Abstract
The thoracic duct lymphocytes from rats previously injected with ultraviolet-light-irradiated allogeneic lymphocytes were grown for 4 days with alloantigen, with or without Con A-induced lymphokine factors, and then for 3 days with the lymphokines alone. They were then tested for their cytoxicity and for their capacity to make interleukin 2 (IL-2) in response to antigen. The results show that T helper cells specific for both class I and class II antigens of the major histocompatibility complex were removed from the circulation by the injection of ultraviolet-irradiated alloantigen. However, precursors of cytotoxic cells remained and appeared to lose their OX-19 markers during activation. We have interpreted the results by using a speculative model that involves separate pathways of induction to cytotoxicity and IL-2 synthesis. We propose that the OX-19 marker is associated with the interleukin 1 receptor and that the latter is required for the IL-2 production pathway but not for activation to cytotoxicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Bach M. L., Sondel P. M. Differential function of major histocompatibility complex antigens in T-lymphocyte activation. Nature. 1976 Jan 29;259(5541):273–281. doi: 10.1038/259273a0. [DOI] [PubMed] [Google Scholar]
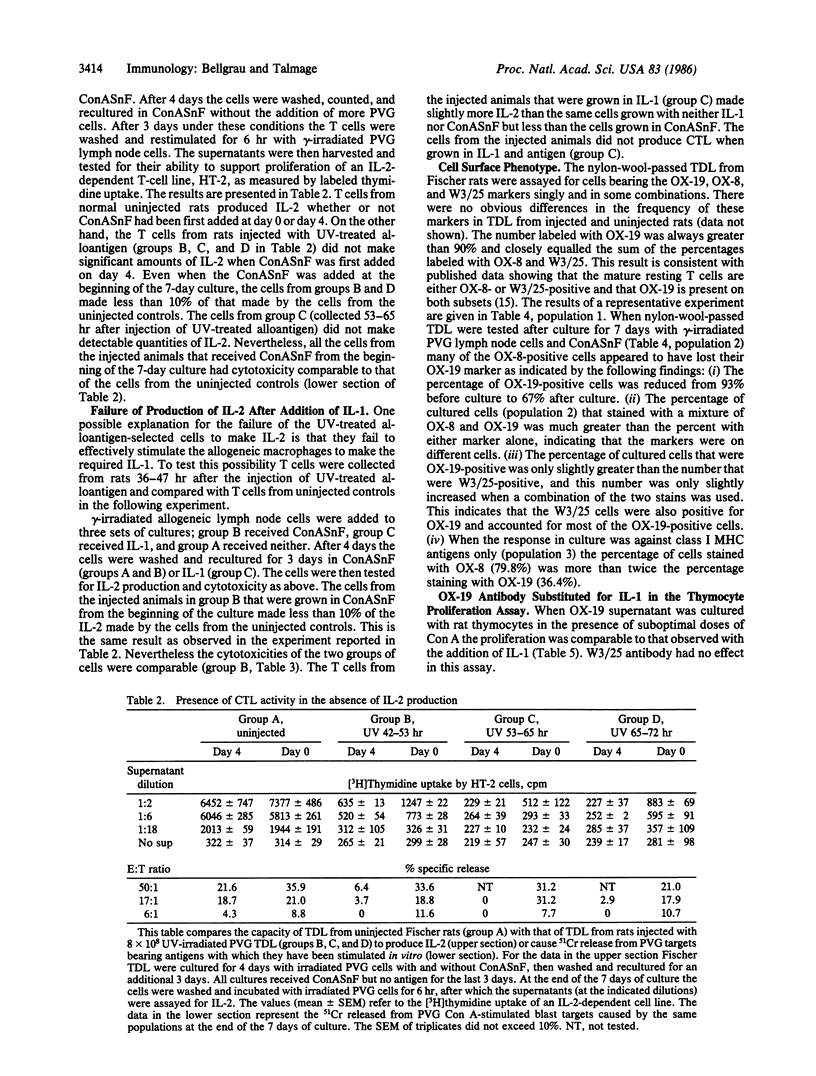
- Bellgrau D. Induction of cytotoxic T cell precursors in vivo. Role of T helper cells. J Exp Med. 1983 May 1;157(5):1505–1515. doi: 10.1084/jem.157.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
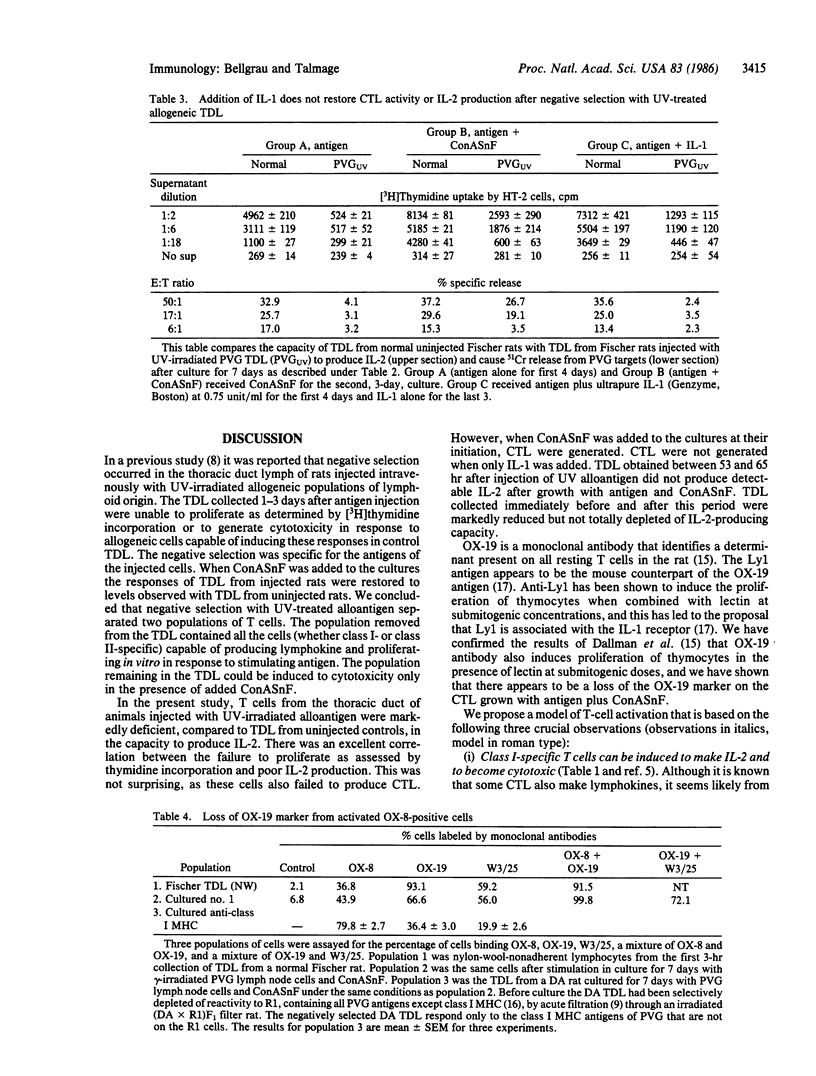
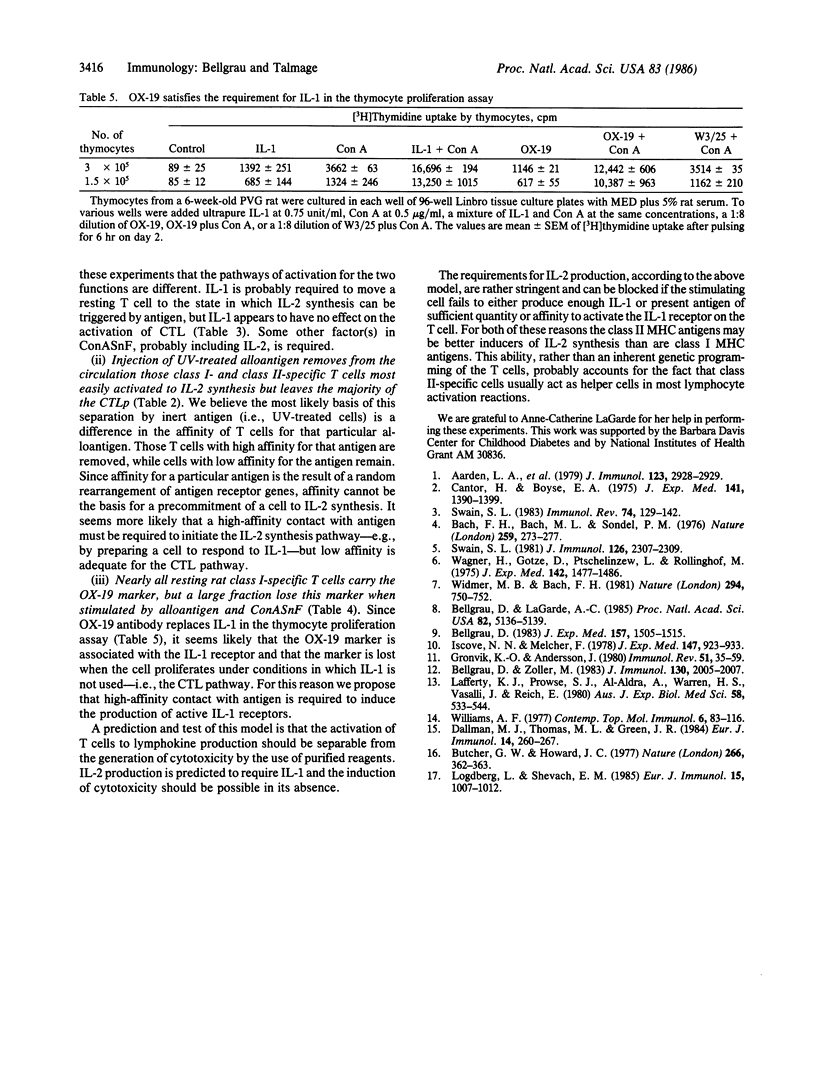
- Bellgrau D., Lagarde A. C. In vivo separation of two classes of T cells as determined by negative selection after the injection of UV-treated allogeneic lymphoid cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5136–5139. doi: 10.1073/pnas.82.15.5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrau D., Zöller M. Cytotoxic T lymphocyte responses to spontaneous tumors: immunogenicity dependent on the recognition of processed tumor antigens. J Immunol. 1983 May;130(5):2005–2007. [PubMed] [Google Scholar]
- Butcher G. W., Howard J. C. A recombinant in the major histocompatibility complex of the rat. Nature. 1977 Mar 24;266(5600):362–364. doi: 10.1038/266362a0. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975 Jun 1;141(6):1390–1399. doi: 10.1084/jem.141.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Thomas M. L., Green J. R. MRC OX-19: a monoclonal antibody that labels rat T lymphocytes and augments in vitro proliferative responses. Eur J Immunol. 1984 Mar;14(3):260–267. doi: 10.1002/eji.1830140311. [DOI] [PubMed] [Google Scholar]
- Grönvik K. O., Andersson J. The role of T cell growth stimulating factors in T cell triggering. Immunol Rev. 1980;51:35–59. doi: 10.1111/j.1600-065x.1980.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty K. J., Prowse S. J., Al-Adra A., Warren H. S., Vasalli J., Reich E. An improved assay for interleukin 2 (lymphocyte growth factor) produced by mitogen-activated lymphocytes. Aust J Exp Biol Med Sci. 1980 Dec;58(6):533–544. doi: 10.1038/icb.1980.55. [DOI] [PubMed] [Google Scholar]
- Lögdberg L., Shevach E. M. Role of the Ly 1 antigen in interleukin 1-induced thymocyte activation. Eur J Immunol. 1985 Oct;15(10):1007–1013. doi: 10.1002/eji.1830151009. [DOI] [PubMed] [Google Scholar]
- Revised nomenclature for antigen-nonspecific T cell proliferation and helper factors. J Immunol. 1979 Dec;123(6):2928–2929. [PubMed] [Google Scholar]
- Swain S. L. Significance of class 1 and class 2 major histocompatibility complex antigens: help to allogeneic K and D antigens does not involve I recognition. J Immunol. 1981 Jun;126(6):2307–2309. [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Wagner H., Götze D., Ptschelinzew L., Röllinghoff M. Induction of cytotoxic T lymphocytes against I-region-coded determinants: in vitro evidence for a third histocompatibility locus in the mouse. J Exp Med. 1975 Dec 1;142(6):1477–1487. doi: 10.1084/jem.142.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer M. B., Bach F. H. Antigen-driven helper cell-independent cloned cytolytic T lymphocytes. Nature. 1981 Dec 24;294(5843):750–752. doi: 10.1038/294750a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F. Differentiation antigens of the lymphocyte cell surface. Contemp Top Mol Immunol. 1977;6:83–116. doi: 10.1007/978-1-4684-2841-4_3. [DOI] [PubMed] [Google Scholar]


