Abstract
Background
There are differences in outcomes in blacks, compared to whites, with lymph node-negative (pN0) colorectal cancer. Recurrence in pN0 patients suggests the presence of occult metastases undetected by conventional approaches. This study explores the association of racial differences in outcomes with occult tumor burden in regional lymph nodes.
Methods
Lymph nodes (range: 2-159) from 282 prospectively enrolled pN0 colorectal cancer patients followed for a median of 24 months (range: 2-63) were subjected to molecular analysis. Occult tumor burden was estimated by quantifying the expression of the biomarker GUCY2C, a biomarker for metastatic colorectal cancer cells. Risk categories defined using occult tumor burden was the primary outcome measure. Association of prognostic variables and risk were defined by multivariate polytomous logistic regression.
Results
Occult tumor burden stratified this cohort of 259 whites and 23 blacks into categories with low (60%; recurrence rate (RR)=2.3% [95%CI 0.1-4.5%]), intermediate (31%; RR=33.3% [23.7%-44.1%]), and high (9%; RR=68.0% [46.5%-85.1%], p<0.001) risk. Blacks, compared to whites, exhibited 4-fold greater occult metastases in individual nodes (p<0.001). Multivariate analysis revealed that race (p=0.02), T stage (p=0.02), and number of nodes collected (p=0.003) were independent prognostic markers of risk category. Blacks, compared to whites, were more likely to harbor levels of occult tumor burden, associated with the highest recurrence risk (adjusted odds ratio=5.08 [1.69-21.39]; p=0.007).
Conclusions
Racial disparities in stage-specific outcomes in colorectal cancer are associated with differences in occult tumor burden in regional lymph nodes.
Keywords: racial disparities, colorectal cancer, molecular staging, occult tumor burden, guanylyl cyclase C, RT-qPCR
There is a widening racial gap in mortality from colorectal cancer, the 4th most common incident cancer and the 2nd leading cause of cancer death in the U.S.1-4 For example, while disease-specific mortality has decreased 54% for non-Hispanic white (white) men, non-Hispanic black (black) men have experienced an increase of 28%, since 1960.5 Racial differences in mortality reflect tumor clinicopathologic characteristics, including advanced stage of disease at diagnosis associated with poorer outcomes in blacks compared to whites.6-10 In turn, differences in disease stage at diagnosis reflect disparities in socioeconomic status and access to quality health service.5-11 Tumor characteristics, socioeconomic status and health services access contribute about 50% to excess mortality reflecting race.8, 11, 12 Other factors underlying race-based excess mortality in colorectal cancer remain undefined.5
Beyond clinicopathological differences at diagnosis, there is an under-appreciated racial disparity in stage-specific mortality in colorectal cancer.5, 6, 8 For patients with regionally-advanced disease (lymph node-positive; Stage III), blacks experience 10% excess mortality compared to whites.5, 6, 8 This difference is amplified in patients with local disease (lymph node-negative (pN0); Stage I and II), where blacks exhibit 40% excess mortality compared to whites.5,6, 8 These stage-specific disparities appear to be one primary driver of overall differences in mortality in blacks and whites.5, 6, 8 Socioeconomic status is one factor contributing to these racial disparities in stage-specific outcomes.5, 8, 12 Other clinicopathologic processes contributing to these differences have not been defined.5, 6, 8, 11 However, the predominance of this racial gap in the earliest stages (pN0) of disease, which receive minimal post-surgical intervention2, 3, suggests contributions by factors other than therapeutic application, acceptance, or compliance.8
Regardless of the underlying mechanisms, diagnostic methods that categorize prognostic risk could identify patients vulnerable to excess mortality who might benefit from clinical management to diminish this racial gap. The most informative prognostic marker of survival and predictive marker of response to therapy in colorectal cancer is the presence of tumor cells in regional lymph nodes.1-3, 13, 14 Despite their importance, techniques to assess nodal metastases remain imperfect and about 25% of patients with histology-negative lymph nodes die of recurrent disease, reflecting the presence of occult metastases that escape detection by conventional methods.1, 3, 13-18 There is an emerging paradigm in which specific tumor markers are coupled with nucleic acid amplification techniques, like the reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR), to identify clinically meaningful occult metastases.13-18
The intestinal tumor suppressor GUCY2C (guanylyl cyclase C) is the receptor for the paracrine hormones guanylin and uroguanylin, gene products universally lost early in intestinal neoplasia.19, 20 Loss of hormone expression silences GUCY2C signaling which contributes to transformation by promoting proliferation, crypt hypertrophy, metabolic remodeling and genomic instability.20 Highly selective expression by intestinal epithelial cells normally, and universal over-expression by intestinal tumor cells, suggested that GUCY2C might be a specific molecular marker for metastatic colorectal cancer.17, 18, 21-24
Recently, the prognostic utility of occult lymph node metastases detected by GUCY2C RT-qPCR in pN0 colorectal cancer patients was validated in a prospective, multicenter, blinded clinical trial.18, 25 The categorical presence (yes/no) of occult metastases was a powerful independent predictor of time to recurrence and disease-free survival in pN0 patients.18 This utility of GUCY2C for detecting occult metastases in lymph nodes that predict clinical outcomes in pN0 colorectal cancer has been independently validated.26-28 This paradigm was refined to quantify occult tumor burden (how much) across the regional lymph node network, which classified patients with near-zero risk, those with elevated (33%) risk, and those with high (70%) risk of unfavorable outcomes.25 The present study examines the relationship between occult tumor burden and disparities in outcomes in blacks and whites with pN0 colorectal cancer. It considers the relative level of GUCY2C mRNA expression in lymph nodes from black and white patients from the prospective trial.18 Moreover, these analyses consider the contribution of race to prognostic stratification of risk by occult tumor burden.25
Methods
Study Design
This prospective observational trial at nine centers in the U.S. and Canada explored the prognostic utility of GUCY2C RT-qPCR in lymph nodes of pN0 colorectal cancer patients.18, 25 Investigators and clinical personnel were blinded to results of molecular analyses while laboratory personnel and analysts were blinded to patient and clinical information. The original study had at least 80% power to detect a hazard ratio of 1.6 or larger (P≤0.05, 2-sided) based on a categorical (yes/no) assessment of GUCY2C status. The study protocol was approved by the Institutional Review Board of each participating hospital. The 299 pN0 patients who met eligibility criteria provided 7,310 lymph nodes (range 2-159, median 21 lymph nodes/patient) for histopathologic examination, of which 3,093 nodes (range 1-87, median 8 lymph nodes/patient) were obtained by fresh dissection and eligible for analysis by RT-qPCR.18, 25 Disease status, obtained in routine follow-up by treating physicians, was provided for all patients through December 31, 2009.
Patients and Tissues
18,25 Between March 2002and June 2007, we enrolled 299 stage 0-II pN0 colorectal cancer patients who provided informed consent in writing prior to surgery at one of 7 academic medical centers and 2 community hospitals in the U.S. and Canada. Patients were ineligible if they had a previous history of cancer, metachronous extra-intestinal cancer, or perioperative mortality associated with primary resection. For all eligible patients, preoperative and perioperative examinations revealed no evidence of metastatic disease. Lymph nodes, and when available tumor specimens (51%), were dissected from colon and rectum resections and frozen at −80°C within one hour to minimize warm ischemia. Half of each resected lymph node was fixed with formalin and embedded in paraffin for histopathological examination. Lymph node specimens were subjected to molecular analysis if (1) tumor samples, where available, expressed GUCY2C mRNA above background levels in disease-free lymph nodes (>30 copies) and (2) at least one lymph node was provided which yielded RNA of sufficient integrity.18, 24, 25 Analysis of the 3,093 lymph nodes available from the 299 pN0 patients revealed 236 nodes (7.6%) yielding RNA of insufficient integrity, which were omitted from further analysis.18, 25 For two patients, all lymph nodes harvested were of insufficient integrity resulting in their exclusion.18, 25 Moreover, GUCY2C expression in tumors was below detectable levels in 6 patients who also were excluded.18, 25 Of the 291 remaining eligible patients, 23 self-identified as black, 259 as white (Table 1), and 9 were of another race or their race could not be identified. These analyses focus on the 282 white and black patients, since other categories were too small for conclusive analysis.
Table 1. Patient Characteristics by Risk Group.
| Overall | Low Risk (n=170) |
Moderate Risk (n=88) |
High Risk (n=24) |
P† | |
|---|---|---|---|---|---|
| Characteristic | n | %++ | %++ | %++ | |
| Race | 0.007 | ||||
| Black | 23 | 47.8 | 26.1 | 26.1 | |
| White | 259 | 61.4 | 31.7 | 6.9 | |
| Age at Diagnosis | 0.83 | ||||
| <65 | 107 | 62.3 | 29.3 | 8.5 | |
| ≥65 | 175 | 58.9 | 32.6 | 8.5 | |
| Sex | 0.83 | ||||
| Male | 157 | 60.5 | 31.9 | 7.6 | |
| Female | 125 | 60.0 | 30.4 | 9.6 | |
| Location | <0.001 | ||||
| Left | 128 | 50.0 | 44.5 | 5.5 | |
| Right | 115 | 69.6 | 17.4 | 13.0 | |
| Rectal | 39 | 66.7 | 28.2 | 5.1 | |
| Differentiation | 0.69 | ||||
| Poor/unknown | 45 | 60.0 | 33.3 | 6.7 | |
| Moderate | 217 | 59.0 | 31.8 | 9.2 | |
| Well | 20 | 75.0 | 20.0 | 5.0 | |
| T Stage | 0.008 | ||||
| T1/T2 | 117 | 66.7 | 30.8 | 2.5 | |
| T3/T4 | 165 | 55.8 | 31.5 | 12.7 | |
| Lymphovascular Invasion | 0.34 | ||||
| No | 224 | 61.2 | 29.4 | 9.4 | |
| Yes | 58 | 56.9 | 37.9 | 5.2 | |
| Treatment | 0.40 | ||||
| Surgery alone | 218 | 60.6 | 32.1 | 7.3 | |
| Surgery + chemotherapy | 64 | 59.4 | 28.1 | 12.5 | |
| Nodes Harvested | 0.002* | ||||
| <13 | 59 | 18.8 | 29.9 | 0.0 | |
| ≥13 | 223 | 81.2 | 70.1 | 100.0 | |
row percentage
p value from chi-square test of association.
p value from exact chi-square test of association.
RNA Isolation
RNA was extracted from tissues by a modification of the acid guanidinium thiocyanate-phenol-chloroform extraction method.22, 23 Briefly, individual tissues were pulverized in 1.0 mL Tri-Reagent (Molecular Research Center, Cincinnati, OH) with 12-14 sterile 2.5 mm zirconium beads in a bead mill (Biospec, Bartlesville, OK) for 1-2 min. Phase separation was performed with 0.1 mL bichloropropane, and the aqueous phase re-extracted with 0.5 mL chloroform. RNA was precipitated with 50% isopropanol and washed with 70% ethanol. Air-dried RNA was dissolved in water, concentration determined by spectrophotometry, and stored at −80°C.
RT-QPCR
GUCY2C mRNA was quantified by RT-qPCR employing an established analytically validated assay.24 The EZ RT-PCR kit (Applied Biosystems, Foster City, CA) was employed to amplify GUCY2C mRNA from total RNA in a 50 μL reaction. Optical strip-tubes were used for all reactions, which were conducted in an ABI 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). In addition to the kit components [50 mM Bicine (pH 8.2), 115 mM KOAc, 10 μM EDTA, 60 nM ROX, 8% glycerol, 3 mM Mg(OAc)2, 300 μM each dATP, dCTP, and dGTP, 600 μM dUTP, 0.5 U uracil N-glycosylase, and 5 U rTth DNA polymerase], the reaction master mix contained 900 nM each of forward (ATTCTAGTGGATCTTTTCAATGACCA) and reverse primers (CGTCAGAACAAGGACATTTTTCAT), 200 nM Taqman probe (FAMTACTTGGAGGACAATGTCACAGCCCCTG-TAMRA), and 1 μg RNA template. The housekeeping gene β-actin was amplified employing similar conditions except that forward (CCACACTGTGCCCATCTACG) and reverse (AGGATCTTCATGAGGTAGTCAGTCAG) primers were 300 nM each, while the Taqman probe (FAM-ATGCCC-X(TAMRA)-CCCCCATGCCATCCTGCGTp) was 200 nM. The thermocycler program employed for RT included: 50° × 2 min, 60° × 30 min, 95° × 5 min; and for PCR: 45 cycles of 94° × 20 sec, 62° × 1 min. Reactions were performed at least in duplicate and results averaged.
Statistical Methods
Procedures for reporting statistical methods, including validation procedures for ensuring the accuracy of estimates of hazard ratios and p values, were specifically guided by the REMARK Guidelines.25, 29 Statistical methods for estimating GUCY2C and β-actin mRNA expression by logistic regression analysis have been described.18, 30 A linear mixed effects model of GUCY2C relative expression across all nodes from eligible patients included random effect of patient, and fixed effects of center, and race. This linear model was used to determine differences in GUCY2C expression in patient lymph nodes based on race, after adjusting for potential center-to-center differences and for repeated measures within patient.
The primary clinical endpoint was molecular risk category (low, intermediate, high), based on time to recurrence and recursive partitioning analysis.25 Recursive partitioning was implemented in the R routine RPART.25, 31 This algorithm tests, across all possible variables and levels, for the variable which optimally identifies discrete groups within the study population. The process repeats recursively until a stopping criterion, pre-defined here as the software default of any subgroup with fewer than 20 participants, is achieved.32 Cross-validation (10-fold) during model fitting provided model stability and accuracy and avoided over-fitting. This algorithm was applied using quantitative measures of occult tumor burden as variables for risk stratification. Metrics of occult tumor burden by GUCY2C qRT-PCR included median copy number, maximum copy number, median relative (normalized to β-actin) expression, maximum relative expression, total copy number, and total relative expression across lymph nodes, and the total number of GUCY2C-positive lymph nodes.18, 25, 30 Time to recurrence served as the outcome in these analyses. Categories of low, medium, and high risk for time to recurrence were defined by amalgamation.33
Univariate analysis of association of molecular risk category with demographic and prognostic factors was completed using the chi-square test of association. Multivariate analyses using polytomous logistic regression34 employed risk level and established prognostic variables including T stage, grade, lymphovascular invasion, therapy, anatomical location, number of lymph nodes collected for histopathology, and race.1, 3, 18, 25 Initial multivariate models included all established prognostic measures regardless of significance and a manual backwards stepwise approach was used to establish the final model of association with occult tumor burden risk level. Variables with the least association with outcome were removed one at a time until all remaining variables were significant by a Type 3 test of association at p<0.05. Predicted conditional probabilities and 95% two-sided confidence intervals were estimated from the final multivariate model. These probabilities are reported to demonstrate the contribution of each variable to the final model of molecular risk strata. Exact adjusted odds ratios were calculated and reported for factors with small cell sizes in multivariate models, when appropriate.
Confidence intervals for raw survival rates were computed by the exact method of Clopper-Pearson.35 All tests were two-sided, and p<0.05 was considered statistically significant. All analyses were performed with R v 2.11.2, SAS v9.2.
Results
Patient Characteristics
The 282 black and white pN0 patients had a mean age of 68 years (26-90 years) at diagnosis and 55% were male (Table 1). Clinicopathologic features, including depth of tumor penetration (T1/2, T3, T4), and tumor anatomical location (right, left, rectal) were similar to national experience.1, 3, 4, 18, 25 Patients with colon cancer represented 85.9%, while those with rectal tumors comprised 14.1%. Blacks comprised 7.9% of the total population enrolled, nearly identical to the national average for disease-specific racial distribution.6, 7, 11 There were no significant differences in clinicopathologic characteristics between black and white patients (Table 2).
Table 2. Patient Characteristics by Race.
| Overall | Black (n=23) |
White (n=259) |
p† | |
|---|---|---|---|---|
| Characteristic | n | %‡ | %‡ | |
| Age at Diagnosis | 0.55 | |||
| <65 | 107 | 43.5 | 37.1 | |
| ≥65 | 175 | 56.5 | 62.9 | |
| Sex | 0.22 | |||
| Male | 157 | 43.5 | 56.0 | |
| Female | 125 | 56.5 | 44.0 | |
| Location | 0.27 | |||
| Left | 128 | 35.1 | 46.3 | |
| Right | 115 | 56.5 | 39.4 | |
| Rectal | 39 | 8.7 | 14.3 | |
| Differentiation | 0.49 | |||
| Poor/unknown | 45 | 13.0 | 16.2 | |
| Moderate | 217 | 74.0 | 77.2 | |
| Well | 20 | 13.0 | 6.6 | |
| T Stage | 0.26 | |||
| T1/T2 | 117 | 30.5 | 42.5 | |
| T3/T4 | 165 | 69.5 | 57.5 | |
| Lymphovascular Invasion | 0.35 | |||
| No | 224 | 87.0 | 79.5 | |
| Yes | 58 | 13.0 | 20.5 | |
| Treatment | 0.69 | |||
| Surgery alone | 218 | 73.9 | 77.7 | |
| Surgery + chemotherapy | 64 | 26.1 | 22.3 | |
| Nodes Harvested | 0.69 | |||
| <13 | 59 | 17.4 | 20.9 | |
| ≥13 | 223 | 82.6 | 79.1 | |
P value from chi-square test of association.
% of total for race.
Occult Tumor Burden and Risk Stratification
Clinical outcomes in pN0 colorectal cancer patients were analyzed by recursive partitioning using metrics of occult tumor burden estimated by GUCY2C RT-qPCR.25 Based on time to recurrence, GUCY2C RT-qPCR stratified pN0 patients into categories in which 170 (60%) patients exhibited low (MolLow), 88 (31%) exhibited intermediate (MolInt), and 24 (9%) exhibited high (MolHigh) (p<0.001) risk of disease recurrence (Fig. 1). All but 4 of the MolLow patients remained free of disease during follow-up (recurrence rate (RR)=2.3% [95%CI 0.1-4.5%]); 29 MolInt patients developed recurrent disease (RR=33.3% [23.7%-44.1%]); and 16 RR=68.0% [46.5%-85.1%]) MolHigh patients developed recurrent disease (p<0.001; Fig. 1).
Figure 1. Time to recurrence in patients with pN0 colorectal cancer stratified by occult tumor burden.
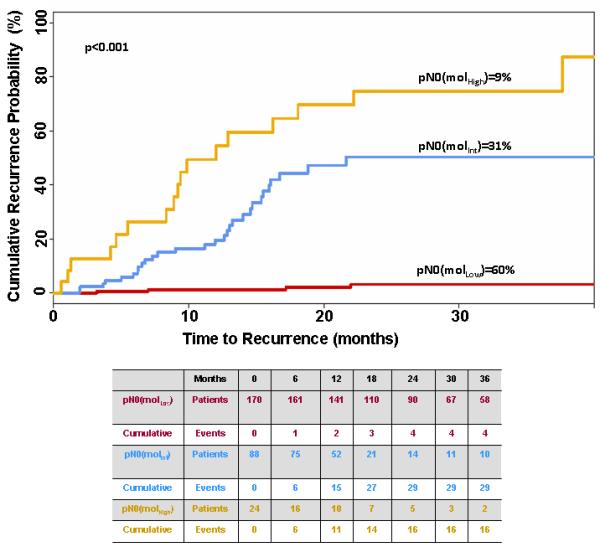
Table summarizes the number of patients at risk as well as cumulative events for each outcome.
Univariate analysis by chi-square test revealed the expected relationship between advanced T stage, occult tumor burden and risk category (p=0.008; Table 1). Similarly, molecular staging depended on collecting 13 or more lymph nodes from each patient (p=0.002; Table 1), recapitulating established enhancements in histopathologic staging by increased nodal harvests.1, 3, 36, 37
Occult Tumor Burden and Race
Individual lymph nodes from blacks compared to whites harbored 4-fold greater quantities of metastatic tumor cells (p<0.001; Fig. 2) identified by GUCY2C RT-qPCR.18, 24 Moreover, blacks harbored a greater burden of occult metastatic tumor across their lymph node network associated with the highest prognostic risk, compared to white patients (p=0.002; Fig. 2; Table 1). Multivariate analyses revealed that blacks exhibited occult tumor burden associated with the greatest prognostic risk regardless of T stage or number of lymph nodes collected (Fig. 3).
Figure 2. Occult tumor burden in black and white patients.
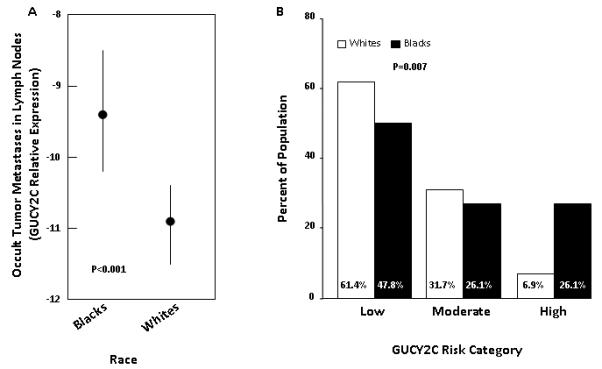
(A) Occult tumor cells in lymph nodes quantified by GUCY2C RT-PCR. Least squares mean and 95% confidence interval of relative GUYC2C expression in lymph nodes18 in blacks and whites. In linear mixed effects model, with random patient effect, controlling for center to center differences, blacks have significantly higher levels of occult tumor cells in lymph nodes (p<0.001). (B) Stratification of prognostic risk by occult tumor burden in blacks and whites. Blacks are significantly more likely to be at high risk for disease recurrence based on occult tumor burden in lymph nodes (p=0.007).
Figure 3. Distribution of black and white pN0 colorectal cancer patients with tumors with different T stages or lymph node collections stratified by occult tumor burden.
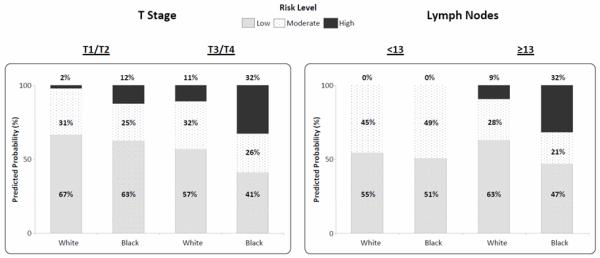
Blacks are significantly more likely to be at high risk (p=0.007) versus low risk for disease recurrence based on occult tumor burden in lymph nodes regardless of T stage (p=0.006) or number of lymph nodes collected (p=0.02). P values reported from multivariate polytomous regression model for High vs Low Risk comparisons.
Occult Tumor Burden is an Independent Prognostic Variable Associated with Racial Disparities in Outcomes
Multivariate analyses employing polytomous logistic regression (Table 3) confirmed that race (p=0.02), T stage (p=0.02), and number of lymph nodes collected (p=0.003) are independently associated with occult tumor burden and stratification into risk categories (Table 3). Patients with T3/T4 tumors were more likely to be categorized as high risk versus low risk (adjusted odds ratio 6.00 [1.69-21.39]; p=0.006) compared to patients with T1/T2 tumors. Similarly, patients providing 13 or more lymph nodes were more likely to be categorized as high risk (adjusted odds ratio 8.10 [1.31-∞]; p=0.02) compared to patients with fewer lymph nodes collected. Importantly, blacks were more likely to be categorized as high risk on the basis of occult tumor burden compared to whites (adjusted odds ratio 5.08 [1.69-21.39] p=0.007).
Table 3. Multivariate Polytomous Logistic Regression Model.
| Overall | Adjusted Odds Ratio |
95% CI |
P† | |
|---|---|---|---|---|
| Characteristic | n | (AOR) | ||
| Race | 0.02‡ | |||
| White | 259 | Referent | --- | |
| Black (Moderate vs Low) | 23 | 1.03 | (0.36, 2.94) | 0.95 † |
| Black (High vs Low) | 5.08 | (1.55, 16.65) | 0.007† | |
| T Stage | 0.02‡ | |||
| T1/T2 | 117 | Referent | --- | |
| T3/T4 (Moderate vs Low) | 165 | 1.25 | (0.74, 2.12) | 0.41 † |
| T3/T4 (High vs Low) | 6.00 | (1.69, 21.39) | 0.006† | |
| Nodes Harvested * | 0.003‡ | |||
| <13 | 59 | Referent | --- | |
| ≥13 (Moderate vs Low) | 223 | 0.43 | (0.28, 1.01) | 0.06 † |
| ≥13 (High vs Low) | 8.10 | (1.31, ∞) | 0.02† | |
P value from multivariable logistic regression model, 1df Wald (exact) chi-square test.
Type 3 overall (exact) test of association from multivariable logistic regression model.
Indicates exact tests reported from multivariable model.
Discussion
There is a well-established racial disparity in disease mortality in blacks compared to whites with colorectal cancer.5-7, 11 One primary contributor to this disparity is stage-specific differences in cancer survival.5-8, 11, 12 Mechanisms underlying differences in stage-specific outcomes in blacks and whites continue to be debated.5, 6, 8, 11 However, there have been no methods, to date, that identify vulnerable patients at increased prognostic risk which eliminate these racial disparities. Here, we demonstrate that blacks compared to whites exhibit higher levels of occult metastatic tumor cells in regional lymph nodes. These metastases are associated with a greater proportion of blacks compared to whites harboring higher levels of occult tumor burden across their regional lymph node networks. In turn, this occult tumor burden is associated with racial disparities in stage-specific prognostic risk. Indeed, occult tumor burden was an independent marker of excess prognostic risk in blacks. These analyses suggest that racial disparities in mortality, in part, reflect differences in clinically undetected tumor metastasis in blacks compared to whites, revealed by occult tumor burden analysis in regional lymph nodes. Importantly, this study suggests that quantifying occult tumor burden in regional lymph nodes can identify patients, regardless of race, that are at greatest risk for developing recurrent disease.
Disproportionate occult metastatic disease in blacks compared to whites could reflect race-based differences in staging accuracy.5, 8 In this model, blacks have a greater risk of being under-staged. For example, blacks with regional metastases (Stage III) might be categorized as having local disease (Stages I or II) more frequently than whites. However, systematic race-based staging misclassification should minimize racial disparities in stage at diagnosis.8 In the context of established differences in stage at diagnosis, a contribution of under-staging to race-based differences in occult metastatic disease seems less likely.5-7, 11 In the present study, patients of both races were treated at the same hospitals by the same healthcare providers. They produced equivalent numbers of lymph nodes for staging which were histologically evaluated by the same pathologists at each institution. These considerations also reduce the likelihood of race-based staging misclassification based on differences in health care services in the present study.5, 8
Differences in occult tumor burden also could reflect race-based disparities in treatment availability, application, or compliance.10, 38, 39 In this paradigm, whites, but not blacks, would receive neoadjuvant therapy prior to surgery that reduce or eliminate metastatic disease in regional lymph nodes inevident by standard histopathology but discernable by molecular analyses. The present study exclusively involved pN0 patients. Colon cancer patients, comprising 86% of the trial cohort, did not receive neoadjuvant treatment, which is required to eliminate nodal metastases at initial staging. Stage II rectal cancer patients typically receive neoadjuvant chemoradiotherapy. However, the 2 blacks with rectal cancer comprised a cohort too small to account for stage-specific racial disparities in occult metastases in this study.
One principle factor contributing to racial disparities in overall mortality in colorectal cancer is the advanced stage of disease at which blacks compared to whites present at the time of diagnosis.5-8, 11 In turn, late stage at presentation reflects a delay in detection and diagnosis.5, 6, 8, 40 Factors contributing to delayed cancer diagnosis are manifold and include access to quality health services, and cultural beliefs, education level and the ability to comply with diagnostic and treatment instructions, to name a few.5, 11, 40 The higher frequency of occult metastases in blacks compared to whites might simply reflect this diagnostic lag. However, most of the factors contributing to delays in detection and diagnosis are related to socioeconomic status5-8, 11, 40, which appears to provide only a partial contribution to stage-specific disparities in outcomes.8, 11, 12 Moreover, a temporal relationship between delays in diagnosis and the incidence of nodal micrometastases, beyond standard staging, has not been observed.
The partial contribution of socioeconomic status to stage-specific racial disparities8, 11, 12, and the unlikely contribution of mis-staging or under-treatment in the present study raises the possibility that biology might contribute to differences in occult tumor burden in blacks compared to whites. Biology may, in part, underlie differences in clinical presentation, for example the earlier onset, greater undifferentiated histology, and right-sided predominance of tumors in black, compared to white, patients.5, 11, 41 In turn, clinical differences may reflect molecular disparities in DNA damage sensing and repair pathways underlying microsatellite, chromosomal, and epigenetic stability in colorectal tumors from blacks compared to whites.42-45 Also, race-related biological differences may be acquired, instead of inherited, reflecting life style, rather than genetic, disparities.5-8, 11 For example, rates of obesity are higher in the blacks compared to whites associated with an increased risk of developing and dying from colon cancer.11 Conversely, it is unlikely that an increase in inherited germline mutations underlies the escalating racial disparity in stage-specific outcomes, which has expanded over the past four decades.5 Moreover, the greater genetic variation within, rather than between, races makes a genetic basis for racial disparities in outcomes unlikely.46, 47 The biologic contribution to racial disparities in outcomes in colorectal cancer will continue to be debated.5, 11, 41-45 However, the present observations associate increased occult tumor burden with poorer outcomes in blacks compared to whites. They suggest future studies exploring the relationship between occult metastases and molecular determinants of tumor aggressiveness, including specific cancer-related gene mutations, microsatellite instability, chromosomal instability, and DNA methylation.48
The present study demonstrates that stage-specific racial disparities in outcomes in pN0 blacks compared to whites with colorectal cancer is associated with greater occult tumor burden in regional lymph nodes. These results demonstrate, for the first time, the impact of occult tumor burden on racial disparities in clinical prognosis. They highlight the importance of validating this emerging approach to identify colorectal cancer patients of all races at increased prognostic risk. The present study was limited to a cohort comprising ~8% African Americans, which is representative of the colon cancer population, but relatively small, demonstrating the need for validation. That validation will be provided by an ongoing study with equally balanced black and white colon cancer patients, which will be critical to understanding the utility of GUCY2C to reduce racial disparities in colorectal cancer outcomes. Moreover, the presence of tumor cells in regional lymph nodes directs therapy in patients with colon cancer. While adjuvant chemotherapy provides a survival benefit to patients with stage III disease, its utility in patients with pN0 colon cancer remains uncertain, with marginal survival benefits in stage II patients in some, but not all, clinical trials.3, 49, 50 Heterogeneous responses to therapy in pN0 patients may reflect, in part, the variable presence of occult metastases. It is tempting to speculate that black and white pN0 patients with excess occult tumor burden might benefit from adjuvant therapy. These considerations underscore the importance of refining the predictive utility of occult tumor burden, to eliminate the widening5 racial gap in stage-specific mortality in colorectal cancer patients.
Acknowledgments
Funding/Support: These studies were supported by grants from NIH (CA75123, CA95026, CA112147, CA146033), the Pennsylvania Department of Health, and Targeted Diagnostic and Therapeutics Inc. SAW is the Samuel M.V. Hamilton Endowed Professor of Thomas Jefferson University.
Footnotes
Disclosures: SAW is the Chair of the Data Safety Monitoring Board for the C-Cure Trial™ sponsored by Cardio Biosciences, and the Chair (uncompensated) of the Scientific Advisory Board to Targeted Diagnostics and Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
References
- 1.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004 Nov-Dec;54(6):295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010 Mar 20;375(9719):1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed Springer; New York: 2009. [Google Scholar]
- 4.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011 Jun 17; doi: 10.3322/caac.20121. 2011:caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Soneji S, Iyer SS, Armstrong K, Asch DA. Racial disparities in stage-specific colorectal cancer mortality: 1960-2005. Am J Public Health. 2010 Oct;100(10):1912–1916. doi: 10.2105/AJPH.2009.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien C, Morimoto LM, Tom J, Li CI. Differences in colorectal carcinoma stage and survival by race and ethnicity. Cancer. 2005 Aug 1;104(3):629–639. doi: 10.1002/cncr.21204. [DOI] [PubMed] [Google Scholar]
- 7.Le H, Ziogas A, Lipkin SM, Zell JA. Effects of socioeconomic status and treatment disparities in colorectal cancer survival. Cancer Epidemiol Biomarkers Prev. 2008 Aug;17(8):1950–1962. doi: 10.1158/1055-9965.EPI-07-2774. [DOI] [PubMed] [Google Scholar]
- 8.Marcella S, Miller JE. Racial differences in colorectal cancer mortality. The importance of stage and socioeconomic status. J Clin Epidemiol. 2001 Apr;54(4):359–366. doi: 10.1016/s0895-4356(00)00316-4. [DOI] [PubMed] [Google Scholar]
- 9.Mayberry RM, Coates RJ, Hill HA, et al. Determinants of black/white differences in colon cancer survival. J Natl Cancer Inst. 1995 Nov 15;87(22):1686–1693. doi: 10.1093/jnci/87.22.1686. [DOI] [PubMed] [Google Scholar]
- 10.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer and race: understanding the differences in outcomes between African Americans and whites. Med Clin North Am. 2005 Jul;89(4):771–793. doi: 10.1016/j.mcna.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 11.White A, Vernon SW, Franzini L, Du XL. Racial disparities in colorectal cancer survival: to what extent are racial disparities explained by differences in treatment, tumor characteristics, or hospital characteristics? Cancer. 2010 Oct 1;116(19):4622–4631. doi: 10.1002/cncr.25395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen VW, Fenoglio-Preiser CM, Wu XC, et al. National Cancer Institute Black/White Cancer Survival Study Group Aggressiveness of colon carcinoma in blacks and whites. Cancer Epidemiol Biomarkers Prev. 1997 Dec;6(12):1087–1093. [PubMed] [Google Scholar]
- 13.Iddings D, Ahmad A, Elashoff D, Bilchik A, Ann Surg Oncol. The prognostic effect of micrometastases in previously staged lymph node negative (N0) colorectal carcinoma: a meta-analysis. 2006 Nov;13(11):1386–1392. doi: 10.1245/s10434-006-9120-y. [DOI] [PubMed] [Google Scholar]
- 14.Nicastri DG, Doucette JT, Godfrey TE, Hughes SJ. Is occult lymph node disease in colorectal cancer patients clinically significant? A review of the relevant literature. J Mol Diagn. 2007 Nov;9(5):563–571. doi: 10.2353/jmoldx.2007.070032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bilchik AJ, Hoon DS, Saha S, et al. Prognostic impact of micrometastases in colon cancer: interim results of a prospective multicenter trial. Ann Surg. 2007 Oct;246(4):568–575. doi: 10.1097/SLA.0b013e318155a9c7. discussion 575-567. [DOI] [PubMed] [Google Scholar]
- 16.Liefers GJ, Cleton-Jansen AM, van de Velde CJ, et al. Micrometastases and survival in stage II colorectal cancer. N Engl J Med. 1998 Jul 23;339(4):223–228. doi: 10.1056/NEJM199807233390403. [DOI] [PubMed] [Google Scholar]
- 17.Mejia A, Schulz S, Hyslop T, Weinberg DS, Waldman SA. Molecular staging estimates occult tumor burden in colorectal cancer. Adv Clin Chem. 2010;52:19–39. doi: 10.1016/s0065-2423(10)52007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldman SA, Hyslop T, Schulz S, et al. Association of GUCY2C expression in lymph nodes with time to recurrence and disease-free survival in pN0 colorectal cancer. JAMA. 2009 Feb 18;301(7):745–752. doi: 10.1001/jama.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, Schulz S, Bombonati A, et al. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007 Aug;133(2):599–607. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Pitari GM, Li P, Lin JE, et al. The paracrine hormone hypothesis of colorectal cancer. Clin Pharmacol Ther. 2007 Oct;82(4):441–447. doi: 10.1038/sj.clpt.6100325. [DOI] [PubMed] [Google Scholar]
- 21.Birbe R, Palazzo JP, Walters R, Weinberg D, Schulz S, Waldman SA. Guanylyl cyclase C is a marker of intestinal metaplasia, dysplasia, and adenocarcinoma of the gastrointestinal tract. Hum Pathol. 2005 Feb;36(2):170–179. doi: 10.1016/j.humpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Cagir B, Gelmann A, Park J, et al. Guanylyl cyclase C messenger RNA is a biomarker for recurrent stage II colorectal cancer. Ann Intern Med. 1999 Dec 7;131(11):805–812. doi: 10.7326/0003-4819-131-11-199912070-00002. [DOI] [PubMed] [Google Scholar]
- 23.Carrithers SL, Barber MT, Biswas S, et al. Guanylyl cyclase C is a selective marker for metastatic colorectal tumors in human extraintestinal tissues. Proc Natl Acad Sci U S A. 1996 Dec 10;93(25):14827–14832. doi: 10.1073/pnas.93.25.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz S, Hyslop T, Haaf J, et al. A validated quantitative assay to detect occult micrometastases by reverse transcriptase-polymerase chain reaction of guanylyl cyclase C in patients with colorectal cancer. Clin Cancer Res. 2006 Aug 1;12(15):4545–4552. doi: 10.1158/1078-0432.CCR-06-0865. [DOI] [PubMed] [Google Scholar]
- 25.Hyslop T, Weinberg DS, Schulz S, Barkun A, Waldman SA. Occult tumor burden predicts disease recurrence in lymph node-negative colorectal cancer. Clin Cancer Res. 2011 May 15;17(10):3293–3303. doi: 10.1158/1078-0432.CCR-10-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beaulieu M, Desaulniers M, Bertrand N, et al. Analytical performance of a qRT-PCR assay to detect guanylyl cyclase C in FFPE lymph nodes of patients with colon cancer. Diagn Mol Pathol. 2010 Mar;19(1):20–27. doi: 10.1097/PDM.0b013e3181ad5ac3. [DOI] [PubMed] [Google Scholar]
- 27.Haince JF, Houde M, Beaudry G, et al. Comparison of histopathology and RT-qPCR amplification of guanylyl cyclase C for detection of colon cancer metastases in lymph nodes. J Clin Pathol. 2010 Jun;63(6):530–537. doi: 10.1136/jcp.2009.072983. [DOI] [PubMed] [Google Scholar]
- 28.Sargent DJ, Resnick MB, Meyers MO, et al. Evaluation of Guanylyl Cyclase C Lymph Node Status for Colon Cancer Staging and Prognosis. Ann Surg Oncol. 2011 May 1; doi: 10.1245/s10434-011-1731-2. [DOI] [PubMed] [Google Scholar]
- 29.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005 Aug 17;97(16):1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 30.Chervoneva I, Li Y, Iglewicz B, Waldman S, Hyslop T. Relative quantification based on logistic models for individual polymerase chain reactions. Stat Med. 2007 Dec 30;26(30):5596–5611. doi: 10.1002/sim.3127. [DOI] [PubMed] [Google Scholar]
- 31.Therneau TM, Atkinson EJ. An introduction to recursive partitioning using the rpart routines. 1997. Mayo Clinic. Technical report 61. [Google Scholar]
- 32.Therneau TM, Atkinson EJ. Technical Report Series No 61, An introduction to recursive partitioning using the rpart routines. Rochester; Minnesota: 1997. Mayo Clinic. Technical report 61. [Google Scholar]
- 33.Sinicrope F, Foster NR, Sargent DJ, Thibodeau SN, Smyrk TC, O’Connell MJ. Model-based prediction of defective DNA mismatch repair using clinicopathological variables in sporadic colon cancer patients. Cancer. 2010 Apr 1;116(7):1691–1698. doi: 10.1002/cncr.24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biesheuvel CJ, Vergouwe Y, Steyerberg EW, Grobbee DE, Moons KG. Polytomous logistic regression analysis could be applied more often in diagnostic research. J Clin Epidemiol. 2008 Feb;61(2):125–134. doi: 10.1016/j.jclinepi.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998 Apr 30;17(8):857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 36.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003 Aug 1;21(15):2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 37.Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003 Jan-Feb;10(1):65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]
- 38.Berry J, Bumpers K, Ogunlade V, et al. Examining racial disparities in colorectal cancer care. J Psychosoc Oncol. 2009;27(1):59–83. doi: 10.1080/07347330802614840. [DOI] [PubMed] [Google Scholar]
- 39.Polite BN, Dignam JJ, Olopade OI. Colorectal cancer model of health disparities: understanding mortality differences in minority populations. J Clin Oncol. 2006 May 10;24(14):2179–2187. doi: 10.1200/JCO.2005.05.4775. [DOI] [PubMed] [Google Scholar]
- 40.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 12;102(8):538–546. doi: 10.1093/jnci/djq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexander D, Jhala N, Chatla C, et al. High-grade tumor differentiation is an indicator of poor prognosis in African Americans with colonic adenocarcinomas. Cancer. 2005 May 15;103(10):2163–2170. doi: 10.1002/cncr.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ashktorab H, Schaffer AA, Daremipouran M, Smoot DT, Lee E, Brim H. Distinct genetic alterations in colorectal cancer. PLoS One. 2010;5(1):e8879. doi: 10.1371/journal.pone.0008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ashktorab H, Smoot DT, Carethers JM, et al. High incidence of microsatellite instability in colorectal cancer from African Americans. Clin Cancer Res. 2003 Mar;9(3):1112–1117. [PubMed] [Google Scholar]
- 44.Ashktorab H, Smoot DT, Farzanmehr H, et al. Clinicopathological features and microsatellite instability (MSI) in colorectal cancers from African Americans. Int J Cancer. 2005 Oct 10;116(6):914–919. doi: 10.1002/ijc.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grizzle WE, Manne U, Weiss HL, Jhala N, Talley L. Molecular staging of colorectal cancer in African-American and Caucasian patients using phenotypic expression of p53, Bcl-2, MUC-1 AND p27(kip-1) Int J Cancer. 2002 Feb 1;97(4):403–409. doi: 10.1002/ijc.1617. [DOI] [PubMed] [Google Scholar]
- 46.Freeman HP. The meaning of race in science--considerations for cancer research: concerns of special populations in the National Cancer Program. Cancer. 1998 Jan 1;82(1):219–225. doi: 10.1002/(sici)1097-0142(19980101)82:1<219::aid-cncr27>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 47.Witzig R. The medicalization of race: scientific legitimization of a flawed social construct. Ann Intern Med. 1996 Oct 15;125(8):675–679. doi: 10.7326/0003-4819-125-8-199610150-00008. [DOI] [PubMed] [Google Scholar]
- 48.Ogino S, Nosho K, Irahara N, et al. Negative lymph node count is associated with survival of colorectal cancer patients, independent of tumoral molecular alterations and lymphocytic reaction. Am J Gastroenterol. Feb;105(2):420–433. doi: 10.1038/ajg.2009.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005 Feb 3;352(5):476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 50.Quasar Collaborative G. Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007 Dec 15;370(9604):2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]


