Abstract
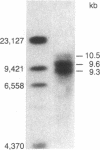
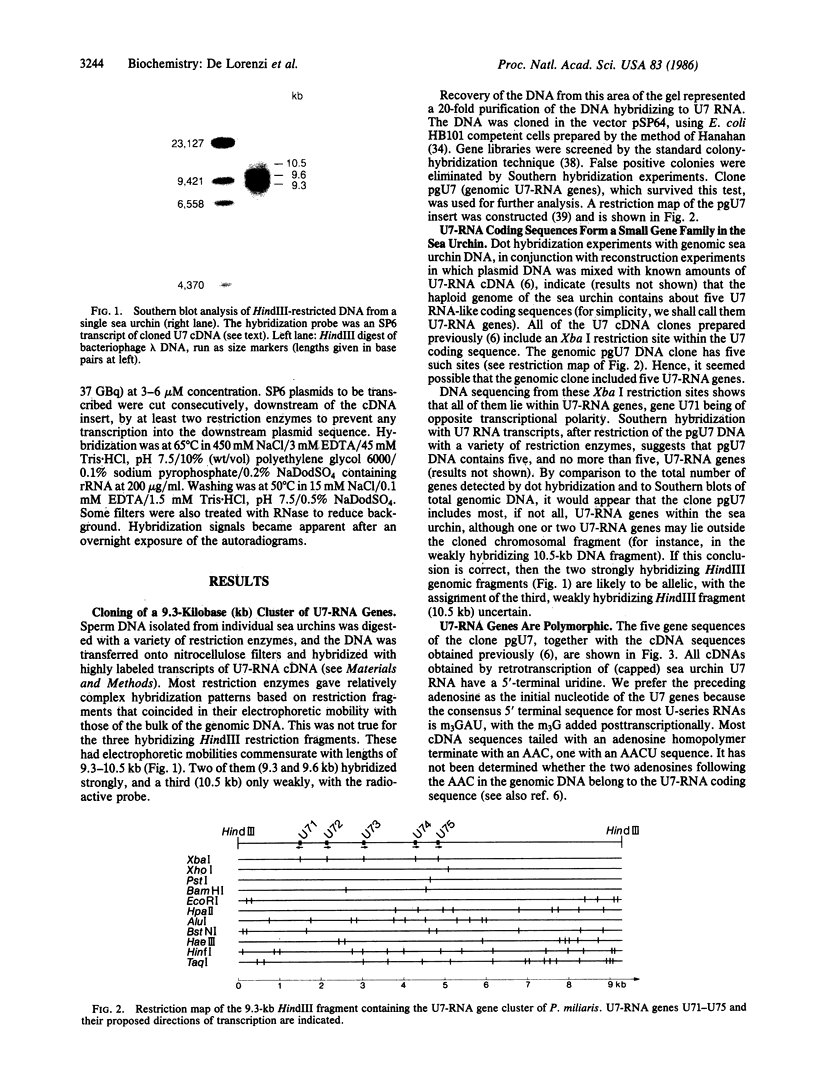
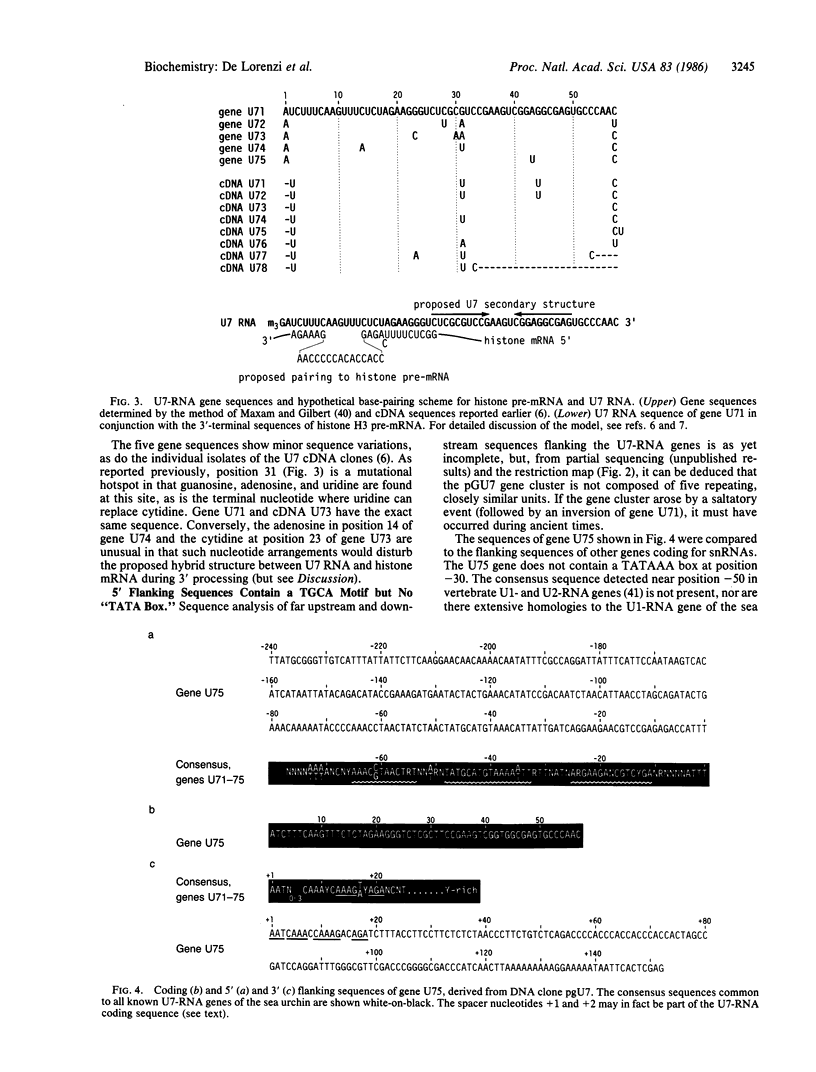
A genomic 9.3-kilobase DNA fragment of the sea urchin Psammechinus miliaris, containing a cluster of five U7-RNA genes (or pseudogenes), has been isolated and analyzed by partial DNA sequencing. The U7-RNA coding sequences differ from one another by one or two nucleotides, one of the five gene sequences being identical to those of the cDNA U73 clone prepared earlier [Strub, K., Galli, G., Busslinger, M. & Birnstiel, M. L. (1984) EMBO J. 3, 2801-2807]. The spacer sequences separating the genes have, on the whole, a low degree of homology; hence, the five genes must have arisen by an ancient duplication event. The sequences preceding the coding portion contain three highly conserved sequence motifs but no "TATA box." The 3' flanking sequences include a highly conserved AAAGNNAGA sequence that is held in common with other U-RNA genes from both sea urchins and vertebrates. Our findings confirm our classification of the U7 RNA as a genuine, if sparsely represented, member of the U-RNA family.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso A., Beck E., Jorcano J. L., Hovemann B. Divergence of U2 snRNA sequences in the genome of D. melanogaster. Nucleic Acids Res. 1984 Dec 21;12(24):9543–9550. doi: 10.1093/nar/12.24.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A., Jorcano J. L., Beck E., Hovemann B., Schmidt T. Drosophila melanogaster U1 snRNA genes. J Mol Biol. 1984 Dec 25;180(4):825–836. doi: 10.1016/0022-2836(84)90259-6. [DOI] [PubMed] [Google Scholar]
- Ares M., Jr, Mangin M., Weiner A. M. Orientation-dependent transcriptional activator upstream of a human U2 snRNA gene. Mol Cell Biol. 1985 Jul;5(7):1560–1570. doi: 10.1128/mcb.5.7.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Folk W., Birnstiel M. L. The terminal RNA stem-loop structure and 80 bp of spacer DNA are required for the formation of 3' termini of sea urchin H2A mRNA. Cell. 1983 Dec;35(2 Pt 1):433–440. doi: 10.1016/0092-8674(83)90176-9. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Black D. L., Chabot B., Steitz J. A. U2 as well as U1 small nuclear ribonucleoproteins are involved in premessenger RNA splicing. Cell. 1985 Oct;42(3):737–750. doi: 10.1016/0092-8674(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Morris G. F., Chodchoy N., Sprecher C., Marzluff W. F. Structure of the sea urchin U1 RNA repeat. Nucleic Acids Res. 1985 Jan 25;13(2):537–556. doi: 10.1093/nar/13.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland R. A., Cooke H. J., Roy K. L., Dahlberg J. E., Lund E. Isolation and characterization of three cloned fragments of human DNA coding for tRNAs and small nuclear RNA U1. Gene. 1983 May-Jun;22(2-3):211–217. doi: 10.1016/0378-1119(83)90105-1. [DOI] [PubMed] [Google Scholar]
- Card C. O., Morris G. F., Brown D. T., Marzluff W. F. Sea urchin small nuclear RNA genes are organized in distinct tandemly repeating units. Nucleic Acids Res. 1982 Dec 11;10(23):7677–7688. doi: 10.1093/nar/10.23.7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberto G., Buckland R., Cortese R., Philipson L. Transcription signals in embryonic Xenopus laevis U1 RNA genes. EMBO J. 1985 Jun;4(6):1537–1543. doi: 10.1002/j.1460-2075.1985.tb03814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley J. M., 3rd, Roebuck K. A., Stumph W. E. Three linked chicken U1 RNA genes have limited flanking DNA sequence homologies that reveal potential regulatory signals. Nucleic Acids Res. 1984 Oct 11;12(19):7411–7421. doi: 10.1093/nar/12.19.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Stunnenberg H. G., Birnstiel M. L. Biochemical complementation with RNA in the Xenopus oocyte: a small RNA is required for the generation of 3' histone mRNA termini. Cell. 1983 Oct;34(3):823–828. doi: 10.1016/0092-8674(83)90539-1. [DOI] [PubMed] [Google Scholar]
- Georgiev O., Birnstiel M. L. The conserved CAAGAAAGA spacer sequence is an essential element for the formation of 3' termini of the sea urchin H3 histone mRNA by RNA processing. EMBO J. 1985 Feb;4(2):481–489. doi: 10.1002/j.1460-2075.1985.tb03654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev O., Mous J., Birnstiel M. L. Processing and nucleo-cytoplasmic transport of histone gene transcripts. Nucleic Acids Res. 1984 Nov 26;12(22):8539–8551. doi: 10.1093/nar/12.22.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harvey R. P., Robins A. J., Wells J. R. Independently evolving chicken histone H2B genes: identification of a ubiquitous H2B-specific 5' element. Nucleic Acids Res. 1982 Dec 11;10(23):7851–7863. doi: 10.1093/nar/10.23.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K. Organization of sequences related to U6 RNA in the human genome. Nucleic Acids Res. 1981 Jul 24;9(14):3379–3388. doi: 10.1093/nar/9.14.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Harada F. Nucleotide sequence of nuclear 5.4 S RNA of mouse cells. Biochim Biophys Acta. 1984 Jun 16;782(2):127–131. doi: 10.1016/0167-4781(84)90015-0. [DOI] [PubMed] [Google Scholar]
- Krol A., Lund E., Dahlberg J. E. The two embryonic U1 RNA genes of Xenopus laevis have both common and gene-specific transcription signals. EMBO J. 1985 Jun;4(6):1529–1535. doi: 10.1002/j.1460-2075.1985.tb03813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer A., Keller W., Appel B., Lührmann R. The 5' terminus of the RNA moiety of U1 small nuclear ribonucleoprotein particles is required for the splicing of messenger RNA precursors. Cell. 1984 Aug;38(1):299–307. doi: 10.1016/0092-8674(84)90551-8. [DOI] [PubMed] [Google Scholar]
- Kunkel G. R., Pederson T. Transcription boundaries of U1 small nuclear RNA. Mol Cell Biol. 1985 Sep;5(9):2332–2340. doi: 10.1128/mcb.5.9.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E., Dahlberg J. E. True genes for human U1 small nuclear RNA. Copy number, polymorphism, and methylation. J Biol Chem. 1984 Feb 10;259(3):2013–2021. [PubMed] [Google Scholar]
- Manser T., Gesteland R. F. Human U1 loci: genes for human U1 RNA have dramatically similar genomic environments. Cell. 1982 May;29(1):257–264. doi: 10.1016/0092-8674(82)90110-6. [DOI] [PubMed] [Google Scholar]
- Marzluff W. F., Brown D. T., Lobo S., Wang S. S. Isolation and characterization of two linked mouse U1b small nuclear RNA genes. Nucleic Acids Res. 1983 Sep 24;11(18):6255–6270. doi: 10.1093/nar/11.18.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I. W., Lienhard S., Jiricny J., De Robertis E. M. An enhancer-like sequence within the Xenopus U2 gene promoter facilitates the formation of stable transcription complexes. Nature. 1985 Jul 11;316(6024):163–167. doi: 10.1038/316163a0. [DOI] [PubMed] [Google Scholar]
- Mattaj I. W., Zeller R. Xenopus laevis U2 snRNA genes: tandemly repeated transcription units sharing 5' and 3' flanking homology with other RNA polymerase II transcribed genes. EMBO J. 1983;2(11):1883–1891. doi: 10.1002/j.1460-2075.1983.tb01675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Steitz J. A. Sequence of U1 RNA from Drosophila melanogaster: implications for U1 secondary structure and possible involvement in splicing. Nucleic Acids Res. 1981 Dec 11;9(23):6351–6368. doi: 10.1093/nar/9.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H., Kornberg R. D. Genes and pseudogenes for mouse U1 and U2 small nuclear RNAs. J Biol Chem. 1983 Jul 10;258(13):8151–8155. [PubMed] [Google Scholar]
- Ohshima Y., Okada N., Tani T., Itoh Y., Itoh M. Nucleotide sequences of mouse genomic loci including a gene or pseudogene for U6 (4.8S) nuclear RNA. Nucleic Acids Res. 1981 Oct 10;9(19):5145–5158. doi: 10.1093/nar/9.19.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestayko A. W., Tonato M., Busch H. Low molecular weight RNA associated with 28 s nucleolar RNA. J Mol Biol. 1970 Feb 14;47(3):505–515. doi: 10.1016/0022-2836(70)90318-9. [DOI] [PubMed] [Google Scholar]
- Reddy R., Busch H. Small nuclear RNAs and RNA processing. Prog Nucleic Acid Res Mol Biol. 1983;30:127–162. doi: 10.1016/s0079-6603(08)60685-6. [DOI] [PubMed] [Google Scholar]
- Reddy R., Henning D., Busch H. Primary and secondary structure of U8 small nuclear RNA. J Biol Chem. 1985 Sep 15;260(20):10930–10935. [PubMed] [Google Scholar]
- Roop D. R., Kristo P., Stumph W. E., Tsai M. J., O'Malley B. W. Structure and expression of a chicken gene coding for U1 RNA. Cell. 1981 Mar;23(3):671–680. doi: 10.1016/0092-8674(81)90430-x. [DOI] [PubMed] [Google Scholar]
- Skuzeski J. M., Lund E., Murphy J. T., Steinberg T. H., Burgess R. R., Dahlberg J. E. Synthesis of human U1 RNA. II. Identification of two regions of the promoter essential for transcription initiation at position +1. J Biol Chem. 1984 Jul 10;259(13):8345–8352. [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroke I. L., Weiner A. M. Genes and pseudogenes for rat U3A and U3B small nuclear RNA. J Mol Biol. 1985 Jul 20;184(2):183–193. doi: 10.1016/0022-2836(85)90372-9. [DOI] [PubMed] [Google Scholar]
- Strub K., Galli G., Busslinger M., Birnstiel M. L. The cDNA sequences of the sea urchin U7 small nuclear RNA suggest specific contacts between histone mRNA precursor and U7 RNA during RNA processing. EMBO J. 1984 Dec 1;3(12):2801–2807. doi: 10.1002/j.1460-2075.1984.tb02212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani T., Watanabe-Nagasu N., Okada N., Ohshima Y. Molecular cloning and characterization of a gene for rat U2 small nuclear RNA. J Mol Biol. 1983 Aug 15;168(3):579–594. doi: 10.1016/s0022-2836(83)80303-9. [DOI] [PubMed] [Google Scholar]
- Van Arsdell S. W., Weiner A. M. Human genes for U2 small nuclear RNA are tandemly repeated. Mol Cell Biol. 1984 Mar;4(3):492–499. doi: 10.1128/mcb.4.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe-Nagasu N., Itoh Y., Tani T., Okano K., Koga N., Okada N., Ohshima Y. Structural analysis of gene loci for rat U1 small nuclear RNA. Nucleic Acids Res. 1983 Mar 25;11(6):1791–1801. doi: 10.1093/nar/11.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Lund E., Murphy J. T., Pettersson U., Dahlberg J. E. Human U2 and U1 RNA genes use similar transcription signals. EMBO J. 1984 Dec 20;3(13):3295–3301. doi: 10.1002/j.1460-2075.1984.tb02293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Zabielski J., Hammarström K., Monstein H. J., Bark C., Pettersson U. Clustered genes for human U2 RNA. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3811–3815. doi: 10.1073/pnas.81.12.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise J. A., Tollervey D., Maloney D., Swerdlow H., Dunn E. J., Guthrie C. Yeast contains small nuclear RNAs encoded by single copy genes. Cell. 1983 Dec;35(3 Pt 2):743–751. doi: 10.1016/0092-8674(83)90107-1. [DOI] [PubMed] [Google Scholar]
- Yuo C. Y., Ares M., Jr, Weiner A. M. Sequences required for 3' end formation of human U2 small nuclear RNA. Cell. 1985 Aug;42(1):193–202. doi: 10.1016/s0092-8674(85)80115-x. [DOI] [PubMed] [Google Scholar]
- Zeller R., Carri M. T., Mattaj I. W., De Robertis E. M. Xenopus laevis U1 snRNA genes: characterisation of transcriptionally active genes reveals major and minor repeated gene families. EMBO J. 1984 May;3(5):1075–1081. doi: 10.1002/j.1460-2075.1984.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]