessels and neurons are known to copattern, and cell surface adhesion molecules represent one means to facilitate their interaction during this process. Accordingly, the communication between neuronal and vascular cell types during remodeling involves integrins expressed on these cell types (McCarty 2009). For example, deletion of αv integrins from neuronal cell lineages leads to vascular defects (McCarty et al. 2005), because αvβ8 expressed on glial cells regulates vascular development in the brain (Zhu et al. 2002). Netrins are secreted neuronal guidance molecules that bind directly to integrins to regulate cell migration during angiogenesis (Nikolopoulos and Giancotti 2005). Semaphorin3A is another secreted protein known for its role in neuronal guidance that also functions as a negative regulator of integrin function (Serini et al. 2003). Studies of Semaphorin3A knockout mice revealed that the dynamic regulation of integrins by spatiotemporal expression of Semaphorin3A is required for proper developmental and pathological angiogenesis (Serini et al. 2003). Exogenous Semaphorin3A blocks VEGF-mediated angiogenesis (Acevedo et al. 2008) and normalizes tumor vasculature (Maione et al. 2009). Additional Semaphorin family members share the ability to modulate integrin function. Semaphorin3C, binds integrins directly and generates intracellular signals that modulate integrin function (Serini et al. 2008), whereas Semaphorin4A inhibits angiogenesis because of its ability to suppress VEGF-mediated Rac activation and integrin-dependent cell adhesion (Toyofuku et al. 2007).
Growth Factors and Growth Factor Receptors
New evidence suggests that integrins directly bind to a number of growth factors. For example, α9β1, α3β1, and αvβ3 integrins directly bind several VEGF isoforms (Hutchings et al. 2003; Rahman et al. 2005) and a handful of different integrins bind angiopoietin (Serini et al. 2008). Furthermore, a number of growth factor receptors can be stimulated via their interaction with integrins even in the absence of growth factor signaling (Giancotti and Tarone 2003). For example, αvβ3 and VEGFR2 form a physical complex and synergize to promote angiogenesis induced by either ECM or growth factor ligand binding (Mahabeleshwar et al. 2007), and α5β1 forms a complex with the angiopoietin receptor Tie-2 to amplify angiogenic signals (Cascone et al. 2005). In contrast, expression of α2β1 increases VEGFR1 expression, which acts in opposition to the proangiogenic VEGFR2 signals (Zhang et al. 2008). Because integrins bind to select growth factors and growth factor receptors, perturbing integrin function likely alters the balance of signaling pathways on multiple cell types involved in the angiogenic cascade.
Integrin Internalization and Recycling
Because integrins function as cell surface receptors, their membrane expression levels, and extent of clustering will determine their availability to bind ligands. Accordingly, trafficking of integrins to and from the cell surface has a powerful influence over integrin function (Caswell et al. 2009). Integrin internalization often involves clathrin or caveolin along with their related adaptors. To allow a cell to move, the integrins associated with focal adhesions undergo endocytosis in a clathrin-dependent manner (Ezratty et al. 2009). Integrin trafficking can also modulate the internalization of growth factor receptors such as EGFR (Caswell et al. 2008) or VEGFR (Reynolds et al. 2009). For example, low nanomolar doses of an αvβ3 antagonist activate the Rab4 pathway, which promotes the rapid recycling of internalized VEGFR2 (Reynolds et al. 2009). This rapid recycling prevents VEGFR2 degradation and shuttles VEGFR2 back to the plasma membrane, thus amplifying the cellular response to VEGF.
Because RGD integrin-binding peptides are rapidly internalized by endothelial cells, RGD-targeted nanoparticles have gained popularity for delivery of therapeutic and imaging agents to tumor-associated endothelial cells. In addition, a new internalizing-RGD (iRGD) peptide has the ability to home to tumor-associated blood vessels within minutes (by targeting αvβ3) and then proceed to shuttle into the tumor microenvironment within several hours (Sugahara et al. 2009). The tissue penetration properties of iRGD are mediated by a cryptic C-end rule (CendR) motif that is proteolytically cleaved to allow binding to neuropilin-1, a receptor for VEGF. Previous studies have shown that neuropilin-1 and αvβ3 associate in a VEGF-dependent manner, and that the presence of αvβ3 on endothelial cells limits the participation of neuropilin-1 during VEGF-mediated angiogenesis (Robinson et al. 2009). Neuropilin-1 also regulates the internalization of α5β1 integrin in endothelial cells (Valdembri et al. 2009) and the expression levels of α2β1 in breast carcinoma cells (Pan et al. 2009). It is therefore possible that the iRGD internalization process may have some additional impact on the function of the β1 and β3 integrins present within angiogenic blood vessels.
Endogenous Inhibitors of Angiogenesis
During physiological or pathological angiogenesis, a balance of pro- and antiangiogenic factors determines the progress of vascular remodeling. Whereas integrin binding to the extracellular matrix promotes adhesion and survival by activating canonical integrin-mediated signaling pathways, integrin binding to soluble fragments acts as a decoy to disrupt the physical connection and suppress the signaling events that lead to cell survival, migration, and proliferation. A number of enzyme or ECM fragments have the ability to act as endogenous inhibitors of angiogenesis through their ability to disrupt integrin-mediated adhesion and signaling (Nyberg et al. 2005; Folkman 2006; Ribatti 2009). For example, Angiostatin is a plasmin fragment that binds αvβ3 (Tarui et al. 2001), Canstatin is a type IV collagen fragment that binds αvβ3 and αvβ5 (Magnon et al. 2005), Endostatin is a collagen XVIII fragment that binds αvβ3, αvβ5, and α5β1 (Rehn et al. 2001), and Tumstatin is a type IV collagen fragment that binds αvβ3 and α5β1 (Sudhakar et al. 2003). Proteolytic degradation of specific extracellular matrix proteins can therefore produce a variety of soluble fragments that can perturb the function of specific integrins, to provide control over the location and amount of angiogenic remodeling that occurs in very distinct microenvironments (Fig. 1).
In addition to soluble matrix fragments binding to and modulating integrin function, the expression of integrins can also be controlled at the mRNA level. MicroRNAs (or miRs) are short RNA sequences that bind to, recruit a silencing complex, and block translation of specific sites on the 3′ untranslated region (3′UTR) of target genes. This process lends itself to many levels of control, because a single miR can suppress expression of multiple genes, and a single gene can be regulated by a number of different miRs. A recent review lists potential miR interactions with cell adhesion molecules, angiogenic factors, and metalloproteinases (Dalmay and Edwards 2006). Although only a handful of miRs have been experimentally linked to integrins to date, there are numerous predicted miR binding sites on integrin 3′UTRs that are well preserved between species. Some of these miR/integrin pairings have been validated experimentally. For example, both miR-92a and mikR-31 suppress expression of integrin α5 (Bonauer et al. 2009; Valastyan et al. 2009), and miR-124 blocks expression of β1 integrin (Cao et al. 2007). During melanoma progression, loss of miR-let-7a contributes to increased β3 expression and the acquisition of an invasive phenotype (Muller and Bosserhoff 2008). Although miR-mediated regulation of integrin gene expression has yet to be fully appreciated, it could provide a unique means to investigate mechanistic aspects of integrin function and/or lead to novel therapeutic strategies. This relationship is especially interesting because a number of pro and antiangiogenic microRNAs have been experimentally validated to date (Kuehbacher et al. 2008; Fish and Srivastava 2009; Suarez and Sessa 2009).
αv INTEGRINS IN CANCER
Many integrins including αvβ3, αvβ5, αvβ6, α2β1, α5β1, α6β1, and α6β4 have been implicated in cancer growth and invasion. Because a number of these have recently been reviewed (Lu et al. 2008; Bandyopadhyay and Raghavan 2009; Caccavari et al. 2010; Sroka et al. 2010), this section will focus primarily on αvβ3 and αvβ5.
Tumor Cell Expression of Integrins and Integrin Ligands Drives Angiogenesis
There are multiple examples of how tumor cells can manipulate the host stroma, and it is likely that the integrin expression profile of a given tumor cell can influence the angiogenic response within the tumor microenvironment. For example, expression of β3 in metastatic prostate cancer cells drives angiogenesis by driving tumor cell production and release of VEGF (De et al. 2005). This relationship may require specific environmental cues, because β3 drives VEGF production for breast carcinoma cells growing in the brain but not for the same cells growing in the mammary fat pad (Lorger et al. 2009). Similarly, αvβ3 antagonism shows a strong antiangiogenic and antitumor effect for glioblastoma cells growing orthotopically in the brain, but not the same cells growing on the flanks of the same mice (MacDonald et al. 2001). Together, these studies confirm integrins as specialized cell adhesion molecules with the ability to integrate environmental cues that modulate tumor growth and sensitivity to therapeutics.
Integrin Signaling Promotes Diverse Functions in Multiple Tumor-Associated Cell Types
Integrin signaling pathways within tumor cells promote many cellular functions required for tumor growth and metastasis, including migration, invasion, proliferation, and survival (Desgrosellier and Cheresh 2010). Many of these integrin signaling pathways are similar to those observed in activated endothelial cells that require the same functional properties to remodel during development, physiological angiogenesis, and pathological vascular hyperproliferation. Similar integrin-mediated signaling is found in tumor-associated fibroblasts and inflammatory cells that are recruited to foster tumor progression (Rainger et al. 1999; Zhu et al. 2007). In simplistic terms, integrin/ligand recognition prevents cells from spreading beyond their appropriate boundaries within normal tissues, but these same gatekeepers will allow cells to travel to and survive within an atypical environment on receiving new environmental cues (outside-in signaling) or cellular reprogramming (inside-out signaling). Although integrins are not oncogenic, their elevated expression is often associated with or required for tumor growth and invasion. Because integrins lack kinase activity, their contribution to cell signaling pathways often involves their recruitment of kinases, scaffolding signaling modules, and cytoskeletal proteins. Certain integrins are typically expressed at only low levels in normal epithelial cells, but show high levels in some tumors. For example, αvβ3 expression is associated with disease progression and metastasis in melanoma (Nip et al. 1992; Danen et al. 1994) and pancreatic (Hosotani et al. 2002), prostate (McCabe et al. 2007), breast (Felding-Habermann et al. 2001), ovarian (Landen et al. 2008), and cervical (Gruber et al. 2005) cancers.
Tumor Cell Expression of β3 Integrin Promotes Anchorage-Indpendent Growth and Metastatic Potential
Expression of αvβ3 integrin by tumor cells has been linked to enhanced transendothelial migration (Kikkawa et al. 2002; Bauer et al. 2007) and production of MMP-2 (Baum et al. 2007), both of which confer metastatic potential. Indeed, roughly half of patients with pancreatic cancer show elevated expression of αvβ3, and this is positively correlated with lymph node metastasis (Hosotani et al. 2002). In experimental mouse models, pancreatic tumor growth and metastatic spread is accelerated by exogenous expression of β3 in pancreatic carcinoma cell lines lacking β3, and slowed by silencing of β3 expression in cell lines with endogenous expression (Desgrosellier et al. 2009). A novel αvβ3/Src oncogenic unit is responsible for this activity, and its mechanism of action represents an unexpected twist on typical integrin function. If a cell expressing β1 integrins encounters an inappropriate extracellular environment (i.e., one lacking β1 ligands), the unligated integrin will trigger a cell death pathway to prevent unauthorized cell movement. However, a cell expressing β3 integrin has an enhanced capacity for anchorage-independent survival. Instead of producing no signal or a prodeath signal, an unligated β3 integrin recruits Src kinase and drives Src activation and Crk-associated substrate (CAS) phosphorylation to promote anchorage-independent growth and survival (Desgrosellier et al. 2009). Similar to pancreatic cancer, αvβ3 expression is detected in 56% of breast tumors examined, and in 72% of breast cancer lymph node metastases (Desgrosellier et al. 2009). These studies suggest that αvβ3 expression confers a tumor cell with the ability to invade and survive in what would be an incompatible environment, and suggest that blocking αvβ3 function in breast or pancreatic cancers may have the ability to suppress the activity of the most aggressive and metastatic tumor cells within these tumors. The surprising aspect of this work is the fact that these integrin signaling events occur in the absence of ligation to extracellular matrix ligands.
The Role of αvβ5 in Carcinoma Invasion and Metastasis
As opposed to αvβ3 signaling which promotes both primary tumor growth and metastatic spread by promoting anchorage-independent survival, αvβ5 signaling uniquely drives invasive and metastatic properties of tumor cells (Klemke et al. 1994; Ricono et al. 2009). Whereas β3-positive tumor cells can migrate and metastasize spontaneously, β3-negative tumor cells require cytokine or growth factor stimulation to drive these activities. Exposure to either epithelial growth factor (EGF) (Klemke et al. 1994) or insulin-like growth factor (IGF) (Brooks et al. 1997) stimulates tumor cell migration and invasion through αvβ5. Importantly, ligation of both αvβ5 and cytokine receptors are required for spontaneous pulmonary metastasis of multiple tumor types but not for primary tumor growth (Brooks et al. 1997). When stimulated by EGF, Src activates p130Cas and drives the metastatic cascade. In particular, p130Cas couples to the adaptor protein Crk, and this complex localizes to membrane ruffles to drive cell migration (Klemke et al. 1998). Src-mediated p130Cas phosphorylation also activates the small GTPase Rap1, which is required for activation of αvβ5 (Ricono et al. 2009). Together, these findings show cross-talk between tyrosine kinase receptors and integrin αvβ5 that is critical for carcinoma cell invasion and metastasis, but not for growth of the primary tumor.
RGD-Targeted Nanoparticles Deliver Imaging or Therapeutic Agents to Tumors
RGD-mimetic peptides or small molecules act as potent antiangiogenic compounds by disrupting αvβ3/αvβ5 integrin-ligand interactions. The antiangiogenic capabilities of such agents were first tested in the early 1990s (Saiki et al. 1990; Nicosia and Bonanno 1991), and a recent review summarizes patents using these agents to target αvβ3 (Hsu et al. 2007). These agents may ultimately be the most useful as targeting agents for tumor-specific drug delivery or imaging (Meyer et al. 2006; Dijkgraaf et al. 2009; Haubner and Decristoforo 2009). Nanoparticles targeted to αvβ3-expressing tumor blood vessels can block tumor growth or metastasis by delivering chemotherapy compounds (Murphy et al. 2008; Sugahara et al. 2009), gene therapy (Hood et al. 2002), or siRNA (Schiffelers et al. 2004). The tumor penetrating iRGD peptide can significantly enhance the delivery of drugs or imaging agents that are either directly conjugated or simply coadministered to tumor-associated blood vessels, stroma, and tumor (Sugahara et al. 2009, 2010).
CLINICAL DEVELOPMENT OF αv INTEGRIN ANTAGONISTS
Because integrin-mediated signaling pathways allow endothelial and tumor cells to migrate, invade, and survive within remodeling environments, numerous approaches have been developed to target these pathways therapeutically in man (Hehlgans et al. 2007; Silva et al. 2008). Table 1 briefly describes αv integrin antagonists currently undergoing testing in clinical trials. For additional information, refer to a recent review article (Avraamides et al. 2008) and www.clinicaltrials.gov.
Table 1:
Clinical development of αv integrin antagonists
| Cyclic RGD peptide: |
| Nearly 20 trials are underway to test Cilengitide (EMD 121974), a cyclic RGD peptide inhibitor of αvβ3 and αvβ5, for advanced brain and CSN tumors, leukemia, and lymphoma. Cilengitide was first synthesized by Kessler and colleagues (Aumailley et al. 1991) and screened using a cell-free receptor assay for the inhibition of integrins αvβ3 and αvβ5 but not αIIbβ3 (Smith et al. 1990). |
| αvβ3 antibody: |
| The anti-αvβ3 antibody LM609 was fully humanized and first known as Vitaxin, then MEDI-522, and is currently being developed by Astra-Zeneca as etaracizumab (Abegrin). Abegrin was well tolerated in Phase I trials (Delbaldo et al. 2008). Although no objective response was observed in a Phase II trial for melanoma (Hersey et al. 2010), Abegrin is currently undergoing testing in eight clinical trials for solid tumors, psoriasis, and rheumatoid arthritis. In addition, Abegrin has shown promise in preclinical models as a targeting ligand for molecular imaging agents (Liu et al. 2010). |
| αv antibody: |
| This fully human monoclonal antibody that inhibits αv has antiangiogenic and antitumor effects in preclinical models (Trikha et al. 2004; Chen et al. 2008) and was well tolerated in Phase I trials (Mullamitha et al. 2007). Unlike some other angiogenesis inhibitors, CNTO 95 does not appear to inhibit normal physiologic angiogenesis or wound healing processes (Martin et al. 2005). CNTO 95 is currently undergoing testing for prostate cancer and melanoma. |
Development of Cilengitide as an Antitumor Agent for Glioblastoma
Preclinical studies have provided rationale for the development of integrin antagonists as both antitumor and antiangiogenic agents (Brooks et al. 1994a,b, 1995a; Montgomery et al. 1994). The most advanced αvβ3 candidate is currently being tested in a Phase III clinical trial to test the RGD peptide Cilengitide for patients with newly diagnosed glioblastoma multiforme (recently reviewed by Tabatabai et al. 2010). In glioblastoma, αvβ3 is highly expressed on both the tumor astrocytes and endothelial cells within these highly invasive tumors (Gladson and Cheresh 1991; Gladson et al. 1995, 1999; Bello et al. 2001). The αvβ3 ligands vitronectin and tenascin colocalize with αvβ3 expression on both tumor and endothelial cells, in contrast to fibronectin (a ligand for β1 integrins), which shows diffuse staining throughout (Gladson and Cheresh 1991; Gladson et al. 1995; Gladson 1999; Bello et al. 2001). αvβ3 expression on the host cells in particular supports both the angiogenic response and the infiltration of macrophages (Kanamori et al. 2006). Early clinical trials for Cilengitide have shown antitumor activity, including durable remissions, and increased survival for a subset of patients. Promoter methylation of the methylated-DNA-protein-cysteine methyltransferase (MGMT) DNA repair gene is a prognostic marker in glioblastoma, because it is correlated with increased chemosensitivity to alkylating agents such as temozolomide (TMZ). The new Phase III study (CENTRIC) will test standard TMZ/radiation therapy +/– Cilengitide only for patients with a methylated MGMT promoter, because this subset of patients showed the most sensitivity to Cilengitide in previous trials. The efficacy of Cilengitide for glioblastoma will likely be a function of blocking αvβ3 function on both tumor cell and endothelial cell compartments, and thus understanding how distinct cell types are impacted by integrin antagonism will enable the logical design of the most effective combination therapies.
CONCLUDING REMARKS
The future success of αv integrin antagonism as a therapeutic approach for cancer and angiogenesis will likely be a function of understanding why these integrins are expressed on the surface of particular cell types within a distinct tissue microenvironment. This knowledge will enable the logical design of therapeutic strategies to enhance or suppress integrin signaling, and might explain the efficacy or lack of activity for some current approaches. Controlling integrin expression via microRNAs may emerge as an additional tool, although this field is currently in its infancy. Antiangiogenic strategies targeting integrins will likely require combination with additional agents, as antivascular agents rarely show activity as single agents (e.g., Avastin). Indeed, preclinical studies suggest that integrin antagonists function well in combination with chemotherapy or radiotherapy. Radiotherapy increases endothelial cell expression of αvβ3 (Abdollahi et al. 2005), which may sensitize cells to αvβ3 antagonism. Combination therapy may also reduce drug resistance mechanisms, as αvβ3 antagonism combined with multiple angiostatic drugs blocks the growth of typically resistant glioblastomas by preventing compensatory up-regulation of other proangiogenic factors (Dorrell et al. 2007). In summary, effective use of αv integrin antagonism in man will require an understanding of which integrins to target on which cells, and thus approaches to selectively target these events within different tissue compartments such as angiogenic endothelial cells or tumor-associated stromal cells will offer a significant advantage.
ACKNOWLEDGMENTS
D.A.C. is supported by NIH grants CA50286, CA104898, CA45726, and HL57900.
Footnotes
Editors: Michael Klagsbrun and Patricia D’Amore
Additional Perspectives on Angiogenesis available at www.perspectivesinmedicine.org
REFERENCES
- Abdollahi A, Griggs DW, Zieher H, Roth A, Lipson KE, Saffrich R, Grone HJ, Hallahan DE, Reisfeld RA, Debus J, et al. 2005. Inhibition of α(v)β3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res 11: 6270–6279 [DOI] [PubMed] [Google Scholar]
- Acevedo LM, Barillas S, Weis SM, Gothert JR, Cheresh DA 2008. Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 111: 2674–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi A, Hood JD, Frausto R, Stupack DG, Cheresh DA 2003. Role of Raf in vascular protection from distinct apoptotic stimuli. Science 301: 94–96 [DOI] [PubMed] [Google Scholar]
- Argraves WS, Pytela R, Suzuki S, Millan JL, Pierschbacher MD, Ruoslahti E 1986. cDNA sequences from the α subunit of the fibronectin receptor predict a transmembrane domain and a short cytoplasmic peptide. J Biol Chem 261: 12922–12924 [PubMed] [Google Scholar]
- Aumailley M, Gurrath M, Muller G, Calvete J, Timpl R, Kessler H 1991. Arg-Gly-Asp constrained within cyclic pentapeptides. Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett 291: 50–54 [DOI] [PubMed] [Google Scholar]
- Avraamides CJ, Garmy-Susini B, Varner JA 2008. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 8: 604–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A, Raghavan S 2009. Defining the role of integrin αvβ6 in cancer. Curr Drug Targets 10: 645–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer K, Mierke C, Behrens J 2007. Expression profiling reveals genes associated with transendothelial migration of tumor cells: A functional role for αvβ3 integrin. Int J Cancer 121: 1910–1918 [DOI] [PubMed] [Google Scholar]
- Baum O, Hlushchuk R, Forster A, Greiner R, Clezardin P, Zhao YS, Djonov V, Gruber G 2007. Increased invasive potential and up-regulation of MMP-2 in MDA-MB-231 breast cancer cells expressing the B3 integrin subunit. Int J Oncol 30: 325–332 [PubMed] [Google Scholar]
- Bello L, Francolini M, Marthyn P, Zhang J, Carroll RS, Nikas DC, Strasser JF, Villani R, Cheresh DA, Black PM 2001. α(v)β3 and α(v)β5 integrin expression in glioma periphery. Neurosurgery 49: 380–389; discussion 390 [DOI] [PubMed] [Google Scholar]
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et al. 2009. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324: 1710–1713 [DOI] [PubMed] [Google Scholar]
- Boudreau N, Andrews C, Srebrow A, Ravanpay A, Cheresh DA 1997. Induction of the angiogenic phenotype by Hox D3. J Cell Biol 139: 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Clark RA, Cheresh DA 1994a. Requirement of vascular integrin αvβ3 for angiogenesis. Science 264: 569–571 [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA 1994b. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 79: 1157–1164 [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA 1995a. Anti-integrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 96: 1815–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA 1995b. Anti-integrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 96: 1815–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Klemke RL, Schon S, Lewis JM, Schwartz MA, Cheresh DA 1997. Insulin-like growth factor receptor cooperates with integrin α v β 5 to promote tumor cell dissemination in vivo. J Clin Invest 99: 1390–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccavari F, Valdembri D, Sandri C, Bussolino F, Serini G 2010. Integrin signaling and lung cancer. Cell Adh Migr 4: 124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH 2007. A functional study of miR-124 in the developing neural tube. Genes Dev 21: 531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascone I, Napione L, Maniero F, Serini G, Bussolino F 2005. Stable interaction between α5β1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol 170: 993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC 2008. Rab-coupling protein coordinates recycling of α5β1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol 183: 143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Vadrevu S, Norman JC 2009. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 10: 843–853 [DOI] [PubMed] [Google Scholar]
- Chen Q, Manning CD, Millar H, McCabe FL, Ferrante C, Sharp C, Shahied-Arruda L, Doshi P, Nakada MT, Anderson GM 2008. CNTO 95, a fully human anti αv integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin Exp Metastasis 25: 139–148 [DOI] [PubMed] [Google Scholar]
- Clark RA, Tonnesen MG, Gailit J, Cheresh DA 1996. Transient functional expression of αVβ3 on vascular cells during wound repair. Am J Pathol 148: 1407–1421 [PMC free article] [PubMed] [Google Scholar]
- Dalmay T, Edwards DR 2006. MicroRNAs and the hallmarks of cancer. Oncogene 25: 6170–6175 [DOI] [PubMed] [Google Scholar]
- Danen EH, Ten Berge PJ, Van Muijen GN, Van’t Hof-Grootenboer AE, Brocker EB, Ruiter DJ 1994. Emergence of α5β1 fibronectin- and αvβ3 vitronectin-receptor expression in melanocytic tumour progression. Histopathology 24: 249–256 [DOI] [PubMed] [Google Scholar]
- De S, Razorenova O, McCabe NP, O’Toole T, Qin J, Byzova TV 2005. VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci 102: 7589–7594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbaldo C, Raymond E, Vera K, Hammershaimb L, Kaucic K, Lozahic S, Marty M, Faivre S 2008. Phase I and pharmacokinetic study of etaracizumab (Abegrin), a humanized monoclonal antibody against αvβ3 integrin receptor, in patients with advanced solid tumors. Invest New Drugs 26: 35–43 [DOI] [PubMed] [Google Scholar]
- Desgrosellier JS, Cheresh DA 2010. Integrins in cancer: Biological implications and therapeutic opportunities. Nat Rev Cancer 10: 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA 2009. An integrin αvβ3-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med 15: 1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkgraaf I, Beer AJ, Wester HJ 2009. Application of RGD-containing peptides as imaging probes for αvβ3 expression. Front Biosci 14: 887–899 [DOI] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Scheppke L, Barnett FH, Friedlander M 2007. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci 104: 967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ, Cheresh DA, Little CD 1995. An antagonist of integrin αvβ3 prevents maturation of blood vessels during embryonic neovascularization. J Cell Sci 108 (Pt 7): 2655–2661 [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG 2009. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol 187: 733–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, et al. 2001. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci 98: 1853–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, McCabe NP, Mahabeleshwar GH, Somanath PR, Phillips DR, Byzova TV 2008. The angiogenic response is dictated by β3 integrin on bone marrow-derived cells. J Cell Biol 183: 1145–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Srivastava D 2009. MicroRNAs: Opening a new vein in angiogenesis research. Sci Signal 2: pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J 2006. Angiogenesis. Annu Rev Med 57: 1–18 [DOI] [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA 1995. Definition of two angiogenic pathways by distinct α v integrins. Science 270: 1500–1502 [DOI] [PubMed] [Google Scholar]
- Friedlander M, Theesfeld CL, Sugita M, Fruttiger M, Thomas MA, Chang S, Cheresh DA 1996. Involvement of integrins αvβ3 and αvβ5 in ocular neovascular diseases. Proc Natl Acad Sci 93: 9764–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG, Tarone G 2003. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu Rev Cell Dev Biol 19: 173–206 [DOI] [PubMed] [Google Scholar]
- Gladson CL 1999. The extracellular matrix of gliomas: modulation of cell function. J Neuropathol Exp Neurol 58: 1029–1040 [DOI] [PubMed] [Google Scholar]
- Gladson CL, Cheresh DA 1991. Glioblastoma expression of vitronectin and the αvβ3 integrin. Adhesion mechanism for transformed glial cells. J Clin Invest 88: 1924–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladson CL, Wilcox JN, Sanders L, Gillespie GY, Cheresh DA 1995. Cerebral microenvironment influences expression of the vitronectin gene in astrocytic tumors. J Cell Sci 108 (Pt 3): 947–956 [DOI] [PubMed] [Google Scholar]
- Gruber G, Hess J, Stiefel C, Aebersold DM, Zimmer Y, Greiner RH, Studer U, Altermatt HJ, Hlushchuk R, Djonov V 2005. Correlation between the tumoral expression of β3-integrin and outcome in cervical cancer patients who had undergone radiotherapy. Br J Cancer 92: 41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E 2006. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell 124: 175–189 [DOI] [PubMed] [Google Scholar]
- Haubner R, Decristoforo C 2009. Radiolabelled RGD peptides and peptidomimetics for tumour targeting. Front Biosci 14: 872–886 [DOI] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, Cordes N 2007. Signalling via integrins: Implications for cell survival and anticancer strategies. Biochim Biophys Acta (BBA) - Reviews on Cancer 1775: 163–180 [DOI] [PubMed] [Google Scholar]
- Hersey P, Sosman J, O’Day S, Richards J, Bedikian A, Gonzalez R, Sharfman W, Weber R, Logan T, Buzoianu M, et al. 2010. A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin αvβ3,+ or – dacarbazine in patients with stage IV metastatic melanoma. Cancer 116: 1526–1534 [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO 1999. β3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest 103: 229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA 2002. Tumor regression by targeted gene delivery to the neovasculature. Science 296: 2404–2407 [DOI] [PubMed] [Google Scholar]
- Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA 2003. Differential αv integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol 162: 933–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M 2002. Expression of integrin αVβ3 in pancreatic carcinoma: Relation to MMP-2 activation and lymph node metastasis. Pancreas 25: e30–e35 [DOI] [PubMed] [Google Scholar]
- Hsu AR, Veeravagu A, Cai W, Hou LC, Tse V, Chen X 2007. Integrin αvβ3 antagonists for anti-angiogenic cancer treatment. Recent Pat Anticancer Drug Discov 2: 143–158 [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ 2006. Integrin ligands at a glance. J Cell Sci 119: 3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings H, Ortega N, Plouet J 2003. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. Faseb J 17: 1520–1522 [DOI] [PubMed] [Google Scholar]
- Kanamori M, Kawaguchi T, Berger MS, Pieper RO 2006. Intracranial Microenvironment reveals independent opposing functions of host αvβ3 expression on glioma growth and angiogenesis. J Biol Chem 281: 37256–37264 [DOI] [PubMed] [Google Scholar]
- Kikkawa H, Kaihou M, Horaguchi N, Uchida T, Imafuku H, Takiguchi A, Yamazaki Y, Koike C, Kuruto R, Kakiuchi T, et al. 2002. Role of integrin αvβ3 in the early phase of liver metastasis: PET and IVM analyses. Clin Exp Metastasis 19: 717–725 [DOI] [PubMed] [Google Scholar]
- Klemke RL, Yebra M, Bayna EM, Cheresh DA 1994. Receptor tyrosine kinase signaling required for integrin αvβ5-directed cell motility but not adhesion on vitronectin. J Cell Biol 127: 859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Leng J, Molander R, Brooks PC, Vuori K, Cheresh DA 1998. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol 140: 961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehbacher A, Urbich C, Dimmeler S 2008. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci 29: 12–15 [DOI] [PubMed] [Google Scholar]
- Landen CN, Kim TJ, Lin YG, Merritt WM, Kamat AA, Han LY, Spannuth WA, Nick AM, Jennnings NB, Kinch MS, et al. 2008. Tumor-selective response to antibody-mediated targeting of αvβ3 integrin in ovarian cancer. Neoplasia 10: 1259–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Jia B, Zhao H, Chen X, Wang F 2010. Specific targeting of human integrin αvβ 3 with (111) in-labeled abegrin in nude mouse models. Mol Imaging Biol 13: 112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorger M, Krueger JS, O’Neal M, Staflin K, Felding-Habermann B 2009. Activation of tumor cell integrin αvβ3 controls angiogenesis and metastatic growth in the brain. Proc Natl Acad Sci 106: 10666–10671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Lu D, Scully M, Kakkar V 2008. The role of integrins in cancer and the development of anti-integrin therapeutic agents for cancer therapy. Perspect Med Chem 2: 57–73 [PMC free article] [PubMed] [Google Scholar]
- Luo BH, Carman CV, Springer TA 2007. Structural basis of integrin regulation and signaling. Annu Rev Immunol 25: 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TJ, Taga T, Shimada H, Tabrizi P, Zlokovic BV, Cheresh DA, Laug WE 2001. Preferential susceptibility of brain tumors to the antiangiogenic effects of an αv integrin antagonist. Neurosurgery 48: 151–157 [DOI] [PubMed] [Google Scholar]
- Magnon C, Galaup A, Mullan B, Rouffiac V, Bidart J-M, Griscelli F, Opolon P, Perricaudet M 2005. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with αvβ3 and αvβ5 integrins. Cancer Res 65: 4353–4361 [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV 2006. Integrin signaling is critical for pathological angiogenesis. J Exp Med 203: 2495–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV 2007. Mechanisms of integrin vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res 101: 570–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabeleshwar GH, Chen J, Feng W, Somanath PR, Razorenova OV, Byzova TV 2008. Integrin affinity modulation in angiogenesis. Cell Cycle 7: 335–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, Seano G, Serini G, Bussolino F, Giraudo E 2009. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest 119: 3356–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PL, Jiao Q, Cornacoff J, Hall W, Saville B, Nemeth JA, Schantz A, Mata M, Jang H, Fasanmade AA, et al. 2005. Absence of adverse effects in cynomolgus macaques treated with CNTO 95, a fully human anti-αv integrin monoclonal antibody, despite widespread tissue binding. Clin Cancer Res 11: 6959–6965 [DOI] [PubMed] [Google Scholar]
- McCabe NP, De S, Vasanji A, Brainard J, Byzova TV 2007. Prostate cancer specific integrin αvβ3 modulates bone metastatic growth and tissue remodeling. Oncogene 26: 6238–6243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH 2009. Integrin-mediated regulation of neurovascular development, physiology and disease. Cell Adh Migr 3: 211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO 2005. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development 132: 165–176 [DOI] [PubMed] [Google Scholar]
- Meyer A, Auernheimer J, Modlinger A, Kessler H 2006. Targeting RGD recognizing integrins: Drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Des 12: 2723–2747 [DOI] [PubMed] [Google Scholar]
- Montgomery AM, Reisfeld RA, Cheresh DA 1994. Integrin αvβ3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci 91: 8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EA, Schneider JG, Baroni TE, Uluckan O, Heller E, Hurchla MA, Deng H, Floyd D, Berdy A, Prior JL, et al. 2009. Dissection of platelet and myeloid cell defects by conditional targeting of the β3-integrin subunit. FASEB J 24: 1117–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, Roberts C, Buonaccorsi GA, Watson Y, Davies K, Cheung S, et al. 2007. Phase I evaluation of a fully human anti-αv integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res 13: 2128–2135 [DOI] [PubMed] [Google Scholar]
- Muller DW, Bosserhoff AK 2008. Integrin β3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene 27: 6698–6706 [DOI] [PubMed] [Google Scholar]
- Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA 2008. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci 105: 9343–9348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia RF, Bonanno E 1991. Inhibition of angiogenesis in vitro by Arg-Gly-Asp-containing synthetic peptide. Am J Pathol 138: 829–833 [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Giancotti FG 2005. Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle 4: e131–e135 [PubMed] [Google Scholar]
- Nip J, Shibata H, Loskutoff DJ, Cheresh DA, Brodt P 1992. Human melanoma cells derived from lymphatic metastases use integrin αvβ3 to adhere to lymph node vitronectin. J Clin Invest 90: 1406–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg P, Xie L, Kalluri R 2005. Endogenous inhibitors of angiogenesis. Cancer Res 65: 3967–3979 [DOI] [PubMed] [Google Scholar]
- Pan H, Wanami LS, Dissanayake TR, Bachelder RE 2009. Autocrine semaphorin3A stimulates α2β1 integrin expression/function in breast tumor cells. Breast Cancer Res Treat 118: 197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E 1984a. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309: 30–33 [DOI] [PubMed] [Google Scholar]
- Pierschbacher MD, Ruoslahti E 1984b. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci 81: 5985–5988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E 1985a. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci 82: 5766–5770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Ruoslahti E 1985b. Identification and isolation of a 140-kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell 40: 191–198 [DOI] [PubMed] [Google Scholar]
- Rahman S, Patel Y, Murray J, Patel KV, Sumathipala R, Sobel M, Wijelath ES 2005. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainger GE, Buckley CD, Simmons DL, Nash GB 1999. Neutrophils sense flow-generated stress and direct their migration through αvβ3-integrin. Am J Physiol 276: H858–864 [DOI] [PubMed] [Google Scholar]
- Rehn M, Veikkola T, Kukk-Valdre E, Nakamura H, Ilmonen M, Lombardo C, Pihlajaniemi T, Alitalo K, Vuori K 2001. Interaction of endostatin with integrins implicated in angiogenesis. Proc Natl Acad Sci 98: 1024–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM 2002. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat Med 8: 27–34 [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, Bodary SC, Hodivala-Dilke KM 2004. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in β}-integrin-deficient mice. Cancer Res 64: 8643–8650 [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Conti FJ, Lucas M, Grose R, Robinson S, Stone M, Saunders G, Dickson C, Hynes RO, Lacy-Hulbert A, et al. 2005. Accelerated re-epithelialization in β3-integrin-deficient mice is associated with enhanced TGF-β1 signaling. Nat Med 11: 167–174 [DOI] [PubMed] [Google Scholar]
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, et al. 2009. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med 15: 392–400 [DOI] [PubMed] [Google Scholar]
- Ribatti D 2009. Endogenous inhibitors of angiogenesis: A historical review. Leukemia Res 33: 638–644 [DOI] [PubMed] [Google Scholar]
- Ricono JM, Huang M, Barnes LA, Lau SK, Weis SM, Schlaepfer DD, Hanks SK, Cheresh DA 2009. Specific cross-talk between epidermal growth factor receptor and integrin αvβ5 promotes carcinoma cell invasion and metastasis. Cancer Res 69: 1383–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM 2004. β3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol 24: 2108–2114 [DOI] [PubMed] [Google Scholar]
- Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, Graca da Silva R, Tavora B, Baker M, Marshall JF, Hodivala-Dilke KM 2009. αvβ3-integrin limits the contribution of neuropilin-1 to VEGF-induced angiogenesis. J Biol Chem 284: 33966–33981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki I, Murata J, Makabe T, Nishi N, Tokura S, Azuma I 1990. Inhibition of tumor angiogenesis by a synthetic cell-adhesive polypeptide containing the Arg-Gly-Asp (RGD) sequence of fibronectin, poly(RGD). Jpn J Cancer Res 81: 668–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffelers RM, Ansari A, Xu J, Zhou Q, Tang Q, Storm G, Molema G, Lu PY, Scaria PV, Woodle MC 2004. Cancer siRNA therapy by tumor selective delivery with ligand-targeted sterically stabilized nanoparticle. Nucl Acids Res 32: e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. 2003. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424: 391–397 [DOI] [PubMed] [Google Scholar]
- Serini G, Napione L, Arese M, Bussolino F 2008. Besides adhesion: New perspectives of integrin functions in angiogenesis. Cardiovasc Res: cvn045. [DOI] [PubMed] [Google Scholar]
- Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE 2008. Integrins: The keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol 28: 1703–1713 [DOI] [PubMed] [Google Scholar]
- Smith JW, Ruggeri ZM, Kunicki TJ, Cheresh DA 1990. Interaction of integrins αvβ3 and glycoprotein IIb-IIIa with fibrinogen. Differential peptide recognition accounts for distinct binding sites. J Biol Chem 265: 12267–12271 [PubMed] [Google Scholar]
- Sroka IC, Anderson TA, McDaniel KM, Nagle RB, Gretzer MB, Cress AE 2010. The laminin binding integrin α6β1 in prostate cancer perineural invasion. J Cell Physiol 224: 283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storgard CM, Stupack DG, Jonczyk A, Goodman SL, Fox RI, Cheresh DA 1999. Decreased angiogenesis and arthritic disease in rabbits treated with an αvβ3 antagonist. J Clin Invest 103: 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez Y, Sessa WC 2009. MicroRNAs as novel regulators of angiogenesis. Circ Res 104: 442–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R 2003. Human tumstatin and human endostatin exhibit distinct antiangiogenic activities mediated by αvβ3 and α5β1 integrins. Proc Natl Acad Sci 100: 4766–4771 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E 2009. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 16: 510–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E 2010. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328: 1031–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Argraves WS, Pytela R, Arai H, Krusius T, Pierschbacher MD, Ruoslahti E 1986. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci 83: 8614–8618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai G, Weller M, Nabors B, Picard M, Reardon D, Mikkelsen T, Ruegg C, Stupp R 2010. Targeting integrins in malignant glioma. Target Oncol 5: 175–181 [DOI] [PubMed] [Google Scholar]
- Tarui T, Miles LA, Takada Y 2001. Specific interaction of angiostatin with integrin αvβ3 in endothelial cells. J Biol Chem 276: 39562–39568 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M 2007. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J 26: 1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikha M, Zhou Z, Nemeth JA, Chen Q, Sharp C, Emmell E, Giles-Komar J, Nakada MT 2004. CNTO 95, a fully human monoclonal antibody that inhibits αv integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer 110: 326–335 [DOI] [PubMed] [Google Scholar]
- Valastyan S, Benaich N, Chang A, Reinhardt F, Weinberg RA 2009. Concomitant suppression of three target genes can explain the impact of a microRNA on metastasis. Genes Dev 23: 2592–2597 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Valdembri D, Caswell PT, Anderson KI, Schwarz JP, Konig I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, et al. 2009. Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells. PLoS Biol 7: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Lindquist JN, Barnes LA, Lutu-Fuga KM, Cui J, Wood MR, Cheresh DA 2007. Cooperation between VEGF and β3 integrin during cardiac vascular development. Blood 109: 1962–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng S, Zemany L, Standley KN, Novack DV, La Regina M, Bernal-Mizrachi C, Coleman T, Semenkovich CF 2003. β3 integrin deficiency promotes atherosclerosis and pulmonary inflammation in high-fat-fed, hyperlipidemic mice. Proc Natl Acad Sci 100: 6730–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ramirez NE, Yankeelov TE, Li Z, Ford LE, Qi Y, Pozzi A, Zutter MM 2008. α2β1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell-specific manner. Blood 111: 1980–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF 2002. β8 integrins are required for vascular morphogenesis in mouse embryos. Development 129: 2891–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CQ, Popova SN, Brown ER, Barsyte-Lovejoy D, Navab R, Shih W, Li M, Lu M, Jurisica I, Penn LZ, et al. 2007. Integrin α11 regulates IGF2 expression in fibroblasts to enhance tumorigenicity of human non-small-cell lung cancer cells. Proc Natl Acad Sci 104: 11754–11759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W 2004. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res 94: 230–238 [DOI] [PubMed] [Google Scholar]