Abstract
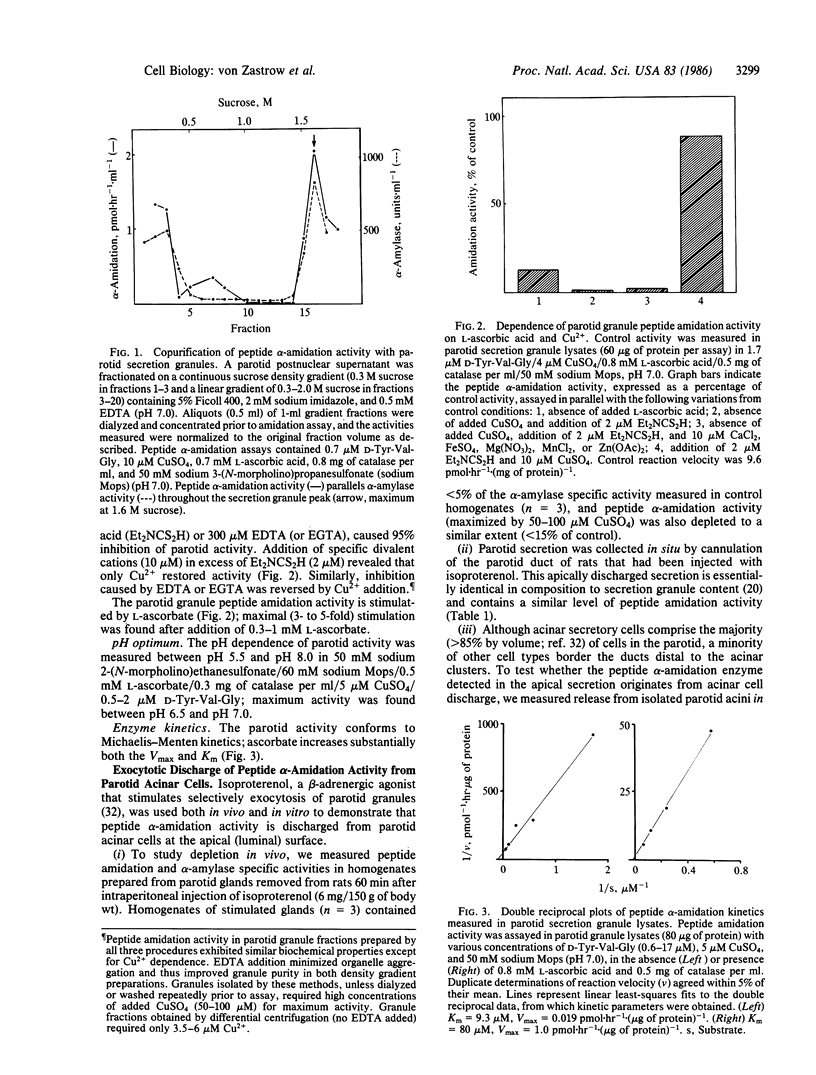
Exocrine secretion granules from the rat parotid gland contain a carboxyl-terminal peptide alpha-amidation enzyme resembling closely an enzyme from the pituitary (peptidyl-glycine alpha-amidating monooxygenase) that functions in post-translational processing of secretory polypeptides within neural and endocrine secretion granules. alpha-Amidation is a characteristic (often essential) chemical feature of a variety of biologically active regulatory peptides in animals. The parotid and pituitary activities exhibit very similar ascorbate and copper requirements, pH dependence, and kinetic properties. Further, like the pituitary enzyme(s), the parotid activity is found predominantly in secretion granule content and is discharged by exocytosis. These results establish the presence of a novel enzyme in exocrine secretion granules and suggest a potential role of the L-ascorbic acid contained in parotid granules. Two additional findings--the detection of similar levels of amidation activity in purified secretion granule fractions from other exocrine glands and the observation, in parotid granule fractions, of a B-type carboxypeptidase activity similar to that involved in post-translational processing in other systems--form a rational basis for considering whether exocrine secretion granules (like their neural and endocrine counterparts) serve as post-translational processing sites. The identity and functional role of the modified polypeptides remain to be determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvan P., Rudnick G., Castle J. D. Osmotic properties and internal pH of isolated rat parotid secretory granules. J Biol Chem. 1984 Nov 10;259(21):13567–13572. [PubMed] [Google Scholar]
- Bernardi L., Bosisio G., Goffredo O., De Castiglione R. Synthesis of physalaemin. Experientia. 1964 Sep 15;20(9):490–492. doi: 10.1007/BF02154065. [DOI] [PubMed] [Google Scholar]
- Bradbury A. F., Finnie M. D., Smyth D. G. Mechanism of C-terminal amide formation by pituitary enzymes. Nature. 1982 Aug 12;298(5875):686–688. doi: 10.1038/298686a0. [DOI] [PubMed] [Google Scholar]
- Cameron R. S., Castle J. D. Isolation and compositional analysis of secretion granules and their membrane subfraction from the rat parotid gland. J Membr Biol. 1984;79(2):127–144. doi: 10.1007/BF01872117. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Glembotski C. C., Mains R. E. Bovine intermediate pituitary alpha-amidation enzyme: preliminary characterization. Peptides. 1983 Nov-Dec;4(6):921–928. doi: 10.1016/0196-9781(83)90091-8. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E., Glembotski C. C. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Snyder S. H. Purification and characterization of enkephalin convertase, an enkephalin-synthesizing carboxypeptidase. J Biol Chem. 1983 Sep 25;258(18):10950–10955. [PubMed] [Google Scholar]
- Fricker L. D., Supattapone S., Snyder S. H. Enkephalin convertase: a specific enkephalin synthesizing carboxypeptidase in adrenal chromaffin granules, brain, and pituitary gland. Life Sci. 1982 Oct 18;31(16-17):1841–1844. doi: 10.1016/0024-3205(82)90224-7. [DOI] [PubMed] [Google Scholar]
- Glembotski C. C., Eipper B. A., Mains R. E. Characterization of a peptide alpha-amidation activity from rat anterior pituitary. J Biol Chem. 1984 May 25;259(10):6385–6392. [PubMed] [Google Scholar]
- Glembotski C. C. The alpha-amidation of alpha-melanocyte stimulating hormone in intermediate pituitary requires ascorbic acid. J Biol Chem. 1984 Nov 10;259(21):13041–13048. [PubMed] [Google Scholar]
- Herzog V., Farquhar M. G. Luminal membrane retrieved after exocytosis reaches most golgi cisternae in secretory cells. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5073–5077. doi: 10.1073/pnas.74.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook I. B., Molan P. C. The identification of a peptide in human parotid saliva particularly active in enhancing the glycolytic activity of the salivary micro-organisms. Biochem J. 1975 Aug;149(2):489–492. doi: 10.1042/bj1490489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V. Y., Loh Y. P. Carboxypeptidase B-like converting enzyme activity in secretory granules of rat pituitary. Proc Natl Acad Sci U S A. 1984 May;81(9):2776–2780. doi: 10.1073/pnas.81.9.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizer J. S., Busby W. H., Jr, Cottle C., Youngblood W. W. Glycine-directed peptide amidation: presence in rat brain of two enzymes that convert p-Glu-His-Pro-Gly-OH into p-Glu-His-Pro-NH2 (thyrotropin-releasing hormone). Proc Natl Acad Sci U S A. 1984 May;81(10):3228–3232. doi: 10.1073/pnas.81.10.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreil G., Mollay C., Kaschnitz R., Haiml L., Vilas U. Prepromelittin: specific cleavage of the pre- and the propeptide in vitro. Ann N Y Acad Sci. 1980;343:338–346. doi: 10.1111/j.1749-6632.1980.tb47262.x. [DOI] [PubMed] [Google Scholar]
- Krieger D. T. Brain peptides: what, where, and why? Science. 1983 Dec 2;222(4627):975–985. doi: 10.1126/science.6139875. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Secretion and regulation of two biosynthetic enzyme activities, peptidyl-glycine alpha-amidating monooxygenase and a carboxypeptidase, by mouse pituitary corticotropic tumor cells. Endocrinology. 1984 Nov;115(5):1683–1690. doi: 10.1210/endo-115-5-1683. [DOI] [PubMed] [Google Scholar]
- Russell J. T., Levine M., Njus D. Electron transfer across posterior pituitary neurosecretory vesicle membranes. J Biol Chem. 1985 Jan 10;260(1):226–231. [PubMed] [Google Scholar]
- SANDRIN E., BOISSONNAS R. A. Synthesis of eledoisin. Experientia. 1962 Feb 15;18:59–61. doi: 10.1007/BF02138251. [DOI] [PubMed] [Google Scholar]
- SCHRAMM M., DANON D. The mechanism of enzyme secretion by the cell. I. Storage of amylase in the zymogen granules of the rat-parotis gland. Biochim Biophys Acta. 1961 Jun 10;50:102–112. doi: 10.1016/0006-3002(61)91065-4. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Isemura S., Sanada K. Complete amino acid sequence of a basic proline-rich peptide, P-D, from human parotid saliva. J Biochem. 1983 Feb;93(2):495–502. doi: 10.1093/oxfordjournals.jbchem.a134204. [DOI] [PubMed] [Google Scholar]
- Saitoh E., Isemura S., Sanada K. Complete amino acid sequence of a basic proline-rich peptide, P-F, from human parotid saliva. J Biochem. 1983 Mar;93(3):883–888. doi: 10.1093/jb/93.3.883. [DOI] [PubMed] [Google Scholar]
- Schnaar R. L., Weigel P. H., Kuhlenschmidt M. S., Lee Y. C., Roseman S. Adhesion of chicken hepatocytes to polyacrylamide gels derivatized with N-acetylglucosamine. J Biol Chem. 1978 Nov 10;253(21):7940–7951. [PubMed] [Google Scholar]
- Schramm M., Selinger Z. The function of alpha- and beta-adrenergic receptors and a cholinergic receptor in the secretory cell of rat parotid gland. Adv Cytopharmacol. 1974;2:29–32. [PubMed] [Google Scholar]
- Stack G., Fricker L. D., Snyder S. H. A sensitive radiometric assay for enkephalin convertase and other carboxypeptidase B-like enzymes. Life Sci. 1984 Jan 9;34(2):113–121. doi: 10.1016/0024-3205(84)90581-2. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Takemoto T., Tani T., Miwa T. Letter: Gastrin-like immunoreactivity in salivary gland and saliva. Lancet. 1973 Oct 20;2(7834):920–920. doi: 10.1016/s0140-6736(73)92063-1. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Gresik E. W., Barka T. Immunocytochemical localization of amylase in the parotid gland of developing and adult rats. J Histochem Cytochem. 1981 Oct;29(10):1189–1195. doi: 10.1177/29.10.6170667. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Carlquist M., Mutt V. Neuropeptide Y--a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982 Apr 15;296(5858):659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Chemical determination of polypeptide hormones. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4115–4119. doi: 10.1073/pnas.75.9.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Isolation and characterization of the intestinal peptide porcine PHI (PHI-27), a new member of the glucagon--secretin family. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6603–6607. doi: 10.1073/pnas.78.11.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Wallensten K. Gastrin release and HCl secretion induced by electrical vagal stimulation in the cat. Acta Physiol Scand Suppl. 1976;438:1–39. [PubMed] [Google Scholar]
- von Zastrow M., Tritton T. R., Castle J. D. Identification of L-ascorbic acid in secretion granules of the rat parotid gland. J Biol Chem. 1984 Oct 10;259(19):11746–11750. [PubMed] [Google Scholar]


