Background: Nucleolin is a multifunctional nucleolar protein. Its function in ESCs remains unknown.
Results: Knockdown of nucleolin induces cell cycle arrest, apoptosis, and differentiation in ESCs by up-regulating p53 protein level.
Conclusion: Nucleolin regulates ESC self-renewal through a p53-dependent pathway.
Significance: Learning distinct functions of nucleolin is of great importance for elucidating the molecular basis of ESC self-renewal and differentiation.
Keywords: Apoptosis, Cell Cycle, Differentiation, Embryonic Stem Cell, p53, Nucleolin, Self-renewal
Abstract
Embryonic stem cells (ESCs) can undergo unlimited self-renewal and retain pluripotent developmental potential. The unique characteristics of ESCs, including a distinct transcriptional network, a poised epigenetic state, and a specific cell cycle profile, distinguish them from somatic cells. However, the molecular mechanisms underlying these special properties of ESCs are not fully understood. Here, we report that nucleolin, a nucleolar protein highly expressed in undifferentiated ESCs, plays an essential role for the maintenance of ESC self-renewal. When nucleolin is knocked down by specific short hairpin RNA (shRNA), ESCs display dramatically reduced cell proliferation rate, increased cell apoptosis, and G1 phase accumulation. Down-regulation of nucleolin also leads to evident ESC differentiation as well as decreased self-renewal ability. Interestingly, expression of pluripotency markers (Oct4 and Nanog) is unaltered in these differentiated cells. Mechanistically, depletion of nucleolin up-regulates the p53 protein level and activates the p53-dependent pathway, at least in part, via increasing p53 protein stability. Silencing of p53 rescues G1 phase accumulation and apoptosis caused by nucleolin deficiency entirely, although it partially blocks abnormal differentiation in nucleolin-depleted ESCs. It is noteworthy that knocking down nucleolin in NIH3T3 cells affected cell survival and proliferation in a much milder way, despite the comparable silencing efficiency obtained in ESCs and NIH3T3 cells. Collectively, our data demonstrate that nucleolin is a critical regulator of ESC self-renewal and that suppression of the p53-dependent pathway is the major molecular mechanism underlying functions of nucleolin in ESCs.
Introduction
Embryonic stem cells (ESCs)2 can undergo unlimited self-renewal and retain the ability to differentiate into any cell type in the body, making them attractive for fundamental research and regenerative medicine (1). A distinct transcriptional hierarchy, a poised epigenetic state, and a specific cell cycle profile distinguish ESCs from somatic cells (2). A comprehensive understanding of the molecular mechanisms underlying these special properties of ESCs is required to achieve the goal of clinical applications. Mouse ESCs can be maintained in an undifferentiated self-renewal state in the presence of leukemia inhibitory factor (LIF). Withdrawal of LIF results in extensive ESC differentiation with reduced expression of pluripotency-associated factors (Oct4, Sox2, and Nanog), which play crucial roles in the maintenance of ESC properties (3–6). Recently, the Oct4-centered transcriptional and protein interaction networks have been intensively investigated (7–9). Roles of epigenetic regulators in the control of ESC self-renewal and pluripotency have also been reported (10–12). In contrast, the regulation of cell cycle progression and survival of ESCs has not been highly regarded, although they are important determinants for maintaining self-renewal. Mouse ESCs proliferate extraordinarily rapidly and take only 8–12 h to progress through a whole cell cycle, because of a lack of G0 phase and G1 checkpoint as well as a shortened G1 phase (2, 13). However, the factors responsible for the distinct cell cycle characteristics and survival of ESCs remain largely unknown. Continued research and identification of critical regulators for the balance between ESC self-renewal and lineage commitment are essential for efficient differentiation of ESCs into clinically useful cells as well as reprogramming of somatic cells into the pluripotent state.
Recently, several studies have indicated that nucleolar proteins play pivotal roles in controlling stem cell proliferation and viability. For example, nucleostemin was shown to participate in controlling cell proliferation and survival in ESCs and adult stem cells (14–16). In addition, studies from our group and other groups have shown that nucleophosmin 1 (NPM1) is essential for mouse ESC growth (17, 18). Recently, we reported that another nucleolar protein Ly1 antibody reactive clone (LYAR) is required for cell proliferation and viability in ESCs (19). Interestingly, LYAR interacts with and inhibits the auto-cleavage activity of a nucleolar phosphoprotein, nucleolin, to stabilize its protein level. Nucleolin is known to be highly expressed in actively dividing cells (20), although it degrades because of auto-cleavage when cells become quiescent (21–23). Since the first description (24), numerous studies have focused on the structure, localization, and functions of nucleolin (25–27). This multiple domain-containing protein has a broad range of localizations and interacts with various RNAs, DNAs, and proteins, all of which are compatible with its multiple functions involved in the regulation of ribosome biogenesis and maturation, cell cycle, proliferation, apoptosis, transcription, and nucleogenesis (27, 28). Despite the great progress, the role of nucleolin in controlling ESC self-renewal has not been well defined.
In this study, we generated tetracycline (Tc)-inducible nucleolin short hairpin RNA (shRNA) expressing ESC lines to study the function and underlying mechanism of nucleolin in mouse ESCs. Our data demonstrate that nucleolin is essential for maintaining the self-renewal ability of ESCs, because of its role in regulating cell cycle progression, proliferation, as well as prevention of apoptosis and differentiation. Mechanistically, knockdown of nucleolin resulted in an elevation in the protein level and activity of p53, a key transcription factor in controlling cell cycle progression and apoptosis. The elevated p53 protein level was, at least in part, due to the enhanced p53 protein stability. Activated p53 pathways led to the accumulation of cells in G1 phase, increased apoptosis, and obvious cell differentiation. Functionally, silencing of p53 expression completely canceled out G1 phase accumulation and apoptosis caused by depletion of nucleolin, whereas the abnormal differentiation phenomenon observed in nucleolin-deficient ESCs was partially rescued when p53 was knocked down. In contrast, nucleolin silencing affected cell proliferation and survival slightly in the differentiated cell type, NIH3T3 cells. Therefore, this study uncovers an important new regulator in the maintenance of the ESC self-renewal ability and establishes the functional link between nucleolar protein nucleolin and transcriptional factor p53 in mouse ESCs.
EXPERIMENTAL PROCEDURES
Plasmids and siRNA Oligos
For knockdown constructs, 19-bp shRNA sequences, corresponding to EGFP (5′-GGC TAC GTC CAG GAG CGC A-3′), nucleolin 1 (5′-AGG AAG AGA TAG TAA GAA A-3′), nucleolin 2 (5′-AGA GAA AGG TCA AAG GCA A-3′) were synthesized and constructed into the pTER+ vector, a kind gift from Hans Clevers (29). The sequences of all plasmids were verified by DNA sequencing. The sequences for p53 siRNA oligo (Stealth RNAiTM duplex oligoribonucleotides, Invitrogen) are as follows: p53i-1 (5′-AGU ACG UGC ACA UAA CAG ACU UGG C-3′) and p53i-2 (5′-AUA UCC GAC UGU GAC UCC UCC AUG G-3′).
Cell Culture
All cell lines used in this study were cultured as described previously (19). Tc-inducible shRNA ESC lines or NIH3T3 cell lines were cultured in the medium for CGR8 cells or NIH3T3 cells, supplemented with 1 μg/ml puromycin (Sigma) and 50 μg/ml Zeocin (Invitrogen) in the absence or presence of Tc at the concentration of 100–300 ng/ml.
Embryoid Body (EB) Formation
CGR8 ESCs were plated at the density of 1 × 106 per 10-cm Petri dish and suspended to form EB without LIF for the indicated time.
Cell Transfection
The establishment of inducible shRNA stable cell lines has been described previously (18). siRNA oligos were transfected into shRNA EGFP and shRNA nucleolin ESCs by Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Quantitative Real Time PCR (qPCR)
Total RNA was extracted from cells according to the manufacturer's instructions using TRIzol reagent (Invitrogen) and reverse-transcribed into cDNA using oligo(dT)15 and ReverTra Ace reverse transcriptase (Toyobo). qPCR was performed in the ABI PRISM 7900 using SYBR Green PCR master mix (ABI), and the data were analyzed by the Sequence Detection System 2.3 software (ABI). Each sample was analyzed in triplicate with Gapdh as the internal control. The primer sequences for different genes are listed in Table 1.
TABLE 1.
Primer sequences for qPCR analysis
F indicates forward and R is reverse.
| Gene name | Sequences |
|---|---|
| Oct4 | F, 5′-ATGGCATACTGTGGACCTCA-3′ |
| R, 5′-AGCAGCTTGGCAAACTGTTC-3′ | |
| Nanog | F, 5′-CTCATCAATGCCTGCAGTTTTTCA-3′ |
| R, 5′-CTCCTCAGGGCCCTTGTCAGC-3′ | |
| Cdx2 | F, 5′-CCTGCGACAAGGGCTTGTTTAG-3′ |
| R, 5′-TCCCGACTTCCCTTCACCATAC-3′ | |
| Gata6 | F, 5′-ACAGCCCACTTCTGTGTTCCC-3′ |
| R, 5′-GTGGGTTGGTCACGTGGTACAG-3′ | |
| brachyury | F, 5′-CTCTAATGTCCTCCCTTGTTGCC-3′ |
| R, 5′-TGCAGATTGTCTTTGGCTACTTTG-3′ | |
| Fgf5 | F, 5′-AAAGTCAATGGCTCCCACGAA-3′ |
| R, 5′-GGCACTTGCATGGAGTTTTCC-3′ | |
| nestin | F, 5′-TGAGGGTCAGGTGGTTCTG-3′ |
| R, 5′-AGAGCAGGGAGGGACATTC-3′ | |
| Gapdh | F, 5′-GTCGTGGAGTCTACTGGTGTC-3′ |
| R, 5′-GAGCCCTTCCACAATGCCAAA-3′ | |
| nucleolin | F, 5′-CTTGCTGTTGTGGATGTC-3′ |
| R, 5′-CATGGCGTCTTCAAACAC-3′ | |
| p53 | F, 5′-TGCTACAGAGGAGTCTGGAGAC-3′ |
| R, 5′-GTGTCTCAGCCCTGAAGTCATAA-3′ | |
| P21 | F, 5′-CCGTGGACAGTGAGCAGTT-3′ |
| R, 5′-CAGCAGGGCAGAGGAAGTA-3′ | |
| cyclin G1 | F, 5′-GCATGGCAGCACATGCCTTTA-3′ |
| R, 5′-TGTAGACCAGCCTGGCTTTGAAT-3′ | |
| Mdm2 | F, 5′-ATTGCCTGGATCAGGATTCAGTT-3′ |
| R, 5′-ACCTCATCATCCTCATCTGAGA-3′ | |
| Bax | F, 5′-ATGCGTCCACCAAGAAGCTGA-3′ |
| R, 5′-AGCAATCATCCTCTGCAGCTCC-3′ | |
| Wig1 | F, 5′-GTCCTCCATGCGTACATTCC-3′ |
| R, 5′-GCATCCTGGTGACCAAAGAAAC-3′ | |
| Apaf1 | F, 5′-AGAGGATGTGGAGGTGAT-3′ |
| R, 5′-CTGGATGGTGCTGTGAT-3′ |
Western Blot Analysis
Western blot analysis was carried out as described previously (18) using primary antibodies against Oct4, Nanog, Sox2 (1:1000, home-made), nucleolin (1:2000, Abcam), p53 (1:1000, Cell Signaling Technology), caspase 3 (1:1000, Cell Signaling Technology), or α-tubulin (1:2000, Sigma) and horseradish peroxidase-linked secondary anti-rabbit (Santa Cruz Biotechnology) or anti-mouse antibodies (Sigma).
Determination of Turnover of p53
Tc-inducible shRNA ESCs were cultured with or without Tc for 2 days, then treated with cycloheximide (30 μg/ml, Sigma), and harvested at the indicated time points. The p53 protein levels were detected by Western blotting and quantified using ImageJ software.
Bromodeoxyuridine (BrdU) Incorporation Assay
Cells were incubated with BrdU (Sigma) at the concentration of 10 μm for 30 min (ESCs) or 1 h (NIH3T3 cells) before harvesting. The amount of incorporated BrdU was detected by a Flow Cytometer (BD Biosciences) using a FITC-conjugated anti-BrdU monoclonal antibody (BD Biosciences) according to the manufacturer's instructions.
Cell Cycle Analysis
ESCs or NIH3T3 cells were fixed with 70% ethanol for 24 h at 4 °C, followed by RNase (Sigma, 100 μg/ml) treatment for 30 min and propidium iodide (PI) (Sigma, 50 μg/ml) staining. The stained cells were analyzed by a Flow Cytometer (BD Biosciences) to determine the cell cycle distribution pattern.
Annexin V/PI Staining
Tc-inducible shRNA ESCs were cultured with or without Tc for 3 days. Then these cells were harvested, and 105 cells were taken and stained with FITC-conjugated annexin V and PI (BD Biosciences) according to the manufacturer's instructions for flow cytometric analysis.
Alkaline Phosphatase (AKP) Staining
Tc-inducible shRNA ESCs were seeded at a low density in 6-well plates. After culturing with or without Tc for the indicated days, the colonies were fixed and stained with an AKP staining kit (Vector Laboratories) by a standard protocol.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
NIH3T3 cells were seeded onto 96-well plates at a density of 300 cells per well. 12 h after plating, 10 μl per well of 5 mg/ml MTT was added at the indicated time points, and 100 μl of dissolving buffer containing 10% SDS, 5% isopropyl alcohol, and 0.01 m HCl was added after incubation for 24 h. The absorbance was measured at 570 and 630 nm as the absorption and reference wavelength, respectively, after the cells were incubated for additional 12 h.
Microarray Analysis
Tc-inducible shRNA ESCs were treated with or without Tc for 3 days and then lysed in TRIzol for RNA extraction. RNA amplification for array analysis was performed with an Illumina TotalPrep RNA amplification kit (Ambion). Five milligrams of total RNA per sample were amplified and hybridized to Illumina Sentrix Mouse 6 BeadChip arrays according to the Illumina's instruction manual. Raw intensity data from BeadChip were exported to GeneSpring GX 9 and subjected to hierarchical clustering or pathway analysis. Three independent sets of samples were applied to microarray analysis.
Statistical Analysis
All values are shown as means ± S.D. The Student's t test was used to determine the significance of differences in comparisons. Values of p ≤ 0.05 were considered to be statistically significant.
RESULTS
Nucleolin Is Highly Expressed in Undifferentiated ESCs
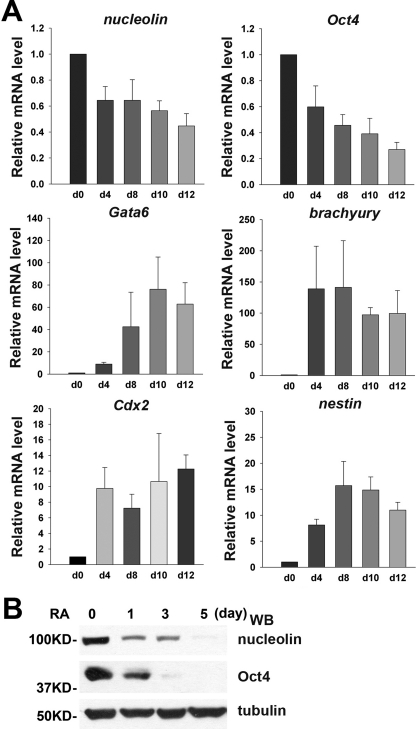
We began by examining the expression pattern of nucleolin during ESC differentiation. When cultured in suspension without LIF, ESCs can spontaneously form aggregates called EBs, which contain heterogeneous cell types representing all three embryonic germ layers (30). As shown in Fig. 1A, the transcript level of nucleolin quickly decreased upon EB formation at day 4 and remained at a low level up to day 12. In line with ESC differentiation, the expression levels of the pluripotency marker Oct4 reduced dramatically, and the levels of differentiation-associated markers (Cdx2, Gata6, brachyury, and nestin) increased significantly during EB formation. In addition, the steady-state level of nucleolin proteins reduced evidently upon retinoic acid (RA) treatment, similar to the reduction in the level of Oct4 protein in RA-treated cells (Fig. 1B). These findings suggest that the expression of nucleolin is associated with the ESC status.
FIGURE 1.
Nucleolin is highly expressed in undifferentiated ESCs and down-regulated during differentiation. A, qPCR analysis of the expression pattern of nucleolin, Oct4, and Nanog, as well as differentiation-associated markers, including Cdx2 (trophectoderm), Gata6 (primitive endoderm), brachyury (mesoderm), Fgf5, and nestin (ectoderm) in EBs aggregated from CGR8 mouse ESCs for the indicated times. All values are shown as means ± S.D. of results from three independent experiments. B, Western blot (WB) analysis of nucleolin and Oct4 protein levels during CGR8 ESC differentiation treated with RA for indicated time. Tubulin was used as a loading control.
Nucleolin Is Required for Cell Proliferation and Survival of ESCs
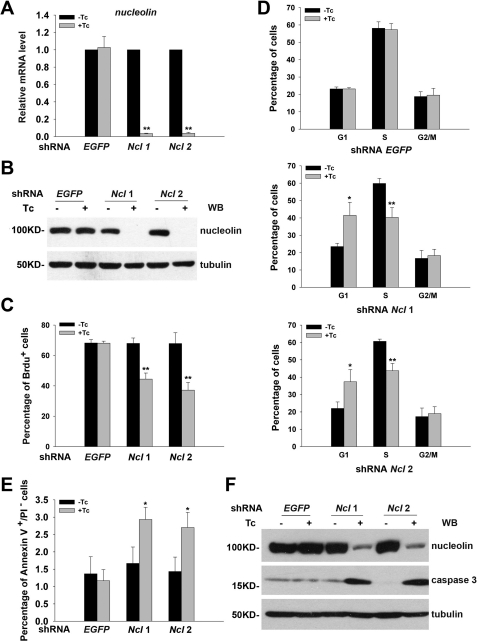
To study the function of nucleolin in ESCs, we established ESC lines stably integrated with Tc-inducible shRNAs targeting nucleolin sequences. To rule out the off-target effect of the shRNA, two sequences (shRNA nucleolin 1 and shRNA nucleolin 2) targeting the nucleolin coding sequence were used. The efficiency of shRNA induced by Tc treatment was validated by both qPCR and Western blot analyses (Fig. 2, A and B). Clearly, Tc treatment remarkably reduced both mRNA and protein levels of nucleolin in either shRNA nucleolin ESC line but did not affect nucleolin expression in shRNA EGFP-expressing cells, indicating that the silencing of nucleolin expression was specifically brought about by Tc-induced expression of shRNA nucleolin but not by Tc treatment per se.
FIGURE 2.
Knockdown of nucleolin leads to impaired cell cycle progression and apoptosis in ESCs. A, relative mRNA level of nucleolin in Tc-inducible shRNA nucleolin ESCs and shRNA EGFP ESCs cultured in the presence or absence of Tc for 3 days. All values are shown as means ± S.D. of results from three independent experiments. ** denotes p < 0.01. B, Western blot (WB) analysis was performed to examine the knockdown efficiency of the shRNA nucleolin as shown in A. Tubulin was used as a loading control. C, BrdU incorporation assay was applied to determine the proliferation ability of shRNA nucleolin ESCs treated with or without Tc for 3 days. All values are shown as means ± S.D. of results from three independent experiments. ** denotes p < 0.01. D, effect of nucleolin depletion on cell cycle distribution was examined by PI staining and fluorescence-activated cell sorting analysis. All values are shown as means ± S.D. of results from three independent experiments. * denotes p < 0.05; ** denotes p < 0.01. E, proportion of annexin V+/PI− cell population was compared between nucleolin-ablated ESCs and control ESCs. * denotes p < 0.05. F, Western blot analysis of the protein level of cleaved caspase 3 in shRNA nucleolin ESCs and shRNA EGFP ESCs treated with or without Tc for 3 days. Tubulin was used as a loading control.
Our previous study reported reduced cell growth and increased apoptosis when nucleolin expression was down-regulated in ESCs (19). To further define the role of nucleolin in controlling ESC proliferation, BrdU incorporation assays were performed. Significantly, knockdown of nucleolin reduced the proportion of BrdU+ cell population in both shRNA nucleolin-expressing ESC lines (Fig. 2C). In agreement with this finding, both nucleolin-deficient ESC lines had a higher percentage of cells in G1 phase with a reduction in S phase cell proportion (Fig. 2D). These results suggest that nucleolin is critical for ESC proliferation and cell cycle progression.
Next, we determined the effect of nucleolin silencing on early apoptosis using annexin V/PI staining approach. As shown in Fig. 2E, Tc-induced down-regulation of nucleolin led to an obvious increase in the annexin V+/PI− early apoptotic cell population. Consistently, Western blot analysis detected an elevation in the protein level of activated caspase 3 (17 kDa) in nucleolin-deficient ESCs (Fig. 2F), arguing for the requirement of nucleolin in the maintenance of ESC viability. Taken together, data obtained in this study demonstrate that the decrease in ESC growth rate caused by nucleolin depletion indicated in our previous study could be attributed to both increased cell apoptosis and reduced cell proliferation as a result from the cell accumulation in the G1 phase.
Nucleolin Plays a Critical Role in Maintaining ESC Self-renewal
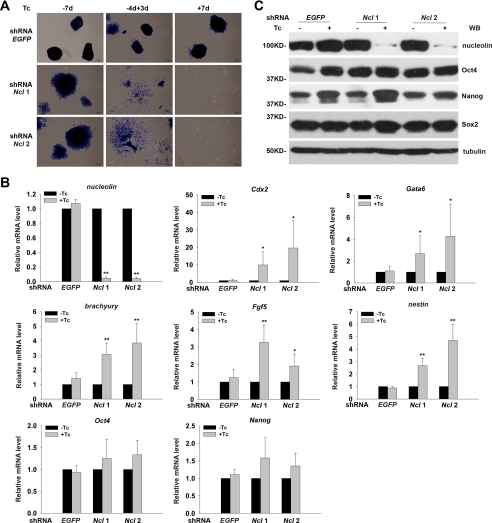
In addition to the abnormality in cell cycle progression and apoptosis, we observed evidently differentiated cell morphology in shRNA nucleolin ESCs under the self-renewal culture conditions, suggesting that nucleolin might play a role in the maintenance of ESC at an undifferentiated state. We conducted ESC colony-forming assays (4, 5) using Tc-inducible ESCs to evaluate the self-renewal ability of ESCs. Strikingly, knockdown of nucleolin expression for 3 days resulted in extensive cell differentiation, manifested as negative AKP staining and the differentiated cell morphology, whereas only few cells survived when the shRNA nucleolin ESCs were treated with Tc for 7 days (Fig. 3A). In contrast, shRNA EGFP ESCs formed typically compact AKP-positive colonies regardless of the presence or absence of Tc (Fig. 3A). This result suggests that nucleolin is required for ESC self-renewal, and its depletion could induce ESC differentiation and concomitant or consequent cell death.
FIGURE 3.
Depletion of nucleolin leads to ESC differentiation toward multiple lineages with continuous expression of pluripotency-associated factors. A, colony forming assay was used to determine the self-renewal ability of shRNA nucleolin and shRNA EGFP ESCs cultured under the indicated conditions. “−7d” indicates the absence of Tc for 7 days; “−4d+3d” indicates the absence of Tc for 4 days followed by Tc treatment for 3 days; “+7d” indicates continuous treatment with Tc for 7 days. B, transcript levels of pluripotency- or differentiation-related markers were determined by qPCR in shRNA nucleolin and shRNA EGFP ESCs treated with or without Tc for 3 days. All values are shown as means ± S.D. of results from three independent experiments. * denotes p < 0.05 and ** denotes p < 0.01. C, protein levels of Oct4, Nanog, and Sox2 were detected by Western blot (WB) analysis in ESCs as shown in B.
We then asked whether the differentiation induced by nucleolin silencing was specific to certain lineages or not. Transcript levels of various pluripotency- and differentiation-associated markers in shRNA nucleolin and shRNA EGFP ESCs treated with or without Tc for 3 days were analyzed. Data from qPCR analysis (Fig. 3B) indicated significant activation of all germ layer markers examined, including Cdx2 (trophectoderm), Gata6 (endoderm), brachyury (mesoderm), Fgf5 (primitive ectoderm), and nestin (ectoderm), upon silencing of nucleolin, implicating that ESC differentiation induced by nucleolin depletion is not biased to a specific lineage. Unexpectedly, the depletion of nucleolin did not reduce the expression of pluripotency genes (Oct4 and Nanog) as often seen in most ESC differentiation models.
To verify the continued expression of pluripotency-associated markers in the nucleolin-depleted and substantially differentiated ESCs, their protein levels were examined. As shown in Fig. 3C, silencing of nucleolin did not affect the steady-state levels of Oct4, Nanog, and Sox2 proteins either. These observations indicate that down-regulation of nucleolin disrupts ESC self-renewal without altering the expression of pluripotency-related genes.
Depletion of Nucleolin Activates p53-dependent Pathway in ESCs
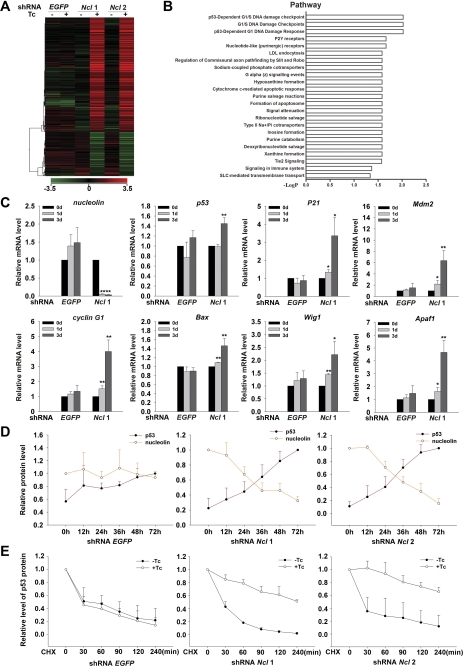
To understand the molecular mechanism responsible for the function of nucleolin in ESCs, we carried out a genome-wide microarray analysis to identify genes or pathways associated with silencing of nucleolin. RNA samples of two shRNA nucleolin ESC lines and shRNA EGFP ESCs cultured with or without Tc for 3 days were subjected to microarray analysis. The expression profiles uncovered 803 genes whose expression levels changed by ≥2-fold (p values ≤0.05) when nucleolin expression was knocked down. These genes were then subjected to the hierarchical clustering analysis (Fig. 4A) and enriched pathway analysis (Fig. 4B) using the GeneSpring GX9 software. Clustering analysis indicated that the number of activated genes (670) was much larger than the number of repressed genes (258) in nucleolin-silenced ESCs (Fig. 4A). The activated genes mostly participate in regulation of cell apoptosis, proliferation, and differentiation processes. The pathway analysis revealed that the p53-dependent G1/S DNA damage checkpoint pathway and the p53-dependent G1 DNA damage-response pathway were enriched after nucleolin expression was silenced (Fig. 4B), suggesting that p53-dependent pathways might be involved in the function of nucleolin in ESCs.
FIGURE 4.
p53-dependent pathways are activated by down-regulation of nucleolin. A, cluster analysis was performed to compare the differentially expressed genes between nucleolin down-regulated ESCs and control ESCs. The expression level of individual gene is indicated by colors, from green (low) to red (high). B, enriched pathways among the differentially expressed genes as shown in A were analyzed using GeneSpring GX 9 software. The microarray data can be viewed in GEO with the accession number GSE23993. C, activation of numerous p53 downstream genes in nucleolin-ablated ESCs observed in array data were confirmed in shRNA nucleolin 1 and shRNA EGFP ESCs treated with Tc for 0, 1, and 3 days by qPCR analysis. All values are shown as means ± S.D. of results from three independent experiments. * denotes p < 0.05 and ** denotes p < 0.01. D, averaged relative protein levels of p53 (the value at 72 h is defined as 1) and nucleolin (the value at 0 h is defined as 1) determined by Western blot analysis in shRNA nucleolin and shRNA EGFP ESCs treated with Tc for the indicated times were normalized against internal control tubulin. All values are shown as means ± S.D. of results from three independent experiments. E, relative protein levels of p53 in shRNA nucleolin and shRNA EGFP ESCs cultured in the absence or presence of Tc for 2 days and treated with cycloheximide (CHX) for the indicated times were detected by Western blotting and quantified by ImageJ (the value at 0 min is defined as 1). All values are shown as means ± S.D. of results from three independent experiments.
To validate the effect of nucleolin depletion on the activation of p53-dependent pathways, we examined the transcript levels of several p53 downstream genes related to its role in cell cycle regulation and apoptosis, including P21, cyclin G1, Bax, Wig1, Apaf1, etc. Data from qPCR analysis showed that all of the p53 target genes examined were significantly up-regulated when Tc induced depletion of nucleolin for 1 day (Fig. 4C), supporting that p53-dependent pathways were indeed activated in shRNA nucleolin ESCs, whereas the transcript level of p53 itself was only slightly elevated when Tc was added for 3 days (Fig. 4C). Consistent with activated p53-dependent pathways, we found that p53 protein levels increased gradually and significantly, whereas nucleolin protein levels decreased in a Tc treatment duration-dependent manner (supplemental Fig. S1A). The quantification data shown in Fig. 4D clearly revealed the inverse relationship between the protein levels of nucleolin and p53.
To understand how nucleolin regulates p53 protein levels, we examined the influence of nucleolin down-regulation on p53 protein stability, as p53 protein levels are primarily controlled by regulation of its protein stability. The turnover rate of p53 protein in control cells and shRNA nucleolin cells was compared in the presence of the protein synthesis inhibitor cycloheximide. We found that the stability of p53 proteins was significantly increased in nucleolin down-regulated ESCs (Fig. 4E and supplemental Fig. S1B). The result indicates that elevated p53 protein levels in shRNA nucleolin cells were, at least in part, due to the increased p53 protein stability.
Silencing of p53 Rescues Nucleolin Depletion-induced G1 Phase Accumulation and Apoptosis Completely
The contribution of activated p53-dependent pathways to nucleolin depletion-induced ESC phenotypes remains unknown, although regulation of p53 protein levels by nucleolin has been previously described in different types of human cells (31–33). To address this issue, we knocked down p53 expression using the synthesized p53 siRNA duplex oligoribonucleotides (oligos). Two p53 siRNA oligos (p53i-1 and p53i-2) as well as a negative control (N.C.) oligo were utilized. As shown in Fig. 5A, both p53i-1 and p53i-2 could substantially reduce the transcript level of p53 to less than 10% that in N.C.-transfected ESCs, without affecting the nucleolin level. Moreover, silencing of p53 expression entirely negated the activation of p53 target genes induced by silencing of nucleolin (Fig. 5A).
FIGURE 5.
Nucleolin ablation-induced cell cycle disruption and apoptosis in ESCs can be completely reversed by p53-specific siRNA oligos. A, quantification of nucleolin, p53, and its downstream genes by qPCR in shRNA nucleolin 1 ESCs transfected with either N.C. oligo or two independent oligos specific for targeting p53 (p53i-1 and p53i-2). All values are shown as means ± S.D. of results from six independent experiments. ** denotes p < 0.01. B, effect of p53 oligos on cell cycle distribution of shRNA nucleolin 1 ESCs treated with or without Tc for 3 days. All values are shown as means ± S.D. of results from three independent experiments. * denotes p < 0.05 and ** denotes p < 0.01. C, protein level of cleaved caspase 3 was examined in shRNA nucleolin 1 and 2 ESCs transfected with either N.C. or p53 siRNA oligos and cultured in the absence or presence of Tc treatment by Western blot (WB) analysis.
We then examined the cell cycle profile of shRNA nucleolin 1 ESCs and shRNA EGFP ESCs transfected with N.C., p53i-1 and p53i-2, respectively, in the absence or presence of Tc. As shown in Fig. 5B, both p53 siRNA oligos completely abolished the abnormal cell cycle profile induced by nucleolin depletion, suggesting a primary role of p53 for the accumulation of cells in G1 phase. Moreover, nucleolin depletion-induced apoptosis totally vanished as indicated by the reduction of the active form of caspase 3 protein levels when either p53 siRNA oligo was introduced into these cells (Fig. 5C). These findings support the notion that the activated p53-dependent pathway is a major contributor to the enhanced apoptosis detected in nucleolin-depleted ESCs.
Down-regulation of p53 Partially Blocks Nucleolin Depletion-induced ESC Differentiation
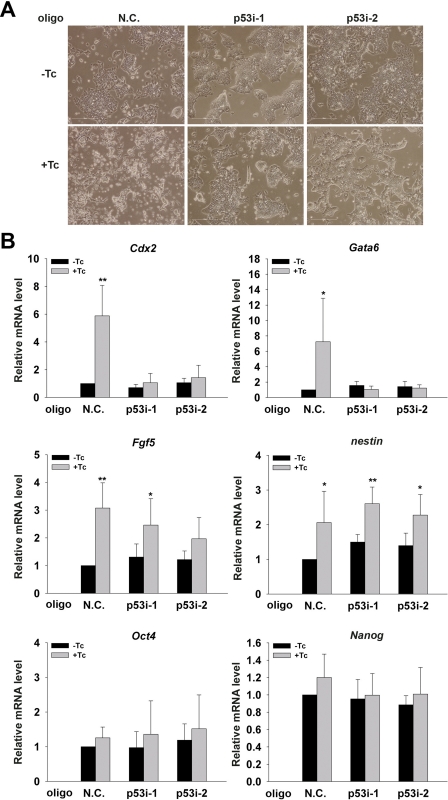
Having shown the predominant role of the activated p53 pathway in the abnormal cell cycle profile and enhanced apoptosis when nucleolin was silenced, we wanted to know whether depletion of p53 could also block nucleolin silencing-mediated disruption of ESC self-renewal. As expected, based on the cell morphology, we found that silencing of nucleolin resulted in severe differentiation in N.C.-transfected ESCs (Fig. 6A). Interestingly, silence of p53 in Tc-treated shRNA nucleolin 1 ESCs partially but obviously recovered the differentiated cell morphology (Fig. 6A), suggesting that p53 may be also responsible for the disrupted self-renewal in the nucleolin-depleted ESCs.
FIGURE 6.
Abnormal differentiation of nucleolin knockdown ESCs can be partially reversed by p53 silencing. A, representative phase-contrast images of shRNA nucleolin 1 ESCs transfected with p53 siRNA oligos or N.C. cultured in the presence or absence of Tc. B, transcript levels of Oct4, Nanog, and differentiation-associated marker genes in N.C. or p53 siRNA oligos transfected with shRNA nucleolin 1 ESCs were quantified by qPCR analysis. All values are shown as means ± S.D. of results from six independent experiments. * denotes p < 0.05 and ** denotes p < 0.01.
To further characterize the role of p53 in nucleolin depletion-mediated disruption of ESC self-renewal, we examined transcript levels of various differentiation-associated markers as well as Oct4 and Nanog. Data from qPCR analysis showed that depletion of p53 completely negated the activation of Cdx2 and Gata6 expression induced by nucleolin deficiency (Fig. 6B). In contrast, it could only partially prevent the nucleolin depletion-induced activation of Fgf5 but could not recover the expression level of nestin (Fig. 6B). Therefore, it appears that activation of the p53-dependent pathway was in part associated with ESC differentiation caused by the silencing of nucleolin. In addition, the mRNA levels of Oct4 and Nanog were not affected by silencing of p53, excluding the possibility that rescue of ESC differentiation under the condition of nucleolin depletion is due to the increase in expression of pluripotency-associated genes.
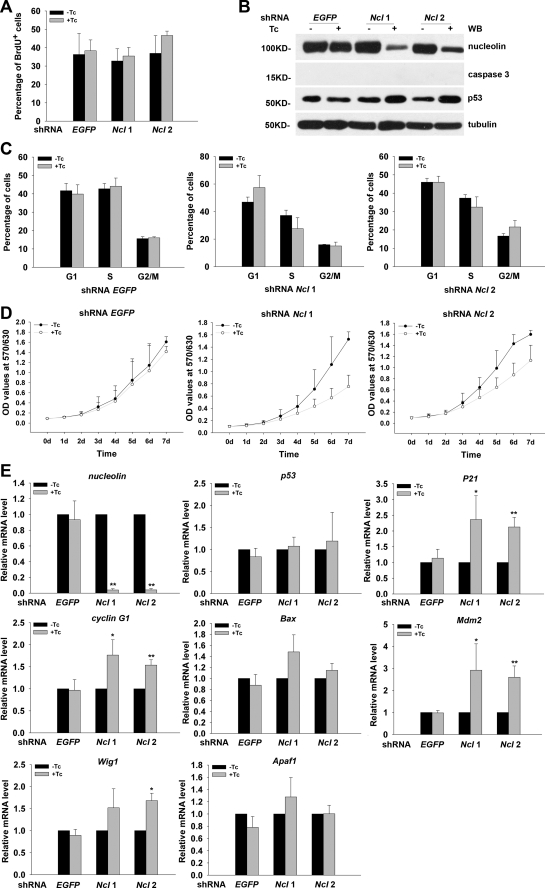
Depletion of Nucleolin Does Not Affect Cell Survival and Proliferation Evidently in NIH3T3 Cells
To determine whether the role of nucleolin in regulation of cell proliferation and viability is unique to ESCs, we generated Tc-inducible shRNA nucleolin NIH3T3 cell lines and examined whether depletion of nucleolin in NIH3T3 could result in similar phenotypes to those observed in ESCs. First, we conducted BrdU incorporation assay to examine the impact of nucleolin deletion on the proliferation ability in NIH3T3 cells, and we found that, after a 3-day Tc treatment, the percentage of BrdU+ cells was comparable between control NIH3T3 cells and nucleolin knockdown NIH3T3 cells (Fig. 7A), although the Tc-induced depletion of nucleolin is as efficient as that in ESCs (Fig. 7, B and E). Analysis of cell cycle profile revealed that nucleolin deficiency did not affect the cell cycle progression in NIH3T3 cells when they were treated with Tc for 3 days (Fig. 7C). Moreover, there were few obvious apoptotic cells in culture, and the protein level of active form caspase 3 was undetectable in NIH3T3 cells after induction of nucleolin shRNA for 3 days (Fig. 7B), indicating that short term nucleolin depletion did not induce apoptosis in NIH3T3 cells. To find out whether knockdown nucleolin for a long time would cause more obvious defects in NIH3T3 cells, we performed MTT assays for measuring the cell growth rate in a 7-day time course. As shown in Fig. 7D, long term induction of nucleolin shRNA expression resulted in a reduced cell growth rate in NIH3T3 cells, although the extent of reduction was much less than that observed in ESCs. These observations suggest that nucleolin might not be so important for the proliferation and survival in NIH3T3 cells as it was in pluripotent ESCs. We next asked whether nucleolin would control p53 protein levels as it did in ESCs. Consistent with its minor influence on cell proliferation and cell survival, knockdown of nucleolin in NIH3T3 cells slightly elevated the p53 protein level (Fig. 7B) and the transcription level of its downstream genes (Fig. 7E). These findings reveal that ESCs and differentiated cells response differently to the silence of nucleolin.
FIGURE 7.
Down-regulation of nucleolin does not affect cell cycle profile and survival in NIH3T3 cells significantly. A, BrdU incorporation assay was performed to determine the proliferation rate of shRNA nucleolin NIH3T3 cells treated with or without Tc for 3 days. All values are shown as means ± S.D. of results from three independent experiments. B, Western blot (WB) analysis was performed to examine the protein levels of nucleolin and active form of caspase 3 and p53 in shRNA EGFP and shRNA nucleolin NIH3T3 cell lines cultured in the presence or absence of Tc for 3 days. Tubulin was used as a loading control. C, effect of nucleolin depletion on cell cycle distribution was examined by PI staining and fluorescence-activated cell sorting analysis. All values are shown as means ± S.D. of results from three independent experiments. D, growth rate of shRNA EGFP or shRNA nucleolin NIH3T3 cell lines cultured with or without Tc for the indicated time was measured by MTT assays. All values are shown as means ± S.D. of results from three independent experiments. E, relative mRNA level of nucleolin, p53, and its target genes in Tc-inducible shRNA nucleolin and shRNA EGFP NIH3T3 cells cultured in the presence or absence of Tc for 3 days. All values are shown as means ± S.D. of results from three independent experiments. *, p < 0.05; **, p < 0.01.
DISCUSSION
Nucleolin is an intensively studied nucleolar protein highly expressed in rapidly dividing cells, and it has been demonstrated to participate in numerous biological processes. Our previous work suggested that nucleolin is situated in the nucleolus of mouse ESCs and might play an important role in maintaining cell growth of undifferentiated mouse ESCs (19). Other than that, no information about the function of nucleolin in ESC has been reported to date. In this study, using the shRNA-mediated depletion approach, we systematically investigated the function of nucleolin in controlling cell proliferation, apoptosis, cell cycle progression, and differentiation of ESCs, and we found that knockdown of nucleolin resulted in enhanced ESC apoptosis and reduced cell proliferation, which probably due to G1 phase accumulation and reduction in S phase cell population. In addition, depletion of nucleolin resulted in extensive ESC differentiation into multiple lineages. Thus, our data demonstrate that nucleolin is required for maintenance of ESC self-renewal, adding a new variety to the already long list of nucleolin functions and a new factor required to sustain the ESC identity.
Numerous reports have implicated the involvement of nucleolin in regulation of cell proliferation and cell growth in many cell types (28, 32). However, the molecular mechanisms responsible for its function remain ambiguous. An elevated protein level of p53 was observed when nucleolin was down-regulated in human HeLa and primary fibroblast cells (32) as well as in MCF-7 cells after DNA damage (33). The increase in p53 protein levels has been considered as a result of the increased p53 mRNA stability or protein translation in nucleolin-depleted human cells. Interestingly, it was also reported that nucleolin stabilized p53 proteins through inhibiting Hdm2 in unstressed human cells (31). In this study, we found that nucleolin silencing could elevate p53 protein levels partially through increasing its protein stability. Therefore, the interplay between these two factors is complex and probably is nucleolin dosage- and cell context-dependent. However, what functional roles the elevated p53 protein level played in the phenotypes caused by the nucleolin depletion in these studies was not tested. Here, we first report that down-regulation of nucleolin in unstressed mouse ESCs is accompanied by the activation of p53-dependent pathway based on the fact that the protein level of p53 and the expression of its major target genes are markedly elevated in nucleolin-deficient ESCs. Most importantly, we experimentally demonstrated that most, if not all, of the phenotypes observed in ESCs depleted of nucleolin could be attributed to activated p53-dependent pathways, because knocking down p53 expression could rescue the majority of cell defects brought about by nucleolin depletion. Therefore, p53 is likely a major mediator downstream of nucleolin to control unique properties of ESCs. Further investigations are required to identify other factors associated with nucleolin deficiency-induced ESC differentiation, as down-regulation of p53 could only partially abrogate the ESC differentiation phenotypes.
In addition to the control of p53 protein levels by nucleolin, nucleolin depletion-induced cellular stress and, in particular, nucleolar disruption could also activate p53-dependent pathways in ESCs. As a major component of the nucleolus, nucleolin probably is required for the survival and proliferation of most, if not all, mammalian cell types. However, it is known that its expression level is higher in tumors and actively dividing cells (34). Here, we report that nucleolin protein levels are higher in undifferentiated ESCs than in differentiated ESCs and are rapidly down-regulated upon differentiation, implying that nucleolin might have a distinct role in controlling ESC proliferation and survival. Supporting this notion, silencing of nucleolin to a comparable extent in differentiated cell type, NIH3T3 fibroblast cells, did not activate p53-dependent pathways and affect cell survival as well as proliferation significantly, as it did in ESCs. Furthermore, down-regulation of nucleolin could not enhance the protein level of p53 markedly in MCF-7 cells in the absence of ionizing irradiation (33). It appears that undifferentiated ESCs are more sensitive to the level of nucleolin than differentiated cell types.
The role of p53 in controlling ESC self-renewal and differentiation as well as somatic cell reprogramming has been a focus point for researchers. Induction of ESC differentiation by p53 has been reported in both mouse and human ESCs when they were exposed to DNA-damaging agents (35, 36). The pro-differentiation role of p53 in mouse ESCs was thought mediated through its repression of Nanog, one of the core transcription factors for pluripotency and self-renewal of ESCs (5). Unexpectedly, we did not observe any reduction in the expression of Nanog, Oct4, and Sox2 at all, despite the obvious differentiation of cell morphology as well as the elevated expression levels of various differentiation associated markers in nucleolin-depleted ESCs. A similar phenomenon was detected in Foxd3-depleted ESCs, in which precocious differentiation along multiple lineages occurred with continuous expression of three core pluripotency-associated transcription factors (37). These data suggest that expression of the pluripotency factor alone is not sufficient to sustain ESC self-renewal. However, our results suggest that p53 triggers ESC differentiation in a Nanog-independent manner. Considering the evident higher percentage of cells in G1 phase in nucleolin-depleted ESCs, we postulate that p53-mediated accumulation of cells in G1 phase may be a determining factor leading to ESC differentiation. It is well known that ESCs possess distinctive cell cycle features (13). In particular, a very short cell cycle and the absence of early G1 in mouse ESCs allow them to avoid differentiation-inducing signals. Further supporting this, Singh and Dalton (38) recently proposed that lengthening of G1 phase appears to be a cause rather than a consequence of ESC differentiation (39). It is possible that nucleolin depletion-mediated activation of p53 pathways causes ESCs to acquire the early G1 phase and thus be able to receive differentiation-inducing stimuli. This may represent the unique response of ESCs to the nucleolin depletion-activated p53-dependent pathways.
Collectively, our study describes an essential role for nucleolin in control of ESC self-renewal and provides experimental evidence of p53 as a major target of nucleolin functions in maintaining the unique cell cycle profile and survival in ESCs. In addition, our study emphasizes the notion that the unique cell cycle feature of ESC is an important mediator in choosing between self-renewal and lineage commitment. Thus, maintaining the unique cell cycle features of ESCs appears to be a critical factor in preventing differentiation.
Supplementary Material
This work was supported by National Natural Science Foundation Grants 91019023, 30730051, 30911130361, and 31100990, National High Technology Research and Development Program of China Grants 2010CB945201, 2011CB965101, 2009CB941103, 2007CB947904, 2007CB948004, and 2011DFB30010, Chinese Academy of Science Grant XDA01010102, China Postdoctoral Science Foundation Grant 20090460626, Shanghai Postdoctoral Research Plan, and Shanghai Leading Academic Discipline Project Grant S30201.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- ESC
- embryonic stem cell
- AKP
- alkaline phosphatase
- EB
- embryoid body
- EGFP
- enhanced green fluorescent protein
- LIF
- leukemia inhibitory factor
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- N.C.
- negative control
- oligo
- oligoribonucleotide
- PI
- propidium iodide
- qPCR
- quantitative real time PCR
- RA
- retinoic acid
- Tc
- tetracycline.
REFERENCES
- 1. Smith A. G. (2001) Annu. Rev. Cell Dev. Biol. 17, 435–462 [DOI] [PubMed] [Google Scholar]
- 2. Boheler K. R. (2009) J. Cell. Physiol. 221, 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burdon T., Smith A., Savatier P. (2002) Trends Cell Biol. 12, 432–438 [DOI] [PubMed] [Google Scholar]
- 4. Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Schöler H., Smith A. (1998) Cell 95, 379–391 [DOI] [PubMed] [Google Scholar]
- 5. Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. (2003) Cell 113, 643–655 [DOI] [PubMed] [Google Scholar]
- 6. Masui S., Nakatake Y., Toyooka Y., Shimosato D., Yagi R., Takahashi K., Okochi H., Okuda A., Matoba R., Sharov A. A., Ko M. S., Niwa H. (2007) Nat. Cell Biol. 9, 625–635 [DOI] [PubMed] [Google Scholar]
- 7. van den Berg D. L., Zhang W., Yates A., Engelen E., Takacs K., Bezstarosti K., Demmers J., Chambers I., Poot R. A. (2008) Mol. Cell. Biol. 28, 5986–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pardo M., Lang B., Yu L., Prosser H., Bradley A., Babu M. M., Choudhary J. (2010) Cell Stem Cell. 6, 382–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen X., Vega V. B., Ng H. H. (2008) Cold Spring Harb. Symp. Quant. Biol. 73, 203–209 [DOI] [PubMed] [Google Scholar]
- 10. Hanna J., Cheng A. W., Saha K., Kim J., Lengner C. J., Soldner F., Cassady J. P., Muffat J., Carey B. W., Jaenisch R. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 9222–9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lagarkova M. A., Volchkov P. Y., Lyakisheva A. V., Philonenko E. S., Kiselev S. L. (2006) Cell Cycle 5, 416–420 [DOI] [PubMed] [Google Scholar]
- 12. Bibikova M., Laurent L. C., Ren B., Loring J. F., Fan J. B. (2008) Cell Stem Cell. 2, 123–134 [DOI] [PubMed] [Google Scholar]
- 13. Orford K. W., Scadden D. T. (2008) Nat. Rev. Genet. 9, 115–128 [DOI] [PubMed] [Google Scholar]
- 14. Kafienah W., Mistry S., Williams C., Hollander A. P. (2006) Stem Cells 24, 1113–1120 [DOI] [PubMed] [Google Scholar]
- 15. Tsai R. Y., McKay R. D. (2002) Genes Dev. 16, 2991–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nomura J., Maruyama M., Katano M., Kato H., Zhang J., Masui S., Mizuno Y., Okazaki Y., Nishimoto M., Okuda A. (2009) Stem Cells 27, 1066–1076 [DOI] [PubMed] [Google Scholar]
- 17. Abujarour R., Efe J., Ding S. (2010) Stem Cells 28, 1487–1497 [DOI] [PubMed] [Google Scholar]
- 18. Wang B. B., Lu R., Wang W. C., Jin Y. (2006) Biochem. Biophys. Res. Commun. 347, 1129–1137 [DOI] [PubMed] [Google Scholar]
- 19. Li H., Wang B., Yang A., Lu R., Wang W., Zhou Y., Shi G., Kwon S. W., Zhao Y., Jin Y. (2009) Stem Cells 27, 1244–1254 [DOI] [PubMed] [Google Scholar]
- 20. Bugler B., Caizergues-Ferrer M., Bouche G., Bourbon H., Amalric F. (1982) Eur. J. Biochem. 128, 475–480 [DOI] [PubMed] [Google Scholar]
- 21. Lapeyre B., Bourbon H., Amalric F. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 1472–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen C. M., Chiang S. Y., Yeh N. H. (1991) J. Biol. Chem. 266, 7754–7758 [PubMed] [Google Scholar]
- 23. Fang S. H., Yeh N. H. (1993) Exp. Cell Res. 208, 48–53 [DOI] [PubMed] [Google Scholar]
- 24. Orrick L. R., Olson M. O., Busch H. (1973) Proc. Natl. Acad. Sci. U.S.A. 70, 1316–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tuteja R., Tuteja N. (1998) Crit. Rev. Biochem. Mol. Biol. 33, 407–436 [DOI] [PubMed] [Google Scholar]
- 26. Ginisty H., Sicard H., Roger B., Bouvet P. (1999) J. Cell Sci. 112, 761–772 [DOI] [PubMed] [Google Scholar]
- 27. Mongelard F., Bouvet P. (2007) Trends Cell Biol. 17, 80–86 [DOI] [PubMed] [Google Scholar]
- 28. Srivastava M., Pollard H. B. (1999) FASEB J. 13, 1911–1922 [PubMed] [Google Scholar]
- 29. van de Wetering M., Oving I., Muncan V., Pon Fong M. T., Brantjes H., van Leenen D., Holstege F. C., Brummelkamp T. R., Agami R., Clevers H. (2003) EMBO Rep. 4, 609–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Desbaillets I., Ziegler U., Groscurth P., Gassmann M. (2000) Exp. Physiol. 85, 645–651 [PubMed] [Google Scholar]
- 31. Saxena A., Rorie C. J., Dimitrova D., Daniely Y., Borowiec J. A. (2006) Oncogene 25, 7274–7288 [DOI] [PubMed] [Google Scholar]
- 32. Ugrinova I., Monier K., Ivaldi C., Thiry M., Storck S., Mongelard F., Bouvet P. (2007) BMC Mol. Biol. 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takagi M., Absalon M. J., McLure K. G., Kastan M. B. (2005) Cell 123, 49–63 [DOI] [PubMed] [Google Scholar]
- 34. Derenzini M., Sirri V., Trerè D., Ochs R. L. (1995) Lab. Invest. 73, 497–502 [PubMed] [Google Scholar]
- 35. Qin H., Yu T., Qing T., Liu Y., Zhao Y., Cai J., Li J., Song Z., Qu X., Zhou P., Wu J., Ding M., Deng H. (2007) J. Biol. Chem. 282, 5842–5852 [DOI] [PubMed] [Google Scholar]
- 36. Lin T., Chao C., Saito S., Mazur S. J., Murphy M. E., Appella E., Xu Y. (2005) Nat. Cell Biol. 7, 165–171 [DOI] [PubMed] [Google Scholar]
- 37. Liu Y., Labosky P. A. (2008) Stem Cells 26, 2475–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Singh A. M., Dalton S. (2009) Cell Stem Cell 5, 141–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lange C., Calegari F. (2010) Cell Cycle 9, 1893–1900 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.