Abstract
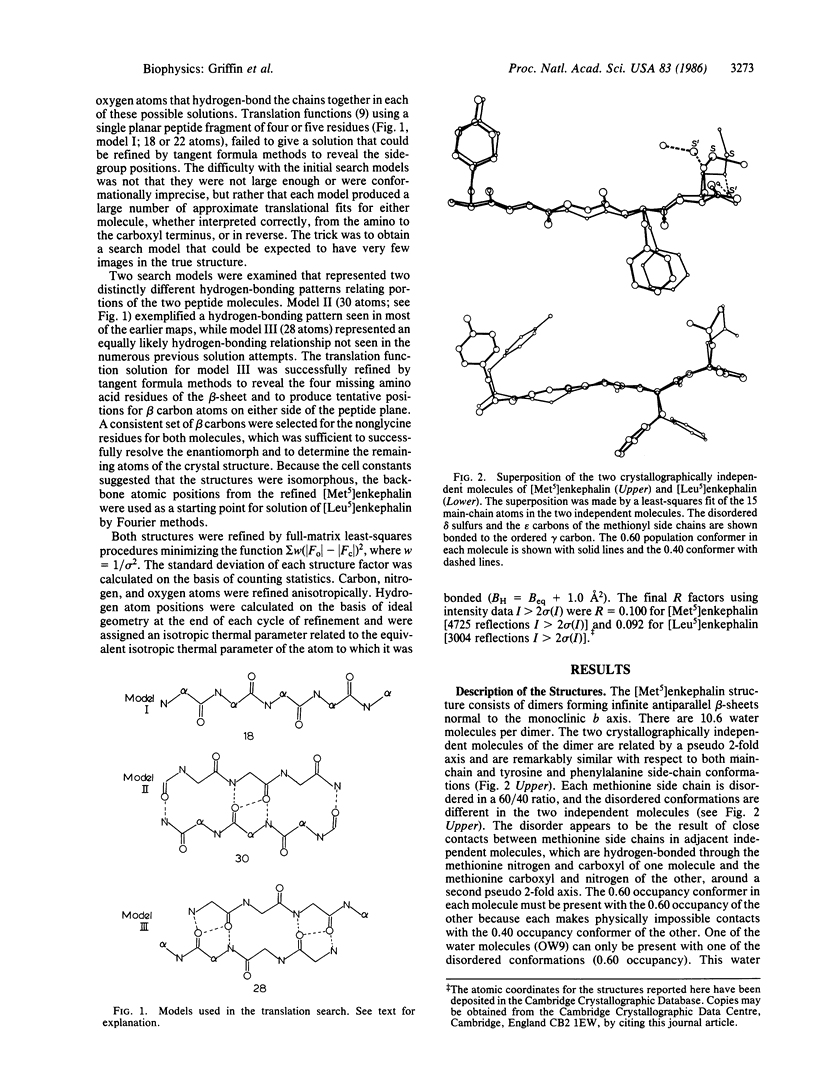
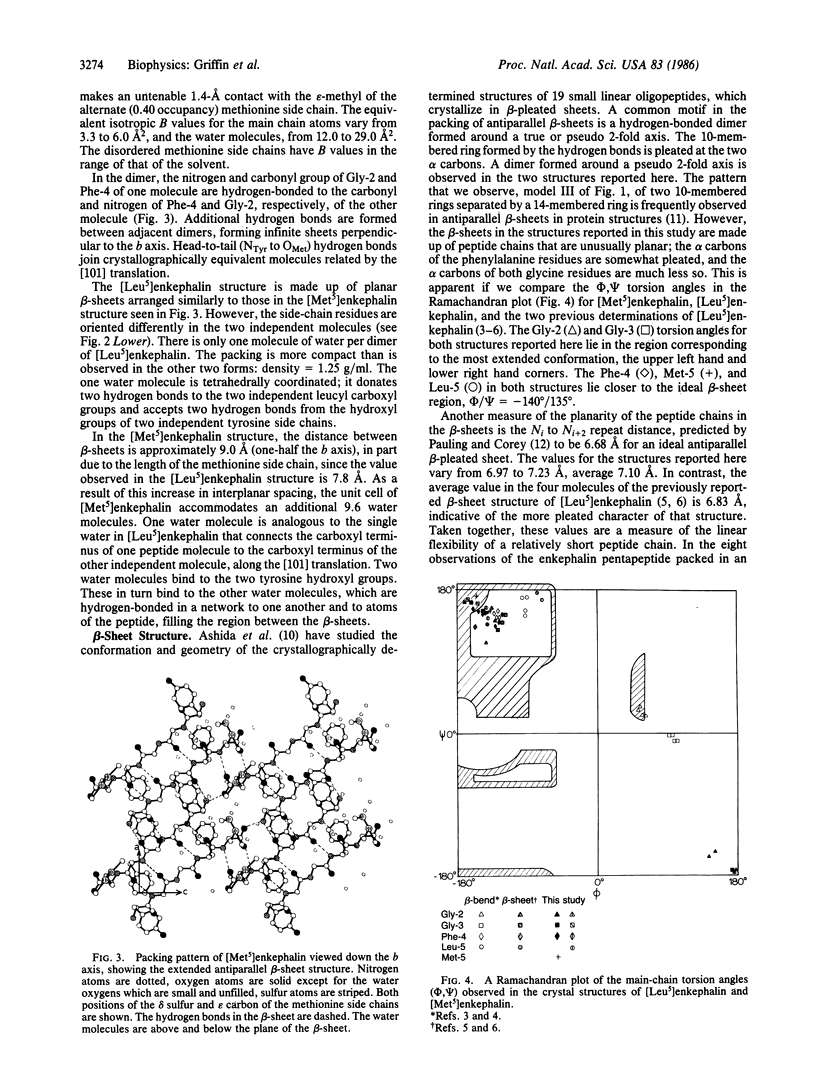
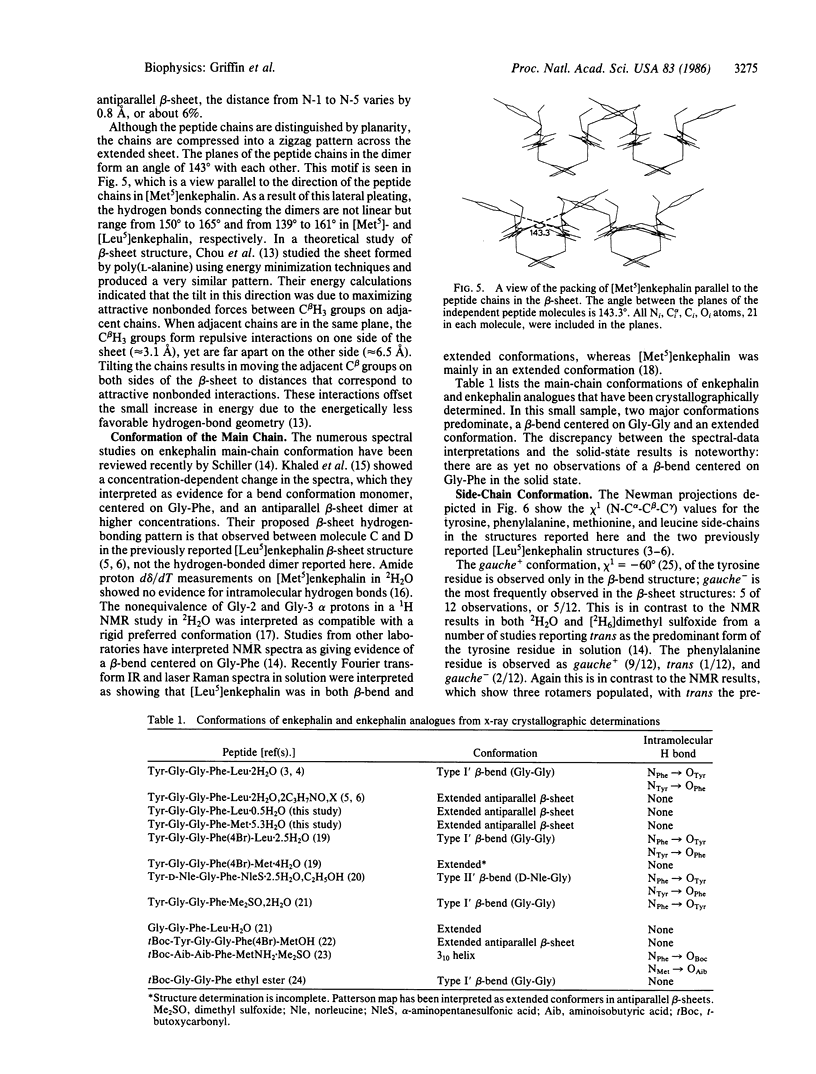
The structures of [Met5]enkephalin (Tyr-Gly-Gly-Phe-Met) and [Leu5]enkephalin (Tyr-Gly-Gly-Phe-Leu) have been determined from single crystal x-ray diffraction data and refined to residuals of 0.100 and 0.092, respectively. The [Met5]enkephalin structure consists of dimers forming antiparallel beta-sheets extending in the monoclinic ac plane with 10.6 water molecules per dimer. The two molecules, related by pseudo two-fold axes, have similar backbone conformations and similar tyrosine and phenylalanine side-chain conformations. Both methionine residues are disordered and the disorder is different in the two independent molecules. Additional hydrogen bonds connect adjacent dimers to form infinite sheets normal to the b axis. The water molecules are found mainly in the interstices between the sheets. [Leu5]Enkephalin crystallizes as a monohydrate that is isomorphous with the [Met5]enkephalin structure with respect to the beta-sheet but different with respect to the tyrosine and phenylalanine side-chain conformations and water content. The peptide chains in both structures are fully extended and more nearly planar than pleated. The planes of the peptide chains in the dimers form an angle of 143.3 degrees with one another in [Met5]enkephalin and 156.0 degrees in [Leu5]enkephalin. This produces a zigzag pattern or pleat in the beta-sheets perpendicular to the direction of the peptide chains and, therefore, perpendicular to the normal beta-sheet pleat. The average repeat distance between Ni and Ni+2 in the peptide chains of both structures is 7.10 A, versus an ideal value of 6.68 A.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashida T., Tanaka I., Yamane T. Beta-pleated sheets in oligopeptide crystals. Int J Pept Protein Res. 1981 Mar;17(3):322–329. doi: 10.1111/j.1399-3011.1981.tb01998.x. [DOI] [PubMed] [Google Scholar]
- Bleich H. E., Cutnell J. D., Day A. R., Freer R. J., Glasel J. A., McKelvy J. F. Preliminary analysis of 1H and 13C spectral and relaxation behavior in methionine-enkephalin. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2589–2593. doi: 10.1073/pnas.73.8.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleich H. E., Day A. R., Freer R. J., Glasel J. A. 360 MHz proton NMR spectra of active and inactive derivatives of methionine-enkephalin. Assignments, derived parameters, conformational implications. Biochem Biophys Res Commun. 1977 Jan 24;74(2):592–598. doi: 10.1016/0006-291x(77)90344-8. [DOI] [PubMed] [Google Scholar]
- Blundell T. L., Hearn L., Tickle I. J., Palmer R. A., Morgan B. A., Smith G. D., Griffin J. F. Crystal structure of [Leu5]enkephalin. Science. 1979 Jul 13;205(4402):220–220. doi: 10.1126/science.451597. [DOI] [PubMed] [Google Scholar]
- Camerman A., Mastropaolo D., Karle I., Karle J., Camerman N. Crystal structure of leucine-enkephalin. Nature. 1983 Dec 1;306(5942):447–450. doi: 10.1038/306447a0. [DOI] [PubMed] [Google Scholar]
- Chou K. C., Pottle M., Némethy G., Ueda Y., Scheraga H. A. Structure of beta-sheets. Origin of the right-handed twist and of the increased stability of antiparallel over parallel sheets. J Mol Biol. 1982 Nov 25;162(1):89–112. doi: 10.1016/0022-2836(82)90163-2. [DOI] [PubMed] [Google Scholar]
- Fournie Zaluski M. C., Prange T., Pascard C., Roques B. P. Enkephalin related fragments. Conformational studies of the tetrapeptides Tyr-Gly-Gly-Phe and Gly-Gly-Phe-X (X = Leu, Met) by X-ray and 1H NMR spectroscopy. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1199–1206. doi: 10.1016/0006-291x(77)91133-0. [DOI] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Ishida T., Kenmotsu M., Mino Y., Inoue M., Fujiwara T., Tomita K., Kimura T., Sakakibara S. X-ray diffraction studies of enkephalins. Crystal structure of [(4'-bromo) Phe4,Leu5]enkephalin. Biochem J. 1984 Mar 15;218(3):677–689. doi: 10.1042/bj2180677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Wodak S. Conformation of amino acid side-chains in proteins. J Mol Biol. 1978 Nov 5;125(3):357–386. doi: 10.1016/0022-2836(78)90408-4. [DOI] [PubMed] [Google Scholar]
- Khaled M. A., Long M. M., Thompson W. D., Bradley R. J., Brown G. B., Urry D. W. Conformational states of enkephalins in solution. Biochem Biophys Res Commun. 1976 May 23;76(2):224–231. doi: 10.1016/0006-291x(77)90715-x. [DOI] [PubMed] [Google Scholar]
- Pauling L., Corey R. B. Configurations of Polypeptide Chains With Favored Orientations Around Single Bonds: Two New Pleated Sheets. Proc Natl Acad Sci U S A. 1951 Nov;37(11):729–740. doi: 10.1073/pnas.37.11.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renugopalakrishnan V., Rapaka R. S., Collette T. W., Carreira L. A., Bhatnagar R. S. Conformational states of Leu5- and Met5-enkephalins in solution. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1029–1035. doi: 10.1016/0006-291x(85)90288-8. [DOI] [PubMed] [Google Scholar]
- Salemme F. R., Weatherford D. W. Conformational and geometrical properties of beta-sheets in proteins. II. Antiparallel and mixed beta-sheets. J Mol Biol. 1981 Feb 15;146(1):119–141. doi: 10.1016/0022-2836(81)90369-7. [DOI] [PubMed] [Google Scholar]
- Smith D., Griffin J. F. Conformation of [Leu5]enkephalin from X-ray diffraction: features important for recognition at opiate receptor. Science. 1978 Mar 17;199(4334):1214–1216. doi: 10.1126/science.204006. [DOI] [PubMed] [Google Scholar]
- Soós J., Berzétei I., Bajusz S., Rónai A. Z. Correlation between circular dichroism data and biological activities of 2,5 substituted enkephalin analogues. Life Sci. 1980 Jul 14;27(2):129–133. doi: 10.1016/0024-3205(80)90454-3. [DOI] [PubMed] [Google Scholar]


