Abstract
Despite 35 years of clinical trials, there is little improvement in one-year survival rates for patients with metastatic melanoma, and the disease is essentially untreatable if not cured surgically. The paucity of chemotherapeutic agents that are effective for treating metastatic melanoma indicates a dire need to develop new therapies. Here, we found a previously unrecognized role for c-Abl and Arg in melanoma progression. We demonstrate that the kinase activities of c-Abl and Arg (c-Abl, Arg) are elevated in primary melanomas (60%), in a subset of benign nevi (33%), and in some human melanoma cell lines. Using siRNA and pharmacological approaches, we show that c-Abl/Arg activation is functionally relevant because it is required for melanoma cell proliferation, survival, and invasion. Significantly, we identify the mechanism by which activated c-Abl promotes melanoma invasion by showing that it transcriptionally upregulates MMP-1, and using rescue approaches we demonstrate that c-Abl promotes invasion via a STAT3→MMP-1 pathway. Additionally, we show that c-Abl and Arg are not merely redundant, as active Arg drives invasion in a STAT3-independent manner, and upregulates MMP-3 and MT1-MMP, in addition to MMP-1. Most importantly, c-Abl and Arg not only promote in vitro processes important for melanoma progression, but also promote metastasis in vivo, as inhibition of c-Abl/Arg kinase activity with the c-Abl/Arg inhibitor, nilotinib, dramatically inhibits metastasis in a mouse model. Taken together, these data identify c-Abl and Arg as critical, novel, drug targets in metastatic melanoma, and indicate that nilotinib may useful in preventing metastasis in patients with melanomas harboring active c-Abl and Arg.
Keywords: c-Abl, Arg, melanoma, invasion, STAT3, MMP
INTRODUCTION
Melanoma diagnoses are increasing faster than any other cancer (Berwick et al 2009). Although melanoma represents 5–7% of all skin malignancies, it accounts for over 75% of skin cancer-related deaths (Berwick et al 2009). The mean survival time from the appearance of distant metastases is 6–9 months, and only 5% of patients survive longer than one year (Mouawad et al 2009). Many chemotherapeutic regimens have been tried in patients with metastatic melanoma; however, none has greater than a 5% success rate or extends survival to more than 10 months, indicating a dire need to develop new therapies (Hodi et al 2010). Melanomas are divided into subtypes based on sun-exposure and location: chronic exposure (face), intermittent (trunk, arms, legs), minimal exposure (palms, soles), and mucosal membranes (Kabbarah and Chin 2005). Melanomas also are classified as radial growth phase (RGP), vertical growth phase (VGP) or metastatic (Clark et al 1984). RGPs are confined to the basement membrane, whereas VGPs have nests of cells that invade the dermis (Clark et al 1984). Fifty-percent of melanomas develop from dysplastic nevi (Shepherd et al 2010). Melanoma subtypes contain distinct molecular alterations. B-Raf mutations are frequent in benign nevi, in melanomas from skin that is intermittently exposed to the sun, and in VGPs rather than RGPs (Kabbarah and Chin 2005, Maldonado et al 2003). In contrast, c-Kit mutations are more frequent in melanomas from mucosal and acral (hands, feet) areas (Kabbarah and Chin 2005, Smalley et al 2009). Drugs targeting B-Raf or c-Kit show promise for treating malignant melanoma (Flaherty et al 2010, Satzger et al 2010).
Matrix metalloproteinases (MMPs) are secreted by stromal and tumor cells as zymogens, which are cleaved by proteases to their active forms, and secretion of MMPs at the site of the progressing tumor promotes progression (Brinckerhoff et al 2000). Interstitial collagenases cleave collagen I, II, III (MMP-1, MMP-8, MMP-13); gelatinases cleave type IV collagen (MMP-2, MMP-9); stromelysins (MMP-3, MMP-10) cleave non-collagen matrices and contribute to activation of the collagenases and MMP-9; and membrane MMPs (e.g. MT1-MMP) cleave and activate other MMPs (MMP-2, MMP-13) and also have some collagenase activity (Brinckerhoff et al 2000). MMP-1 activity is frequently increased in advanced cancers, and its expression is negatively correlated with patient survival (Brinckerhoff et al 2000). In melanomas, acquisition of the VGP phenotype is dependent on MMP expression; MMP-1 is expressed in VGPs; and MMP-1 activity is required for melanoma invasion and metastasis (Blackburn et al 2009). MMP expression is regulated by many transcription factors including NF-κB, AP-1, Ets, and STAT3 (Fanjul-Fernandez et al 2010). STAT3 is often constitutively activated in melanoma, and promotes survival, proliferation, invasion, VGP transition, angiogenesis, and metastasis (Kortylewski et al 2005).
c-Abl and Arg (c-Abl, Arg) are most known for their oncogenic role in leukemia, and drugs targeting oncogenic forms (e.g. BCR-Abl) are successful in treating these diseases (Pendergast 2001, Santos and Ravandi 2009). Imatinib mesylate (STI571, Gleevec), a cAbl/Arg inhibitor that also inhibits c-Kit and PDGFRα,β, induces remission in chronic myelogenous leukemia (CML), which express BCR-Abl (Druker et al 2001) and in gastrointestinal stroma tumors (GIST), which express mutant c-Kit (DeMatteo 2009). Nilotinib, a second generation drug, is effective for CML patients that develop resistance or cannot tolerate imatinib (Deininger 2008). We were the first to demonstrate that c-Abl and Arg also are activated in solid tumors (breast cancer, glioblastoma, MDA-MB-435s cells), downstream of constitutively activated receptor tyrosine kinases and Src kinases, and promote invasion and proliferation (Srinivasan and Plattner 2006, Srinivasan et al 2008, Srinivasan et al 2009). Arlinghaus and colleagues subsequently showed that c-Abl and Arg also are activated in non-small cell lung cancer cells (Lin et al 2007), and Maina and colleagues demonstrated that c-Abl is activated downstream of c-Met in gastric carcinoma cells (Furlan et al 2011). Several lines of evidence suggest that c-Abl and Arg may contribute to melanoma development/progression: 1) MDA-MB-435s, originally thought to be of breast origin, was recently identified as melanoma M14 (Rae et al 2007); 2) imatinib inhibits proliferation of some melanoma cell lines (Mayorga et al 2006). However, the activities of c-Abl and Arg were not examined, and the mechanism of STI571-mediated inhibition of proliferation was not determined (Mayorga et al 2006); and 3) imatinib inhibits murine melanoma tumor growth in a model that lacks expression of c-Kit and PDGFR-α,β (Ogawa et al 2008, Redondo et al 2004). These data prompted us to examine whether cAbl and Arg play a role in human melanoma progression. Here, we demonstrate that cAbl/Arg kinase activities are increased in primary melanomas and in some human melanoma cell lines; their activation is required for proliferation, survival, and invasion; cAbl and Arg promote melanoma invasion via distinct molecular pathways; and c-Abl and Arg drive melanoma metastatic progression. Therefore, c-Abl and Arg are important clinical targets in melanoma, and represent an unexplored avenue for targeted treatment.
RESULTS
c-Abl and Arg are activated in human melanoma cell lines and in primary melanomas
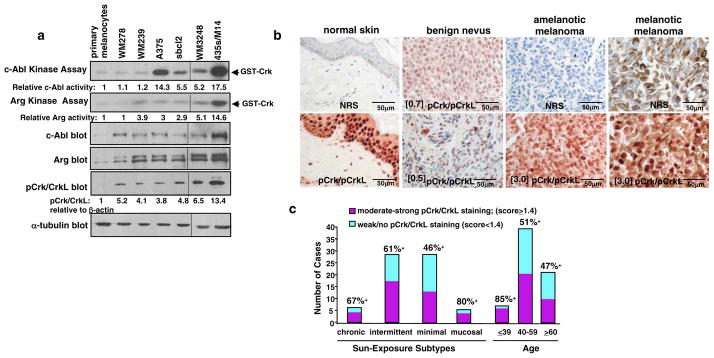
Expression of c-Abl and Arg was dramatically elevated in all melanoma cell lines examined relative to primary melanocytes (Figure 1a). To determine whether c-Abl and Arg are activated in melanoma cell lines, their basal activities were directly assessed by in vitro kinase assay utilizing the known c-Abl/Arg target, Crk, as substrate (Plattner et al 1999, Plattner et al 2003). Interestingly, several melanoma cell lines had high c-Abl and/or Arg activity (sbcl2, A375, WM3248, 435s/M14)(Figure 1a). With the exception of WM278, phosphorylation of Crk/CrkL, c-Abl/Arg targets, paralleled c-Abl/Arg activities (Figure 1a). To test whether c-Abl and Arg are activated in primary melanomas, we performed immunohistochemistry (IHC) on melanoma tissue microarrays. Phospho-specific antibodies to c-Abl (Y412, Y245) cross-react with phospho-PDGFR and phospho-EGFR, and thus, cannot be used to assess activity by IHC, and phospho-specific Arg antibodies are not available. Therefore, we stained melanoma tissue microarrays with an antibody to the c-Abl/Arg phosphorylation sites (Y221/Y207) on c-Abl/Arg substrates, Crk and CrkL. We and others previously showed that Crk/CrkL phosphorylation on Y221/Y207 correlates with c-Abl/Arg activity in cancer cell lines (Figure 1a) (Smith-Pearson et al 2010, Srinivasan and Plattner 2006, Yogalingam and Pendergast 2008). An advantage to this approach is that activation of c-Abl and Arg can be assessed simultaneously. In normal skin, pCrk/CrkL staining was limited to the cytoplasm and nuclei of keratinocytes and nuclei of lymphocytes (Figure 1b). Most benign nevi demonstrated weak nuclear pCrk/CrkL staining (red); although some (33%) exhibited moderate-strong staining (score≥1.4; score=I × P where I=Intensity (1+, 2+, 3+) and P=proportion of positively staining tumor cells; Figure 1b). In primary melanomas, melanin (brown), if present, was localized in the cytoplasm, whereas pCrk/CrkL staining (red) was predominantly nuclear (Figure 1b). Cores with extremely strong melanin expression were excluded due to difficulty in scoring. Sixty-percent (29/48) of melanomas had moderate-strong pCrk/CrkL staining as compared to 33% (6/18) of benign nevi and 47% (9/19) of lymph node metastases (Figure 1b; Table 1). Intense staining was observed in some melanomas from all subtypes; however, there was a trend towards a higher percentage of positive cases in melanomas from chronically and intermittently sun-exposed skin and mucosal areas as opposed to those derived from minimally sun-exposed skin (Figure 1c; Table 1). In addition, there was a trend towards a higher percentage of melanomas with strong c-Abl/Arg activity in younger patients (<39) (Figure 1c; Table 1).
Figure 1. c-Abl and Arg are activated in human melanoma cell lines and primary melanomas.
(a) Basal c-Abl and Arg kinase activities were directly assessed by in vitro kinase assay. c-Abl and Arg, immunoprecipitated from lysates from serum-starved (24h) cells, were incubated in a “hot” in vitro kinase assay using the c-Abl/Arg target, GST-tagged Crk as substrate (top 2 panels). Lysates were blotted with antibodies (bottom 2 panels). One of three representative experiments is shown. (b) Melanoma tissue microarrays were incubated with normal rabbit serum (NRS) or phospho-Crk/CrkL antibody (Y2221/Y207), hematoxylin stained, and visualized with Dako Red. Cores were scored as moderate-strongly positive if the Score was ≥1.4 where Score=Intensity (1+,2+,3+) X proportion of positive-staining tumor cells. Scores for each core are indicated in brackets (e.g. [0.5]). Photographs are 400X magnification, and two pCrk/CrkL-stained nevi are shown. (c) Graphical representation of pCrk/CrkL staining in melanoma subtypes and age groups. Positive cases have Scores ≥1.4 (see above).
Table 1.
Association of pCrk/CrkL phosphorylation with various characteristics.
| Characteristics | Total n | pCrk/CrkL+ (score≥1.4) | pCrk/CrkL− (score<1.4) | p-value | |
|---|---|---|---|---|---|
| Tumor Type | |||||
| Benign Nevi | 18 | 6 (33%) | 12 |
|
0.051 |
| Primary Melanomas | 48 | 29 (60%) | 19 | ||
| Lymph Node Metastases | 19 | 9 (47%) | 10 | ||
| Age | |||||
| Primary and Metastases | |||||
| ≤39 | 7 | 6 (85%) | 1 |
|
0.12 |
| 40–59 | 39 | 20 (51%) | 19 | ||
| ≥60 | 21 | 10 (47%) | 11 | ||
| Sun-Exposure | |||||
| Primary and Metastases | |||||
| chronic | 6 | 4 (67%) | 2 |
|
0.162 |
| intermittant | 28 | 17 (61%) | 11 | ||
| minimally-exposed | 28 | 13 (46%) | 15 | ||
| mucosal | 5 | 4 (80%) | 1 | ||
| Stage | |||||
| Primary | |||||
| T1 | 2 | 1 | 1 |
|
0.492 |
| T2 | 4 | 3 | 1 | ||
| T3 | 5 | 4 | 1 | ||
| T4 | 37 | 21 | 16 | ||
*two-sided p value from χ2 test
one-sided p value from Cochran-Armitage Trend Test
c-Abl and Arg promote melanoma invasion, proliferation and survival
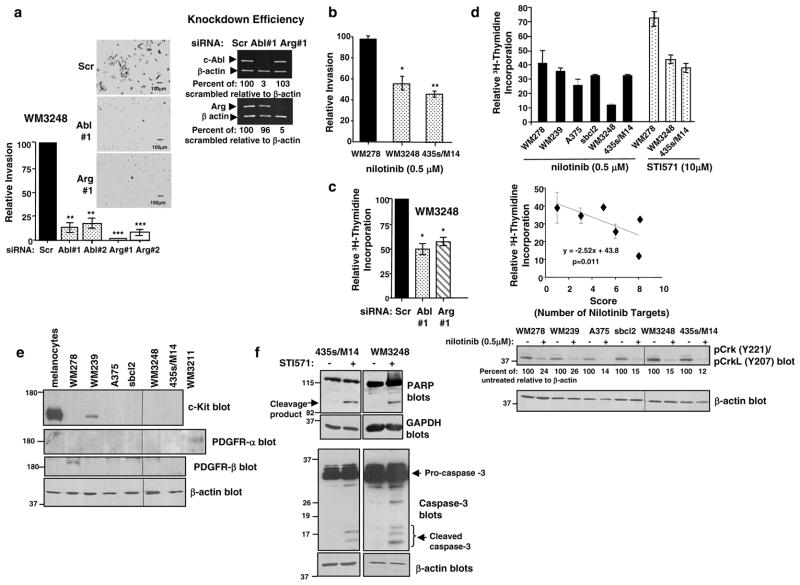
Previously, we showed that c-Abl and Arg promoted 435s/M14 invasion, whereas Arg alone induced proliferation (Srinivasan and Plattner 2006, Srinivasan et al 2008). To determine whether c-Abl and Arg promote these processes in other melanoma cell lines, we studied WM3248 cells, which also contain highly active c-Abl and Arg (Figure 1a). Consistent with our data in 435s/M14 cells, silencing either c-Abl or Arg, with two different siRNAs, dramatically reduced matrigel invasion of WM3248 cells (Figure 2a). Treatment with low dose nilotinib (0.5μM) also reduced invasion of melanoma cells containing highly active c-Abl/Arg (WM3248, 435s/M14), whereas nilotinib had no effect in a cell line containing low c-Abl/Arg activity (WM278; Figure 2b). Utilizing tritiated thymidine assays, we found that unlike in 435s/M14 cells where Arg alone promoted proliferation, both c-Abl and Arg were required for proliferation of WM3248 cells (Figure 2c), whereas STI571-treatment inhibited proliferation/S-phase entry in both cell lines (Figure 2d, top)(Srinivasan et al 2008). Knockdown of c-Abl and Arg was highly efficient in both cell lines (Figure 2a) (Srinivasan and Plattner 2006, Srinivasan et al 2008), and neither cell line expressed c-Kit or PDGFRα,β other targets of imatinib/STI571 and nilotinib (Figure 2e) (Srinivasan and Plattner 2006). A dose of 10μM STI571 was used because this is the lowest dose required to inhibit c-Abl phosphorylation/activity (65–75%) (Srinivasan and Plattner 2006). Melanoma proliferation/S-phase entry also was efficiently inhibited by nilotinib, and a concentration of 0.5μM inhibited proliferation slightly better than 10μM STI571 in 435s/M14 cells, and dramatically better than STI571 in WM3248 cells (Figure 2d, top). Nilotinib-mediated inhibition of proliferation correlated with the level of c-Abl/Arg activity and the number of nilotinib targets expressed in melanoma cell lines (“Score”) (Table 2, Figure 2d, middle). Interestingly, proliferation of WM278 was modestly inhibited by nilotinib, which was consistent with pCrk/CrkL levels but not with c-Abl/Arg kinase activities. These data indicate that in this cell line, pCrk/CrkL may be more indicative of the potential anti-proliferative response to nilotinib than c-Abl/Arg activity, perhaps due to the fact that these cells express PDGFR-β, a nilotinib target (Figure 2e). Nilotinib efficiently inhibited phosphorylation of c-Abl/Arg downstream targets, Crk/CrkL, in all melanoma cell lines; however, nilotinib was slightly more effective in cell lines with the highest c-Abl/Arg activity (Figure 2d, bottom). Activated c-Abl and Arg also prevented PARP and caspase-3 cleavage following prolonged nutrient deprivation, indicating a role for c-Abl and Arg in melanoma cell survival (Figure 2f).
Figure 2. Activation of c-Abl and Arg promotes invasion, proliferation, and survival of melanoma cells.
(a) Serum-starved WM3248 cells, transfected with two independent cAbl or Arg siRNAs, were incubated in matrigel invasion chambers for 48h utilizing IGF-1 (10nM) as chemoattractant. The total number of invaded cells on the undersurface of the membranes were counted and expressed as a percentage of scrambled. Graphs are Mean ± SEM, n=3 independent experiments, performed in duplicate. **0.001≤p<0.01; ***p<0.001. Photographs are at 100X magnification. Knockdown of c-Abl and Arg was assessed by semi-quantitative RT-PCR (right). (b) Melanoma cells were serum-starved and treated with nilotinib (0.5μM) for 16–20h, and incubated in invasion assays as described in (a) with nilotinib in the top and bottom chambers. Graphs are Mean ± SEM, n=3, normalized to untreated. *p<0.05. (c,d) Tritiated thymidine incorporation was assessed in cells treated with STI571, nilotinib, or transfected with siRNAs. CPMs were normalized to vehicle-treated or scrambled-transfected cells. Mean ± SEM, n=3 independent experiments, performed in triplicate. Some error bars are too small to be visualized. (d, middle) The level of c-Abl/Arg activation and the number of nilotinib targets were added together to create a “Score” (Table 2, Fig. 1a, Fig. 2e), which was plotted against tritiated thymidine incorporation results. An inverse correlation between the “Score” (number of nilotinib targets) and the sensitivity to nilotinib was observed. R2=0.45, p=0.011. Lysates from untreated (e) or nilotinib-treated (24h) (d-bottom) cells in serum conditions were blotted with the indicated antibodies. (f) Lysates from detached and attached cells treated with vehicle or STI571 and deprived of serum for 72h (WM3248) or 96h (435s/M14) were probed with the indicated antibodies. Scr=scrambled.
Table 2.
Sensitivity of Melanoma cells to Nilotinib.
| Cell Line | WM278 | WM239 | A375 | sbcl2 | WM3248 | 435s/M14 |
|---|---|---|---|---|---|---|
| Abl activity | − | − | ++++ | +++ | ++++ | ++++ |
| Arg activity | − | ++ | ++ | ++ | ++++ | ++++ |
| c-Kit | − | + | − | − | − | − |
| PDGFR-α | + | − | − | − | − | − |
| PDGFR-β | − | − | − | − | − | − |
| # of nilotinib targets | 1 | 3 | 6 | 5 | 8 | 8 |
| 3H-Thymidine | 41±8.4 | 36±2.07 | 26±2.4 | 39.5±1.5 | 12±0.38 | 32.7±.0.48 |
The level of c-Abl/Arg activities (Fig. 1) and presence of other nilotinib targets were tabulated, and +’s added together to create a “Score”. Outcome of tritiated thymidine incorporation assays using nilotinib (0.5μM) also are shown. An inverse correlation between the number of nilotinib targets and tritiated thymidine incorporation is shown in Figure 2d.
c-Abl and Arg induce transcriptional upregulation and activation of matrix metalloproteinases (MMPs) in melanoma cells
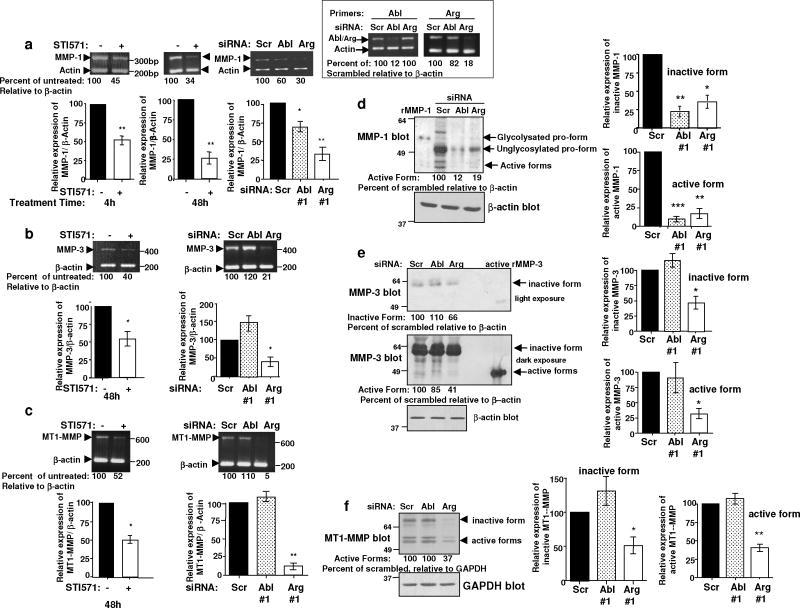
Since invasion is critical for metastasis, and c-Abl and Arg dramatically promoted invasion of melanoma cells, we focused on identifying the mechanism of c-Abl/Arg-dependent invasion. Acquisition of the invasive, VGP phenotype in melanoma cells is dependent on MMP expression. Using semi-quantitative RT-PCR, we found that MMP-1, MMP-3, and MT1-MMP were expressed in 435s/M14 cells, while MMP-2 was not (data not shown). Significantly, expression of MMP-1, MMP-3, and MT1-MMP contributed to the invasiveness of 435s/M14 cells, as silencing any one MMP significantly reduced invasion, although MT1-MMP played a less prominent role (Supplementary Figure 1). Since c-Abl and Arg also potently promote invasion, we determined whether they regulate MMP expression. Significantly, STI571 treatment or expression of c-Abl or Arg siRNAs inhibited MMP-1, MMP-3, and MT1-MMP transcription as assessed by semi-quantitative RT-PCR (Figure 3a–c; Supplementary Figure 2). However, although silencing c-Abl or Arg reduced MMP-1 transcription, only the Arg siRNA decreased MMP-3 and MT1-MMP mRNA levels (Figure 3a–c, right). Next, we examined MMP activation and secretion by blotting conditioned medium with antibodies that recognize active/cleaved forms. Consistent with the RT-PCR results, silencing either c-Abl or Arg reduced secretion and activation of MMP-1 (Figure 3d), whereas silencing Arg alone inhibited MMP-3 and MT1-MMP activation (Figure 3e,f). Thus, c-Abl and Arg upregulate MMPs in melanoma cells, increasing secretion of the active, cleaved forms, which are required for invasion.
Figure 3. Activated c-Abl and Arg induce MMP transcription and secretion in melanoma cells.
RNA extracted from serum-starved 435s/M14 cells, treated with STI571 (10μM) for 4h (a) or 48h (a-c), or from cells transfected with Abl siRNAs and serum-starved for 48h, was subjected to semi-quantitative RT-PCR with MMP and internal control actin primers. Quantitated MMP bands were divided by quantified actin bands and expressed as a percentage of scrambled. Mean ± SEM, n≥3 independent experiments. *0.01≤p<0.05; **0.001≤p<0.01. Knockdown efficiency was determined with c-Abl/Arg primers; a representative experiment is shown (a, right inset). (d,e) Concentrated conditioned media from siRNA-transfected cells were blotted with MMP antibodies that recognize inactive and active forms. Media amounts were normalized to β-actin levels in cell lysates (below). Full-length recombinant MMP-1 (inactive and active forms) and full-length, recombinant, active MMP-3 were used to confirm the location of the active forms. Graphs are Mean ± SEM, n=3 independent experiments. *0.01≤p<0.05; **0.001≤p<0.01; ***p<0.001. (f) Lysates were blotted with antibody that recognizes active MT1-MMP (left). Graphs are Mean ± SEM, n=3 independent experiments. **0.001≤p<0.01.
c-Abl and Arg induce MMP-1 transcription by activating STAT3
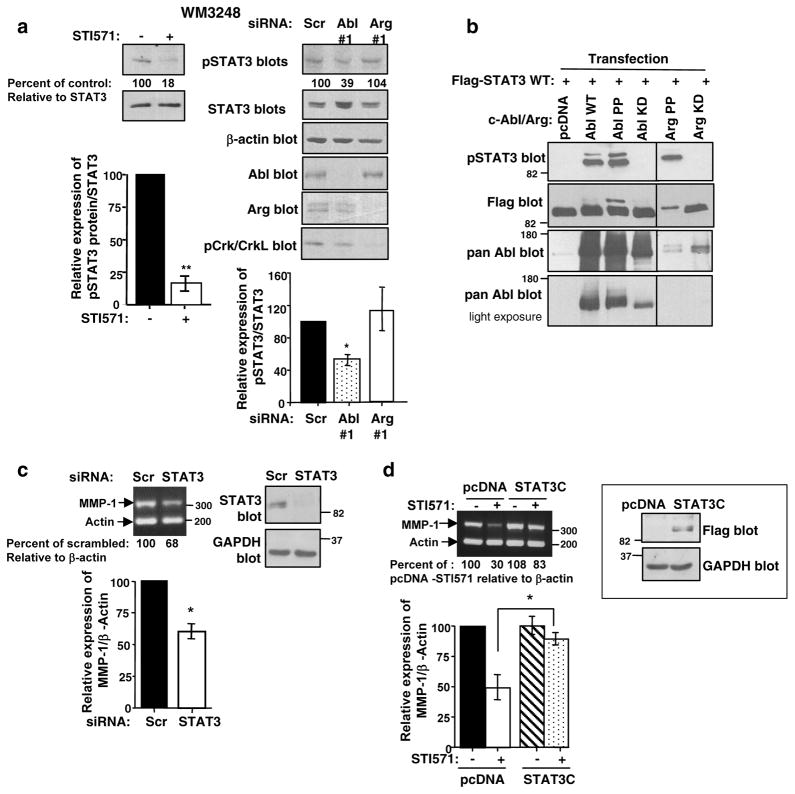
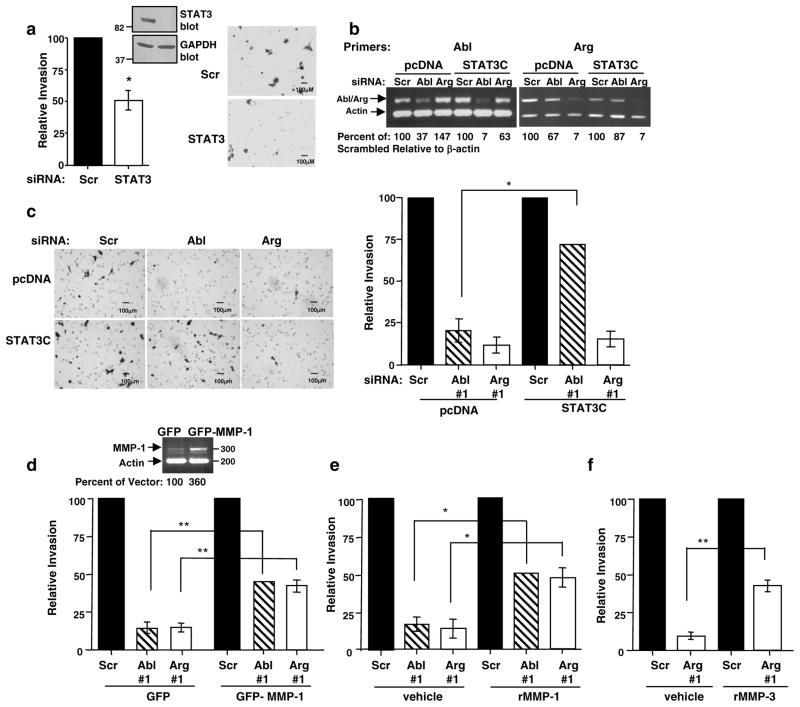
Like MMPs, STAT3 also plays a critical role in progression of melanomas from RGP to VGP, and increases MMP-1 expression in bladder and colon cancer cells (Itoh et al 2006, Tsareva et al 2007). Using STI571 and siRNA approaches, we showed that c-Abl and Arg activate STAT3 in 435s/M14 cells (Srinivasan et al 2008). STI571 and silencing c-Abl also efficiently inhibited STAT3 phosphorylation in WM3248 cells (Figure 4a). To confirm that c-Abl and Arg activate STAT3, we tested whether they induce STAT3 phosphorylation in a heterologous system. High-level overexpression of wild-type c-Abl in 293T cells activates its kinase activity (Pendergast et al 1991). We found that expression of wild-type c-Abl or constitutively active c-Abl or Arg (PP) induced tyrosine phosphorylation of Flag-tagged STAT3 when coexpressed in 293T cells (Figure 4b). STAT3 is known to be phosphorylated by Src and JAK kinases; however, STI571 treatment had no effect on Jak 1,2, or Src phosphorylation in 435s/M14 cells, indicating that c-Abl and Arg induce STAT3 phosphorylation independent of these proteins (Kortylewski et al 2005, Srinivasan and Plattner 2006, Srinivasan et al 2008). Since Src and c-Abl/Arg phosphorylate many of the same substrates (Boyle et al 2007), we investigated whether c-Abl and Arg directly phosphorylate STAT3. We immunoprecipitated constitutively active c-Abl and Arg from transfected 293T cell lysates, and assayed their ability to phosphorylate GST-STAT3 by in vitro kinase assay. Surprisingly, c-Abl and Arg did not appreciably phosphorylate STAT3 in vitro (Supplementary Figure 3), indicating that they indirectly induce STAT3 phosphorylation via an as yet unidentified tyrosine kinase. Since c-Abl and Arg promote activation of MMPs and STAT3, and MMP-1 has STAT3 binding sites in its promoter, we investigated whether c-Abl/Arg upregulate MMP-1 via a STAT3-dependent mechanism using semi-quantitative RT-PCR. Significantly, MMP-1 mRNA levels were reduced following silencing STAT3 (Figure 4c), and expression of a constitutively active form of STAT3 (STAT3C) rescued the inhibition of MMP-1 transcription induced by STI571 treatment (Figure 4d). Taken together, these data indicate that STAT3 lies in a signaling pathway between c-Abl/Arg and MMP-1.
Figure 4. c-Abl upregulates MMP-1 via STAT3.
(a) WM3248 cells were treated with STI571 (10μM) for 48h or transfected with c-Abl/Arg siRNAs, and lysates probed with the indicated antibodies. Graphs are Mean ± SEM, n=3 independent experiments. *p<0.05, **0.001≤p<0.01. (b) 293T cells were cotransfected with flag-tagged STAT3 and wild-type (WT), constitutively active (PP), or kinase-dead (KD) forms of c-Abl or Arg, and lysates were blotted with the indicated antibodies. One of three representative experiments is shown. (c) RNA extracted from 435s/M14 cells, transfected with STAT3 siRNA and serum-starved (48h), was subjected to semi-quantitative RT-PCR (left). Quantitated MMP bands were divided by quantified actin bands and expressed as a percentage of scrambled. Graph is Mean ± SEM, n=3 independent experiments. *p<0.05. An aliquot of cells was lysed, and blotted with STAT3 antibody (right); a representative blot is shown. (d) Clones expressing Flag-tagged STAT3C or vector (pcDNA) were pooled, and expression examined by western blot (right). Serum-starved 435s/M14 cells, stably expressing vector or STAT3C, were treated with STI571 (10μM; 48h), and RNA subjected to semi-quantitative RT-PCR (left). Quantitated MMP bands divided by quantified actin bands were expressed as a percentage of untreated. Graph is Mean ± SEM, n=3 independent experiments. *p<0.05.
c-Abl promotes melanoma invasion via a STAT3→MMP-1 pathway, while Arg drives invasion in a STAT3-independent manner via MMP-1 and MMP-3
Silencing either cAbl or Arg potently inhibited invasion of 435s/M14 and WM3248 melanoma cell lines, demonstrating that both kinases are required for melanoma invasion (Figure 2a) (Srinivasan and Plattner 2006). Since silencing STAT3 also reduced invasion (Figure 5a), we tested whether c-Abl and Arg promote invasion in a STAT3-dependent manner. Significantly, expression of STAT3C rescued the block in invasion induced by silencing cAbl but not Arg, indicating that c-Abl alone promotes invasion via STAT3 (Figure 5b,c). To determine which MMPs mediate c-Abl and Arg-dependent invasion, we performed a series of rescue experiments. Modest constitutive expression of MMP-1 or addition of recombinant MMP-1 partially (45–50%) rescued the block of invasion induced by silencing c-Abl or Arg (Figure 5d,e), and recombinant MMP-3 partially (41%) rescued the inhibitory effect of the Arg siRNA on invasion (Figure 5f). c-Abl and Arg were efficiently silenced in vector and MMP-1 transfected cells (Supplementary Fig. 4). Thus, c-Abl and Arg mediate invasion via distinct mechanisms: c-Abl promotes STAT3-dependent invasion, in part, via MMP-1, whereas, Arg promotes STAT3-independent invasion via MMP-1 and MMP-3.
Figure 5. c-Abl promotes invasion in a STAT3-dependent manner.
Invasion assays were performed on: (a) 435s/M14 cells transfected with scrambled or STAT3 siRNAs; (c) 435s/M14 cells stably expressing vector or STAT3C, transfected with c-Abl/Arg siRNAs; (d) 435s/M14 cells stably expressing GFP or GFP-MMP-1, transfected with c-Abl/Arg siRNAs; and (e,f) 435s/M14 cells, transfected with c-Abl or Arg siRNAs and incubated with recombinant MMP-1 or MMP-3 (25 ng/ml) during the invasion assays. Photographs are at 100X magnification. The total number of invaded cells on the undersurface of the membranes were counted and expressed as a percentage of scrambled. Graphs are Mean ± S EM, n=3 independent experiments, performed in duplicate. *0.01≤p<0.05; **0.001≤p<0.01; ***p<0.001. Some error bars are too small to be visualized. (b, d-top) Knockdown efficiency and MMP-1 expression in stably expressing cells were determined by semi-quantitative RT-PCR (35, 27 cycles, respectively); representative experiments are shown.
Since STAT3 also promotes proliferation and survival of melanoma cells (Kortylewski et al 2005), we tested whether the effects of c-Abl and/or Arg on proliferation or survival are STAT3-dependent. Although silencing STAT3 decreased proliferation as measured by tritiated thymidine assay (Supplementary Figure 5a), expression of constitutively active STAT3C did not rescue Arg siRNA-mediated inhibition of proliferation (Supplementary Figure 5b), and only partially (30–40%) rescued STI571-mediated PARP cleavage following prolonged nutrient deprivation (Supplementary Figure 5c). Therefore, cAbl alone mediates invasion via STAT3; Arg promotes proliferation and invasion in a STAT3-independent manner; and c-Abl and Arg prevent PARP cleavage in nutrient-deprived conditions, in part, via a STAT3-dependent pathway.
c-Abl and Arg promote melanoma metastasis
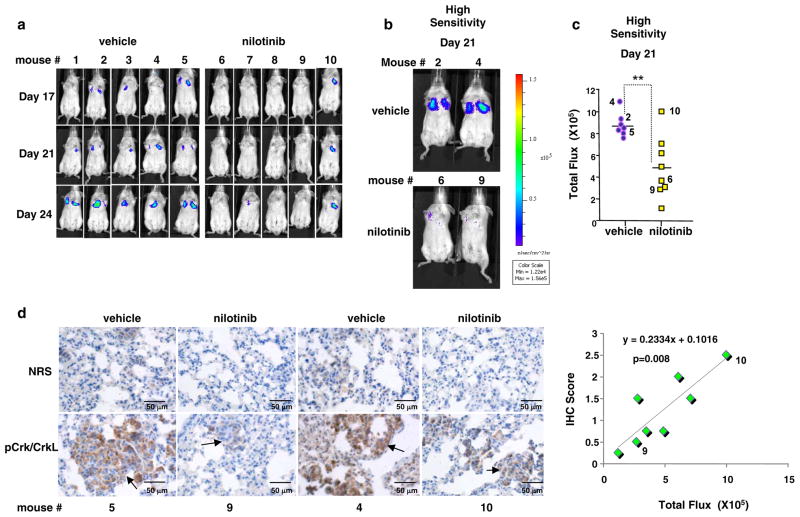
To test whether c-Abl and Arg promote melanoma metastatic progression, we utilized an experimental metastasis model, in which melanoma cells are introduced intravenously into immune-compromised mice, and the ability of cells to metastasize to the lungs is assessed. c-Abl and Arg promote invasion, proliferation, and survival in the absence of nutrients, in vitro, processes which are required for metastasis (intravasation/extravasation, survival in the bloodstream, and growth and survival at the ectopic site). Therefore, to test whether active c-Abl and Arg drive melanoma metastasis, GFP/luciferase-labeled human melanoma cells were injected intravenously into SCID-beige mice, mice were treated with vehicle or STI571, and metastasis was measured by IVIS imaging. STI571 treatment induced significant toxicity in young mice, necessitating a dose reduction, and had no effect on metastasis in a pilot experiment (data not shown). Since the second generation drug, nilotinib, is more specific for c-Abl and Arg, more potent (Figure 2d), and less toxic (Deininger 2008), we initiated a similar study with nilotinib. Significantly, using IVIS imaging, we demonstrate that metastasis was dramatically inhibited in mice treated with nilotinib as compared to vehicle-treated mice (Figure 6a–c). In addition, pathologic examination of the lungs revealed that the small, infrequent lesions found in the lungs of a mouse that responded to nilotinib (mouse #9) had reduced c-Abl/Arg activity (phospho-Crk/CrkL staining) as compared to vehicle-treated mice (Figure 6d, mice #4,5). In contrast, in the numerous metastases from a mouse that did not respond to nilotinib, c-Abl/Arg activity was only minimally suppressed (Figure 6d, left; mouse #10). In addition, c-Abl/Arg kinase activities were inversely correlated with IVIS fluorescence in all nilotinib-treated mice (Figure 6d, right). Taken together, these data demonstrate that the anti-metastatic capability of nilotinib is linked to inhibition of c-Abl/Arg kinase activity, and show for the first time, that active c-Abl and Arg not only promote in vitro processes associated with metastatic progression, but also promote metastasis, in vivo. In addition, nilotinib is a less toxic, more active agent than imatinib/STI571 for inhibiting c-Abl/Arg-dependent melanoma metastatic progression.
Figure 6. Activation of c-Abl and Arg promotes melanoma metastasis.
435s/M14 cells, labeled with GFP and luciferase (2×106), were injected into the tail vein of SCID-beige mice. Mice were treated with vehicle or nilotinib (30mg/kg; b.i.d) by oral gavage, and imaged with IVIS following luciferin D injection (i.p.). (a) IVIS imaging of representative mice on days 17, 21, and 24 post-injection. (b) Representative mice on day 21 imaged with high sensitivity (integration) such that low level fluorescence could be detected. (c) Quantitated fluorescence of all mice on day 21 imaged with high sensitivity. Each symbol indicates one animal. Numbers refer to animals numbers shown in (a, b). **0.001≤p<0.01. (d) Lungs from the indicated mice were fixed in formalin on day 24, paraffin-embedded, sectioned, stained with normal rabbit serum or antibody to phosphorylated Crk/CrkL, visualized with DAB, and hematoxylin-stained. Photographs are 400X magnification. IVIS fluorescence values were plotted against pCrk/CrkL IHC intensity scores (1–3+) in metastases from lungs of nilotinib-treated mice. A positive correlation was observed between the values (R2=0.72; p=0.008).
DISCUSSION
This is the first demonstration that the kinase activities of c-Abl and Arg are elevated in primary melanomas (60%), benign nevi (33%), and in multiple human melanoma cell lines. Abl activation was significantly more frequent in melanomas than in benign nevi. A subset of nevi did contain high c-Abl/Arg activity; however, the percentage was much lower than the prevalence of B-Raf mutations in nevi (82%) (Kong et al 2010). In contrast, the percentage of melanomas containing high c-Abl/Arg activity approximated the prevalence of B-Raf mutations in melanomas (60% vs. 66%) (Kong et al 2010). These data indicate that, unlike B-Raf, activation of Abl kinases is unlikely to be involved in melanoma initiation. It is possible that nevi containing active c-Abl and Arg are more likely to progress to melanomas than nevi lacking active c-Abl and Arg; however, we are unable to test this hypothesis due to lack of clinical data. Interestingly, the presence of B-Raf mutations in benign nevi is not predictive of progression, likely due to its role in promoting senescence (Lin et al 2010, Maldonado et al 2003). We observed high c-Abl/Arg activity in melanomas from all sun-exposure subtypes, although there was a trend towards a lower percentage of positive cases in melanomas from minimally sun-exposed skin. c-Kit is often activated in mucosal melanomas, and some melanomas with activated c-Kit respond to imatinib, whereas others do not (Kabbarah and Chin 2005, Satzger et al 2010, Smalley et al 2009). Since c-Abl and Arg are activated in some melanomas from mucosal areas, activated cAbl and/or Arg and mutated c-Kit may occur simultaneously in some melanomas. Therefore, response to imatinib may depend on the activation status of c-Abl and Arg (i.e. melanomas that have c-Kit mutations AND c-Abl and/or Arg activation may be more sensitive to imatinib due to activation of more than one imatinib target).
We demonstrate here that c-Abl and Arg are both required for the invasive capacity of two human melanoma cell lines, and they induce STAT3 phosphorylation and increase MMP expression/activation. Since activation of STAT3 and MMPs is critical for converting non-invasive RGP melanomas to invasive VGPs, c-Abl and Arg also are likely to play a critical role in this process. Interestingly, although STAT3 and c-Abl and Arg promote proliferation and invasion of melanoma cells, STAT3 only mediates c-Abl-dependent invasion, and is not involved in Arg-dependent invasion or proliferation. We also report for the first time, that c-Abl and Arg signal through distinct pathways to mediate the same biological outcome, indicating that the two proteins are not merely redundant.
A recent report demonstrated that silencing c-Abl and Arg inhibited gelatinase activity in mouse NIH3T3 fibroblasts and MDA-MB-231 breast cancer cells; however, the mechanism was not clear (Smith-Pearson et al 2010). c-Abl and Arg interacted with and induced phosphorylation of MT1-MMP following overexpression in 293T cells, and silencing Arg inhibited MT1-MMP plasma membrane localization in cells that overexpress activated Src. Thus, the authors suggested that c-Abl/Arg-dependent phosphorylation of MT1-MMP promotes its membrane localization/activity. However, endogenous Abl/MT1-MMP complexes and Abl-dependent tyrosine phosphorylation of endogenous MT1-MMP were not demonstrated in untransfected human cancer cells. Here, we identify the mechanism by which endogenous Arg increases endogenous MT1-MMP activity in human melanoma cells by demonstrating that Arg but not c-Abl increases MT1-MMP expression and activity by increasing its transcription.
There is controversy in the literature regarding the role of c-Abl in solid tumors. Whereas we and others show that c-Abl and Arg are activated in some solid tumor cells, and promote invasion, proliferation, survival, PDGF-induced epithelial-mesenchymal transition (EMT), and TGF-β-induced anchorage-independent growth (Furlan et al 2011, Lin and Arlinghaus 2008, Sirvent et al 2008, Smith-Pearson et al 2010, Srinivasan and Plattner 2006, Srinivasan et al 2008, Srinivasan et al 2009), other groups suggest that c-Abl prevents invasion, inhibits TGF-β-induced EMT, and abrogates tumorigenesis (Allington and Schiemann 2011). In studies showing a positive role for c-Abl and Arg in invasion and proliferation, such as those described here, inhibition of c-Abl and/or Arg in cells expressing highly active forms of c-Abl and Arg (due to activation downstream of constitutively active receptor tyrosine kinases and Src kinases or following loss of expression of a negative regulator) abrogated invasion and proliferation in response to growth factors or serum (Furlan et al 2011, Li et al 2010, Lin and Arlinghaus 2008, Mader et al 2011, Sirvent et al 2008, Smith-Pearson et al 2010, Srinivasan and Plattner 2006, Srinivasan et al 2008, Srinivasan et al 2009). In contrast, in studies demonstrating a negative role for c-Abl, researchers inhibited c-Abl in cells with low/basal activity (or it was never analyzed), or they examined the role of c-Abl following stimulation with a factor that inhibits invasion, proliferation, and tumorigenesis (e.g. ephrin B2/EphB4)(Allington et al 2009, Allington and Schiemann 2011, Noren et al 2006). Other differences include: 1) the use of mouse rather than human cells; 2) overexpression of a mutated, constitutively active form of c-Abl, which does not exist naturally in solid tumor cells, in the absence of other molecular alterations normally present in invasive tumor cells; 3) use of kinase-dead c-Abl, which may not act as a dominant-negative since it also has scaffolding functions; 4) lack of examination of the effect of Arg in combination with c-Abl, as Arg activation may modulate c-Abl effects; 5) use of extremely high doses of STI571/imatinib (17–50μM) for in vitro studies, which are likely to have significant off-target effects; and 6) use of low STI571/imatinib doses, administered only once daily, for in vivo studies (Allington et al 2009). It also was suggested that clinical trials using imatinib for the treatment of solid tumors have failed because c-Abl and Arg inhibit rather than promote tumorigenesis (Allington and Schiemann 2011). However, it is important to note that in all of these studies, treatment was not restricted to patients containing tumors with highly active c-Abl and/or Arg (Allington and Schiemann 2011). Therefore, it is clear that one must identify tumors containing highly active c-Abl and/or Arg, and utilize inhibitors only for this population, as treatment of tumors with low activity may have no effect or may even promote tumorigenesis and metastases.
This is the first demonstration that active c-Abl and Arg dramatically promote metastasis of human cancer cells. Thus, the c-Abl/Arg-dependent effects that we observed on in vitro characteristics of melanoma metastatic progression were recapitulated in vivo. Our data predict that metastatic progression of melanomas containing active c-Abl and Arg should be inhibited by anti-Abl therapies. However, in clinical trials using untargeted populations of melanoma patients, imatinib was ineffective (Hofmann et al 2009, Ugurel et al 2005). There are two possible explanations for these results: 1) c-Abl and Arg may not be activated in melanomas from the non-responding patients (described above); and/or 2) imatinib concentrations needed to effectively inhibit c-Abl and Arg were not achieved. CML patients in blast crisis are treated with 600 mg/day STI571, which results in Cmax plasma concentrations of 12–13μM, and patients with gastrointestinal tumors expressing c-Kit receive 800 mg/day (Candelaria et al 2005, le Coutre et al 2004). Therefore, a plasma concentration of 10μM should be able to be achieved in the clinic. However, here we demonstrate that although imatinib and nilotinib both inhibited Abl-dependent processes in vitro, only nilotinib inhibited metastasis, in vivo. This may be because imatinib was toxic to young animals, requiring a dose reduction, likely resulting in suboptimum plasma concentrations levels. Since nilotinib is more potent and selective for c-Abl/Arg and less toxic (Deininger 2008), higher plasma concentrations are likely to have been achieved, resulting in more effective inhibition of c-Abl/Arg kinase activity and dramatic abrogation of metastasis. Since low level phospho-Crk/CrkL staining was observed in small, infrequent metastatic lesions from animals that responded to imatinib, the plasma nilotinib concentration still may not be high enough to completely abrogate c-Abl/Arg activity, and a higher plasma concentration is likely to increase nilotinib’s anti-metastatic effects. In summary, our data demonstrate that c-Abl and Arg are important clinical targets in melanoma, and indicate that nilotinib may be an effective agent for inhibiting metastatic disease in patients with melanomas containing activated c-Abl and Arg.
MATERIALS AND METHODS
Immunohistochemistry
Melanoma tissue microarrays (TMAs; ME1003; Biomax, Rockville, MD) or slides containing paraffin-embedded, sectioned mouse lungs, were heated overnight (60°C), deparaffinized, antigens retrieved (90°C EDTA; 20′), incubated with primary phospho-Crk/CrkL (Y221/Y207) antibody (1:10, TMAs; 1:25, mouse lungs)(Singer et al 2004) or normal rabbit serum (2h), Envision+horseradish peroxidase (HRP) anti-Rabbit HRP-conjugated antibody (30′), followed by Dako Red AEC+ High Sensitivity Chromagen RTU (Figure 1)(15) or DAB (3,3′-diaminobenzidine; Figure 6), and hematoxylin counterstaining (Dako, Real Carpinteria, CA). Photographs were taken on an Olympus BX51 microscope, 40X objective, equipped with a QI cam (Fast 1394), and imaged with QCapture Pro software (Figure 1)(Surrey, BC, Canada), or on a Nikon Eclipse TE200, and imaged with MetaMorph software (Molecular Devices; Sunnyvale, CA; Figure 6). TMAs were blindly scored by the Director of Surgical Pathology (Michael Cibull, M.D.).
MMP expression/activation
Basal, constitutive MMP levels were assessed in serum-starved, similar density, subconfluent cells, since cell density and serum, which contains growth factors (e.g. EGF) and MMPs, can alter MMP expression (Bachmeier et al 2005, Nawrocki Raby et al 2001, Watabe et al 1998). For short-term STI571 treatment (4–8h), cells were serum-starved overnight prior to treatment, while for 24–48h time points, cells were starved and treated simultaneously. siRNA-transfected cells were serum-starved for 24–48h, 3 days after transfection. STI571-treatment of serum-starved cells for <48h did not induce apoptosis (Supplementary Figure 6). Transcript levels were determined by semi-quantitative RT-PCR (Srinivasan and Plattner 2006) (Supplementary Methods), and activation/secretion was assessed by western blot of concentrated media (Ultracel-10K; Millipore; Temecula, CA).
Metastasis Assays
435s/M14 cells were transfected with pcDNA-EGFP-N1 (Clontech, Mountain View, CA), and pcDNA3.1-Zeo-luciferase (cloned from pGL3 (Promega, Madison, WI) into pcDNA3.1-Zeo (Invitrogen, Carlsbad, CA)), followed by zeocin/G418 (600/900μg/ml) selection. Expressing clones were pooled, expanded, and injected (2×106 cells/100μl Hanks Balanced Salt Solution, (HBSS); Invitrogen) into the tail vein of 7–8 week-old SCID-beige mice. Mice were treated with vehicle (0.5% hydroxymethylcelluose/0.05% Tween-80) or nilotinib (30mg/kg; b.i.d.) by oral gavage. On days 17, 21 and 24, mice were injected with luciferin D (100mg/kg; i.p.), and fluorescence measured by IVIS Xenogen Spectrum (Caliper Life Sciences, Hopkinton, MA). Flux values were normalized with Living Image 3.1 software using low level integration in order to observe differences between timepoints, and high level integration (high sensitivity) for quantitation. On day 24, mice were euthanatized, lungs removed, fixed in 100% formalin, paraffin-embedded (Supplemental Methods), sectioned and stained (above). The study was approved by the University of Kentucky Institutional Animal Care and Use Committee, according to NIH guidelines.
Statistics
Student’s t tests were used for group comparisons using SAS software.
Supplementary Material
Acknowledgments
We thank Patty Cross, J. Anthony Brandon, Holly Bennett, and Jennifer Strange for assistance with IHC, IVIS imaging, isolating GST-STAT3, and cell sorting, respectively; John D’Orazio for primary melanocytes; Meenhard Herlyn for WM melanoma cell lines and for genotyping WM cell lines; Suyan Huang for A375; Fernando deCastro for IHC advice; and Paul Manley (Novartis, Switzerland) for STI571 and nilotinib and advice regarding their use.
Footnotes
CONFLICTS OF INTEREST. Dr. Plattner’s work is funded by NIH/NCI. All other authors declare no potential conflicts of interest.
References
- Allington TM, Galliher-Beckley AJ, Schiemann WP. Activated Abl kinase inhibits oncogenic transforming growth factor-beta signaling and tumorigenesis in mammary tumors. FASEB J. 2009;23:4231–4243. doi: 10.1096/fj.09-138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allington TM, Schiemann WP. The Cain and Abl of Epithelial-Mesenchymal Transition and Transforming Growth Factor-beta in Mammary Epithelial Cells. Cells Tissues Organs. 2011;193:98–113. doi: 10.1159/000320163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier BE, Albini A, Vene R, Benelli R, Noonan D, Weigert C, et al. Cell density-dependent regulation of matrix metalloproteinase and TIMP expression in differently tumorigenic breast cancer cell lines. Exp Cell Res. 2005;305:83–98. doi: 10.1016/j.yexcr.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Berwick M, Erdei E, Hay J. Melanoma epidemiology and public health. Dermatol Clin. 2009;27:205–214. viii. doi: 10.1016/j.det.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JS, Liu I, Coon CI, Brinckerhoff CE. A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis. Oncogene. 2009;28:4237–4248. doi: 10.1038/onc.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007;17:445–451. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE, Rutter JL, Benbow U. Interstitial collagenases as markers of tumor progression. Clin Cancer Res. 2000;6:4823–4830. [PubMed] [Google Scholar]
- Candelaria M, de la Garza J, Duenas-Gonzalez A. A clinical and biological overview of gastrointestinal stromal tumors. Med Oncol. 2005;22:1–10. doi: 10.1385/MO:22:1:001. [DOI] [PubMed] [Google Scholar]
- Clark WH, Jr, Elder DE, Guerry Dt, Epstein MN, Greene MH, Van Horn M. A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol. 1984;15:1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- Deininger MW. Nilotinib. Clin Cancer Res. 2008;14:4027–4031. doi: 10.1158/1078-0432.CCR-07-5015. [DOI] [PubMed] [Google Scholar]
- DeMatteo RP. Nanoneoadjuvant therapy of gastrointestinal stromal tumor (GIST) Ann Surg Oncol. 2009;16:799–800. doi: 10.1245/s10434-009-0316-9. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- Fanjul-Fernandez M, Folgueras AR, Cabrera S, Lopez-Otin C. Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta. 2010;1803:3–19. doi: 10.1016/j.bbamcr.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan A, Stagni V, Hussain A, Richelme S, Conti F, Prodosmo A, et al. Abl interconnects oncogenic Met and p53 core pathways in cancer cells. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann UB, Kauczok-Vetter CS, Houben R, Becker JC. Overexpression of the KIT/SCF in uveal melanoma does not translate into clinical efficacy of imatinib mesylate. Clin Cancer Res. 2009;15:324–329. doi: 10.1158/1078-0432.CCR-08-2243. [DOI] [PubMed] [Google Scholar]
- Itoh M, Murata T, Suzuki T, Shindoh M, Nakajima K, Imai K, et al. Requirement of STAT3 activation for maximal collagenase-1 (MMP-1) induction by epidermal growth factor and malignant characteristics in T24 bladder cancer cells. Oncogene. 2006;25:1195–1204. doi: 10.1038/sj.onc.1209149. [DOI] [PubMed] [Google Scholar]
- Kabbarah O, Chin L. Revealing the genomic heterogeneity of melanoma. Cancer Cell. 2005;8:439–441. doi: 10.1016/j.ccr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Kong Y, Kumar SM, Xu X. Molecular pathogenesis of sporadic melanoma and melanoma-initiating cells. Arch Pathol Lab Med. 2010;134:1740–1749. doi: 10.1043/2009-0418-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–327. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- le Coutre P, Kreuzer KA, Pursche S, Bonin M, Leopold T, Baskaynak G, et al. Pharmacokinetics and cellular uptake of imatinib and its main metabolite CGP74588. Cancer Chemother Pharmacol. 2004;53:313–323. doi: 10.1007/s00280-003-0741-6. [DOI] [PubMed] [Google Scholar]
- Li X, Ma Q, Wang J, Liu X, Yang Y, Zhao H, et al. c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3. Cell Death Differ. 2010;17(8):1277–87. doi: 10.1038/cdd.2010.8. [DOI] [PubMed] [Google Scholar]
- Lin J, Sun T, Ji L, Deng W, Roth J, Minna J, et al. Oncogenic activation of c-Abl in non-small cell lung cancer cells lacking FUS1 expression: inhibition of c-Abl by the tumor suppressor gene product Fus1. Oncogene. 2007;26:6989–6996. doi: 10.1038/sj.onc.1210500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Arlinghaus R. Activated c-Abl tyrosine kinase in malignant solid tumors. Oncogene. 2008;27:4385–4391. doi: 10.1038/onc.2008.86. [DOI] [PubMed] [Google Scholar]
- Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The Role of B-RAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-kappaB/Snail/RKIP/PTEN Circuit. Genes Cancer. 2010;1:409–420. doi: 10.1177/1947601910373795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader CC, Oser M, Magalhaes MA, Bravo-Cordero JJ, Condeelis J, Koleske AJ, et al. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011;71:1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- Mayorga ME, Sanchis D, Perez de Santos AM, Velasco A, Dolcet X, Casanova JM, et al. Antiproliferative effect of STI571 on cultured human cutaneous melanoma-derived cell lines. Melanoma Res. 2006;16:127–135. doi: 10.1097/01.cmr.0000215039.30812.9b. [DOI] [PubMed] [Google Scholar]
- Mouawad R, Sebert M, Michels J, Bloch J, Spano JP, Khayat D. Treatment for metastatic malignant melanoma: Old drugs and new strategies. Crit Rev Oncol Hematol. 2009;74(1):27–39. doi: 10.1016/j.critrevonc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Nawrocki Raby B, Polette M, Gilles C, Clavel C, Strumane K, Matos M, et al. Quantitative cell dispersion analysis: new test to measure tumor cell aggressiveness. Int J Cancer. 2001;93:644–652. doi: 10.1002/ijc.1380. [DOI] [PubMed] [Google Scholar]
- Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl-Crk pathway. Nat Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Kawamura T, Furuhashi M, Tsukamoto K, Shimada S. Improving chemotherapeutic drug penetration in melanoma by imatinib mesylate. J Dermatol Sci. 2008;51:190–199. doi: 10.1016/j.jdermsci.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pendergast AM, Muller AJ, Havlik MH, Clark R, McCormick F, Witte ON. Evidence for regulation of the human Abl tyrosine kinase by a cellular inhibitor. Proc Natl Acad Sci USA. 1991;88:5927–5931. doi: 10.1073/pnas.88.13.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast AM. BCR-ABL protein domain, function, and signaling. In: Carella AM, Daley GQ, Eaves CJ, Goldman JM, Helmann R, editors. Chronic Myeloid Leukaemia: Biology and Treatment. Martin Dunitz, Lt; London: 2001. pp. 19–39. [Google Scholar]
- Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–2411. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner R, Irvin BJ, Guo S, Blackburn K, Kazlauskas A, Abraham RT, et al. A New Link Between the c-Abl Tyrosine Kinase and Phosphoinositide Signaling via PLC-γ1. Nat Cell Biol. 2003;5:309–319. doi: 10.1038/ncb949. [DOI] [PubMed] [Google Scholar]
- Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- Redondo P, Lloret P, Andreu EJ, Inoges S. Imatinib mesylate in cutaneous melanoma. J Invest Dermatol. 2004;123:1208–1209. doi: 10.1111/j.0022-202X.2004.23496.x. [DOI] [PubMed] [Google Scholar]
- Santos FP, Ravandi F. Advances in treatment of chronic myelogenous leukemia--new treatment options with tyrosine kinase inhibitors. Leuk Lymphoma. 2009;50(Suppl 2):16–26. doi: 10.3109/10428190903383427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satzger I, Kuttler U, Volker B, Schenck F, Kapp A, Gutzmer R. Anal mucosal melanoma with KIT-activating mutation and response to imatinib therapy--case report and review of the literature. Dermatology. 2010;220:77–81. doi: 10.1159/000265558. [DOI] [PubMed] [Google Scholar]
- Shepherd C, Puzanov I, Sosman JA. B-RAF inhibitors: an evolving role in the therapy of malignant melanoma. Curr Oncol Rep. 2010;12:146–152. doi: 10.1007/s11912-010-0095-2. [DOI] [PubMed] [Google Scholar]
- Singer CF, Hudelist G, Lamm W, Mueller R, Czerwenka K, Kubista E. Expression of tyrosine kinases in human malignancies as potential targets for kinase-specific inhibitors. Endocr Relat Cancer. 2004;11:861–869. doi: 10.1677/erc.1.00801. [DOI] [PubMed] [Google Scholar]
- Sirvent A, Benistant C, Roche S. Cytoplasmic signalling by the c-Abl tyrosine kinase in normal and cancer cells. Biol Cell. 2008;100:617–631. doi: 10.1042/BC20080020. [DOI] [PubMed] [Google Scholar]
- Smalley KS, Nathanson KL, Flaherty KT. Genetic subgrouping of melanoma reveals new opportunities for targeted therapy. Cancer Res. 2009;69:3241–3244. doi: 10.1158/0008-5472.CAN-08-4305. [DOI] [PubMed] [Google Scholar]
- Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM. Abl kinases are required for invadopodia formation and chemokine-induced invasion. J Biol Chem. 2010;285(51):40201–11. doi: 10.1074/jbc.M110.147330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan D, Plattner R. Activation of abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–5655. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008;27:1095–1105. doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
- Srinivasan D, Kaetzel DM, Plattner R. Reciprocal regulation of Abl and receptor tyrosine kinases. Cell Signal. 2009;21:1143–1150. doi: 10.1016/j.cellsig.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsareva SA, Moriggl R, Corvinus FM, Wiederanders B, Schutz A, Kovacic B, et al. Signal transducer and activator of transcription 3 activation promotes invasive growth of colon carcinomas through matrix metalloproteinase induction. Neoplasia. 2007;9:279–291. doi: 10.1593/neo.06820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugurel S, Hildenbrand R, Zimpfer A, La Rosee P, Paschka P, Sucker A, et al. Lack of clinical efficacy of imatinib in metastatic melanoma. Br J Cancer. 2005;92:1398–1405. doi: 10.1038/sj.bjc.6602529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Yoshida K, Shindoh M, Kaya M, Fujikawa K, Sato H, et al. The Ets-1 and Ets-2 transcription factors activate the promoters for invasion-associated urokinase and collagenase genes in response to epidermal growth factor. Int J Cancer. 1998;77:128–137. doi: 10.1002/(sici)1097-0215(19980703)77:1<128::aid-ijc20>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Yogalingam G, Pendergast AM. Abl kinases regulate autophagy by promoting the trafficking and function of lysosomal components. J Biol Chem. 2008;283:35941–35953. doi: 10.1074/jbc.M804543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.