Abstract
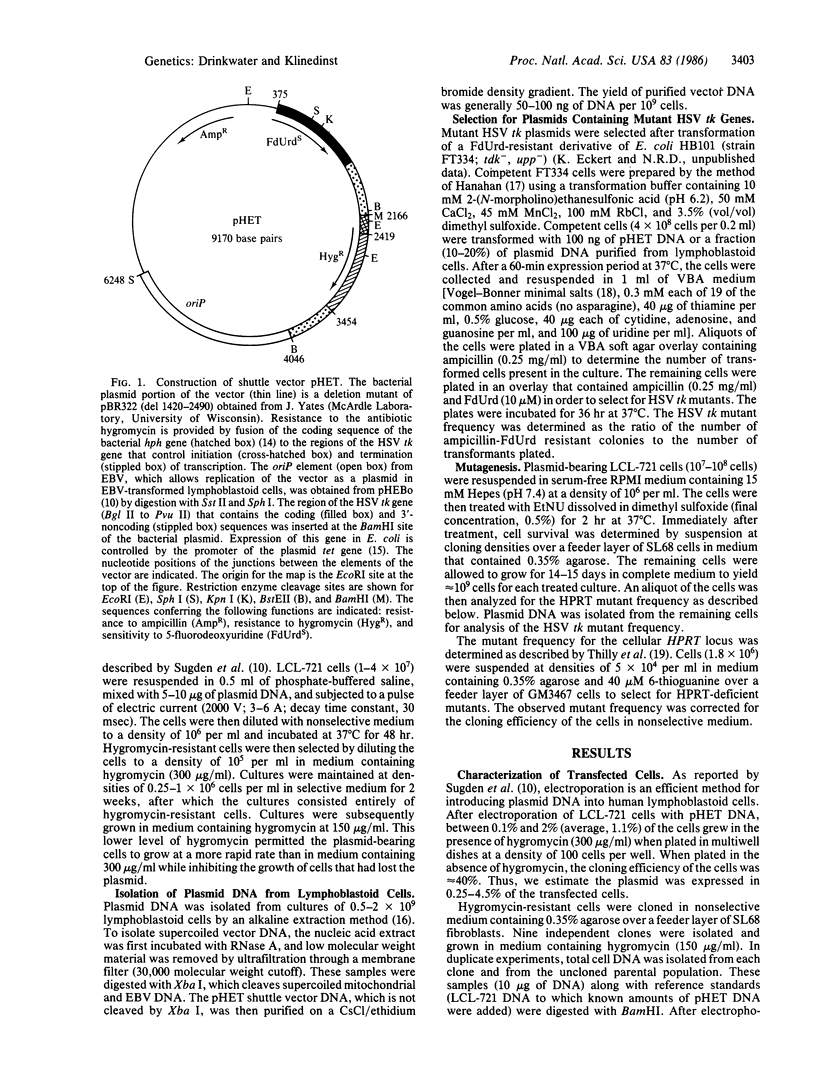
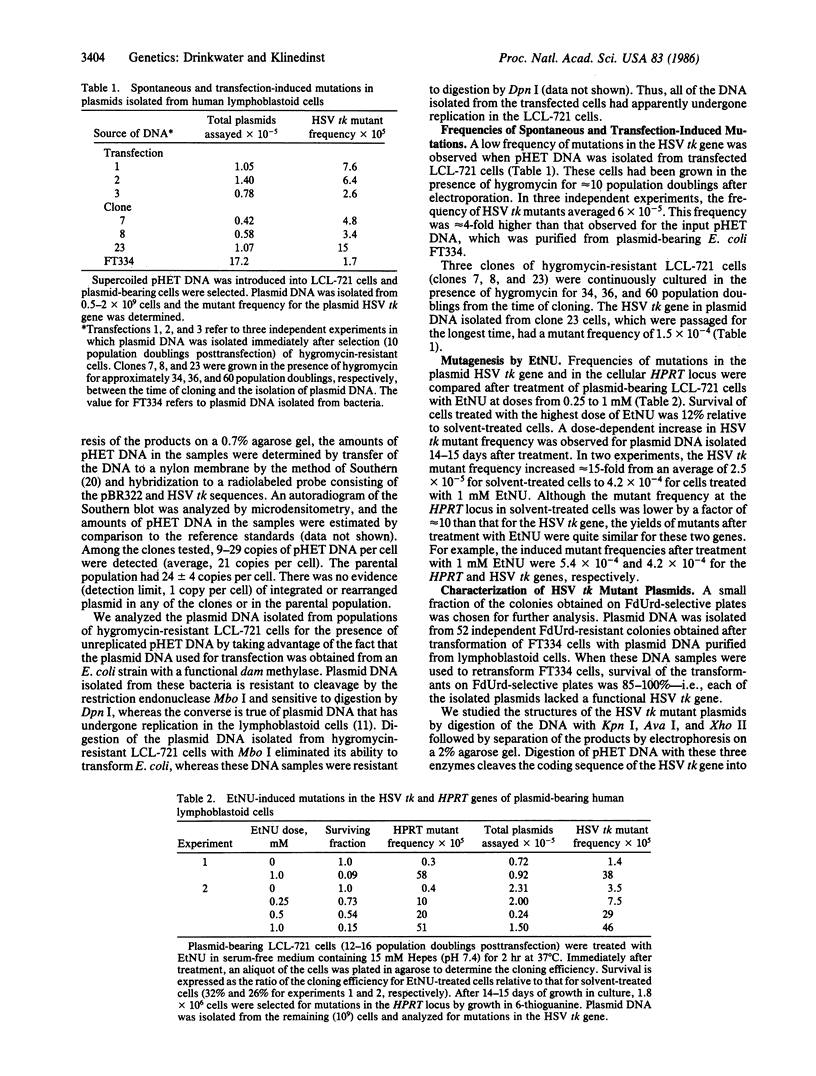
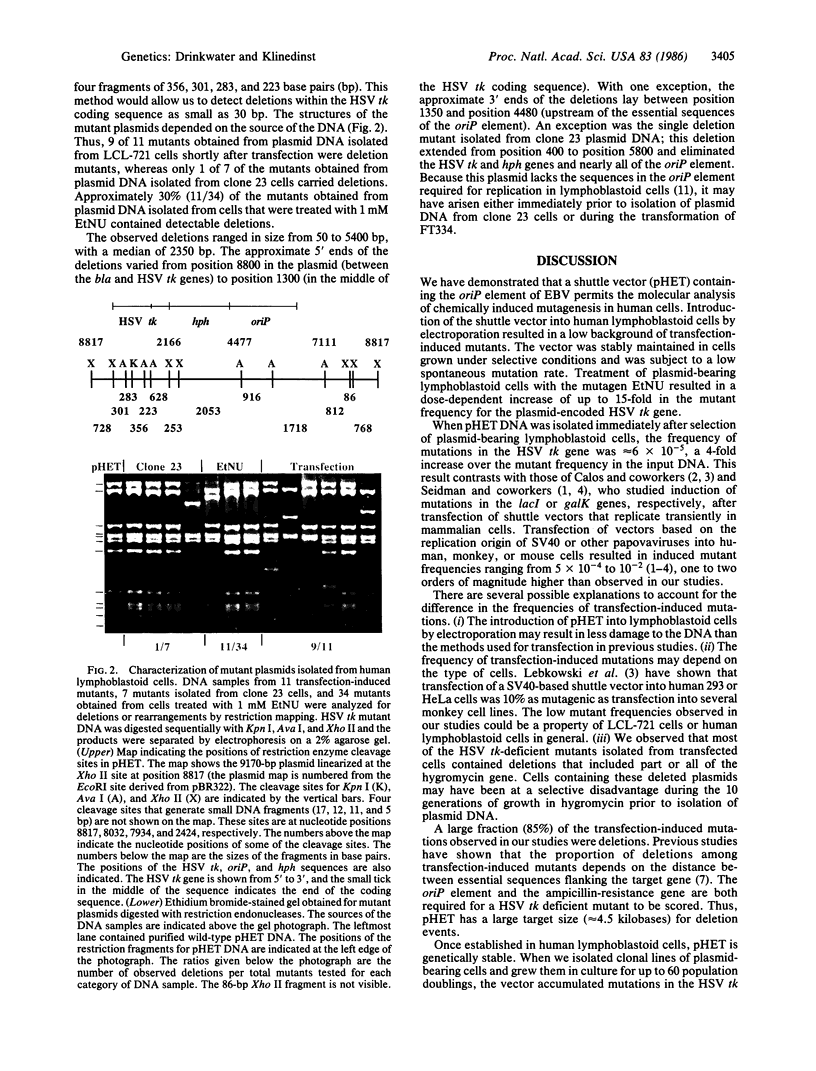
We have developed a recombinant DNA shuttle vector that permits the molecular analysis of mutations induced in human cells by chemical or physical mutagens. The vector is able to replicate as a plasmid in Escherichia coli and in Epstein-Barr virus (EBV)-transformed human lymphoblastoid cell lines and contains the herpes simplex virus type 1 thymidine kinase gene (HSV tk) as the target for mutagenesis studies. After introduction of the vector into an EBV-transformed lymphoblastoid cell line (LCL-721) by electroporation, approximately equal to 2% of the transfected cells expressed the vector-encoded gene for hygromycin resistance. Plasmid DNA isolated from cells immediately after selection for hygromycin resistance (10 population doublings posttransfection) contained mutations in the HSV tk gene at a frequency of 6 X 10(-5). Treatment of plasmid-bearing LCL-721 cells with N-ethyl-N-nitrosourea resulted in a dose-dependent increase of up to 15-fold in the frequency of mutations in the HSV tk gene. The dose-response for the induction of mutations in the plasmid-encoded gene closely paralleled that for the induction of mutations in the cellular gene for hypoxanthine (guanine) phosphoribosyltransferase.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman C. R., Davidson R. L. High spontaneous mutation frequency in shuttle vector sequences recovered from mammalian cellular DNA. Mol Cell Biol. 1984 Nov;4(11):2266–2272. doi: 10.1128/mcb.4.11.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust A. E., Drinkwater N. R., Debien K., Maher V. M., McCormick J. J. Comparison of the frequency of diphtheria toxin and thioguanine resistance induced by a series of carcinogens to analyze their mutational specificities in diploid human fibroblasts. Mutat Res. 1984 Jan;125(1):95–104. doi: 10.1016/0027-5107(84)90036-8. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Joffe S., Seidman M. M. Recombination and deletion of sequences in shuttle vector plasmids in mammalian cells. Mol Cell Biol. 1985 Sep;5(9):2265–2271. doi: 10.1128/mcb.5.9.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkwater N. R., Corner R. C., McCormick J. J., Maher V. M. An in situ assay for induced diphtheria-toxin-resistant mutants of diploid human fibroblasts. Mutat Res. 1982 Dec;106(2):277–289. doi: 10.1016/0027-5107(82)90109-9. [DOI] [PubMed] [Google Scholar]
- Garner R. C., Pickering C., Martin C. N. Mutagenicity of methyl-, ethyl-, propyl- and butylnitrosourea towards Escherichia coli WP2 strains with varying DNA repair capabilities. Chem Biol Interact. 1979 Jul;26(2):197–205. doi: 10.1016/0009-2797(79)90023-1. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Björck E., Bjursell G., Lindahl T. Sequence complexity of circular Epstein-Bar virus DNA in transformed cells. J Virol. 1981 Oct;40(1):11–19. doi: 10.1128/jvi.40.1.11-19.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Tanaka A., Nonoyama M., Derge J. G. Replication of the resident repressed Epstein-Barr virus genome during the early S phase (S-1 period) of nonproducer Raji cells. Proc Natl Acad Sci U S A. 1974 Mar;71(3):631–633. doi: 10.1073/pnas.71.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kavathas P., Bach F. H., DeMars R. Gamma ray-induced loss of expression of HLA and glyoxalase I alleles in lymphoblastoid cells. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4251–4255. doi: 10.1073/pnas.77.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Otsuka H., Qavi H., Kit M. Functional expression of the Herpes simplex virus thymidine kinase gene in Escherichia coli K-12. Gene. 1981 Dec;16(1-3):287–295. doi: 10.1016/0378-1119(81)90084-6. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Clancy S., Miller J. H., Calos M. P. The lacI shuttle: rapid analysis of the mutagenic specificity of ultraviolet light in human cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8606–8610. doi: 10.1073/pnas.82.24.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Lebkowski J. S., Greisen K. S., Calos M. P. Specificity of mutations induced in transfected DNA by mammalian cells. EMBO J. 1984 Dec 20;3(13):3117–3121. doi: 10.1002/j.1460-2075.1984.tb02267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Chakrabarti S., Joffee S., Seidman M. Mutagenesis of a shuttle vector plasmid in mammalian cells. Mol Cell Biol. 1984 Mar;4(3):435–441. doi: 10.1128/mcb.4.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Yates J., Sugden B. A putative origin of replication of plasmids derived from Epstein-Barr virus is composed of two cis-acting components. Mol Cell Biol. 1985 Aug;5(8):1822–1832. doi: 10.1128/mcb.5.8.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thilly W. G., Deluca J. G., Hoppe H., 4th, Penman B. W. Phenotypic lag and mutation to 6-thioguanine resistance in diploid human lymphoblasts. Mutat Res. 1978 Apr;50(1):137–144. doi: 10.1016/0027-5107(78)90068-4. [DOI] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]



