Abstract
Studies have shown that mesenchymal stem cells (MSCs) have low immunogenicity and immune regulation. Human umbilical cord Wharton’s jelly provides a new source for MSCs that are highly proliferative and have multi-differentiation potential. To investigate immunomodulatory effects of human Wharton’s jelly cells (WJCs) on lymphocytes, we successfully isolated MSCs from human umbilical cord Wharton’s jelly. WJCs expressed MSC markers but low levels of human leukocyte antigen (HLA)-ABC and no HLA-DR. These results indicate that WJCs have low immunogenicity. Both WJCs and their culture supernatant could inhibit the proliferation of phytohemagglutinin-stimulated human peripheral blood lymphocytes and mouse splenocytes. Additionally, WJCs suppressed secretion of transforming growth factor-β1 and interferon-γ by human peripheral blood lymphocytes. We conclude that the immunomodulatory effect of WJCs may be related to direct cell contact and inhibition of cytokine secretion by human peripheral blood lymphocytes.
Keywords: Wharton’s jelly, mesenchymal stem cells, lymphocytes, immunomodulatory, interferon-γ, transforming growth factor-β1
1. Introduction
Mesenchymal stem cells (MSCs) are derived from the mesoderm and have self-renewal and multi-differentiation capacity. Under appropriate conditions in vivo and in vitro, they can differentiate into various tissue cells, such as osteoblasts, chondrocytes, adipocytes, muscle cells, and neurocytes (1-7). For these reasons, MSCs are considered important seed cells for tissue engineering and cell transplantation. Until now, the study of MSCs has mainly focused on bone marrow mesenchymal stem cells (BMSCs). However, the invasiveness of the bone marrow aspiration procedure and the age-dependent degradation in quantity and quality of the BMSCs limit their clinical potential (8, 9). Research is ongoing to identify alternative sources of MSCs that can be derived from a wide range of ethically approved sources. The human umbilical cord Wharton’s jelly provides a new source of MSCs that exhibit a high degree of self-renewal capacity and multi-differentiation potential. Wharton’s jelly is the connective tissue that surrounds the umbilical vessels. Wharton’s jelly cells (WJCs) have a wider range of collection sources than do BMSCs and can be easily collected with fewer ethical constraints. As an alternative source of MSCs, WJCs have promising clinical application prospects.
Recent studies have shown that BMSCs have low immunogenicity and do not stimulate T-cell proliferation in vitro. It has been confirmed that MSCs do not express major histocompatibility complex class II (MHC-II) and only express low levels of MHC-I. The function of MHC-I is to protect MSCs from destruction by natural killer cells (NK); MHC-II can aid MSCs in escaping immune recognition by CD4+ T cells (10). Furthermore, MSCs do not express costimulatory molecules such as CD40, CD80, and CD86, which are necessary for T-cell activation (11).
Recent studies have indicated that MSCs can suppress lymphocyte proliferation induced by phytohemagglutinin (PHA) and are not restricted by MHC. Allograft has been proven to minimize the risk of rejection after transplantation, even between unmatched individuals (12). These unique immunological properties of MSCs increase the prospects that they might be useful for treating organ transplant rejection or autoimmune disease. Research has shown that MSCs can effectively reduce the immune rejection after major organ transplantations, such as those for heart and kidney (13, 14). Studies have also shown that MSCs have a potent therapeutic effect in graft-versus-host disease, which is caused by hematopoietic stem cell transplantation (15). In addition, MSCs have been used for the treatment of autoimmune diseases, and their therapeutic effects have already been tested in animal models of systemic lupus erythematosus and experimental autoimmune encephalomyelitis (16-18).
The immunomodulatory effects of MSCs are not only apparent in T lymphocytes but also in B lymphocytes, dendritic cells (DC), and NK. MSCs can inhibit immune cell proliferation, reduce immune cell cytokine secretion, and alter immune cell subtypes (19-22). Although the immunomodulatory effects of MSCs have been confirmed by many experiments, the specific immune suppression mechanisms are debated. Some researchers believe that the contact between cells plays an important role in the immune regulation process. Others think that the immunomodulatory effects of MSCs may be linked to soluble factors, such as hepatocyte growth factor, interleukin (IL)-10, and transforming growth factor (TGF)-β1(23). Further study revealed that some signaling pathways were involved in the immune regulation of MSCs (24-26). Merging these theories portrays the immune regulatory mechanism as a complex interconnected network that combines micro-environment, cytokines, and signaling pathways.
Although the immunogenic behavior of BMSCs has been characterized (10, 27), the immunoregulatory properties of the human WJCs have not been defined. TGF-β1 is an important negative immune regulator that inhibits proliferation and function of lymphocytes. As a critical signal factor, TGF-β1 might shut down the local immune system. Interferon-γ (IFN-γ) activates and promotes lymphocyte function as a positive immune regulator in immune rejection. In this study, we established a protocol for isolation and culture of human WJCs, investigated their effects on lymphocyte proliferation and secretion of TGF-β1 and IFN-γ, and explored whether direct cell-to-cell interactions and soluble factors such as TGF-β1 and IFN-γ are important for balancing WJC-mediated immune regulation.
2. Materials and methods
2.1. Cell isolation and culture
Human umbilical cords were obtained under sterile conditions from full-term infants delivered by caesarean section; residual blood was fully washed by phosphate-buffered saline (PBS). The umbilical cord membrane was stripped, and the umbilical cord blood vessels (two arteries and one vein) were removed to retain the Wharton’s jelly. The Wharton’s jelly was cut into 1 mm3 pieces and then cultured in DMEM/F12 (GIBCO, USA) supplemented with 10% fetal bovine serum (FBS) (GIBCO, USA) and 5 ng/mI basic fibroblast growth factor (bFGF) (Sigma, USA). The cytokine bFGF promotes WJC proliferation by increasing the mitotic activity of the cells. The culture was maintained at 37°C with saturated humidity and 5% (v/v) CO2. The medium was changed every 3 days. WJCs were passaged at 80% confluence after 1-min treatment with 0.25% trypsin (Sigma, USA) and 0.02% EDTA (Sigma, USA) at 37°C, and centrifugation at 1000 rpm for 10 min.
2.2. Immunophenotype analysis
The third passage (P3) of WJCs was selected for its optimal proliferation capability. WJCs were trypsinized, suspended in DMEM/F12 at a concentration of 2×106/ml, and cultured until they reached 80% confluence. Aliquots of cells (100 μl) were incubated for 30 min at 4°C with mouse anti-human monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC)-CD44, FITC-HLA-ABC, FITC-HLA-DR, phycoerythrin-conjugated CD34-PE, PerCP, or CD45-PerCP (BD, USA). After incubation, the cells were washed twice with PBS. Mous e isotypic antibodies PerCP-IgG1, PE-IgG1, and FITC-IgG1(BD, USA) were used as experimental controls. The cells were then resuspended and analyzed by flow cytometry.
2.3. Measurement of WJC proliferation activity
Passages 1, 3, and 5 were selected for their high proliferation capabilities. WJCs were trypsinized, suspended in DMEM/F12 medium supplemented with 10% FBS and 5 ng/mI bFGF at a concentration of 2×104/ml, and grown until they reached 80% confluence. WJCs (100 μl) were then seeded into 96-well plates and incubated at 37°C with 5% CO2 in a fully humidified atmosphere. Cell proliferation was assessed on days 1, 3, 5, 7, and 9. Ten microliters of Cell Counting Kit-8 (CCK-8) solution (Beyotime, China) were added to each well in the absence of light and incubated at 37°C with an environment of 5% CO2 for 2 h. The absorbance of each well at 450 nm was determined using an enzyme-linked immunosorbent assay reader; the growth curve was then drawn accordingly. Each trial was repeated in triplicate.
2.4. Lymphocyte proliferation assay
Human peripheral blood lymphocytes were isolated from healthy donors by Ficoll–Paque (1.077 g/ml) density gradient centrifugation. The cell concentration was adjusted to 1×106 /ml by RPMI-1640 medium (GIBCO, USA) supplemented with 10% FBS for use. Splenocytes were obtained from mice by using a standard protocol. The spleen was removed quickly by aseptic technique and washed thoroughly with PBS. The spleen cells were then released by trituration. After being resuspended in PBS, the spleen mononuclear lymphocytes were obtained by Ficoll–Paque (1.077g/ml) density gradient centrifugation. The pellets were then resuspended in PBS, and the erythrocytes were lysed. Splenocytes were washed two more times with PBS, and the cell concentration was adjusted to 1×106/ml in RPMI-1640 medium supplemented with 10% FBS. Human peripheral blood lymphocyte count, splenocyte count, and viability were assessed by trypan blue prior to final plating.
WJCs at passage 3 were harvested and adjusted to 1×105/ml, 5×104/ml, or 2×104/ml in DMEM/F12 containing 10% FBS. A 100-μl suspension of WJCs was plated into 96-well plates. The plates were incubated for 72 h at 37°C with 5% CO2. After the WJCs reached 70% confluence, the medium was removed, and 100 μl of fresh medium containing 5 μl of mitomycin-C (1 μg/μl; Sigma, USA) were added for 30 min at 37°C to mitotically inactivate the WJCs. After the medium was removed, the inactivated WJCs were washed twice with PBS. WJCs were resuspended in 100 μl of lymphocyte medium (RPMI-1640 containing 10% FBS), co-cultured with 1×105 lymphocytes (splenocytes or human peripheral blood lymphocytes), and stimulated by 5 μl of PHA (Sigma, USA) for 72 h at 37°C and 5% CO2.The groups were divided as follows: lymphocytes + PHA (positive control); WJCs (1×104 ) + lymphocytes; WJCs (5×103) + lymphocytes; WJCs (2×103) + lymphocytes; WJCs (1×104) + lymphocytes + PHA; WJCs (5×103) + lymphocytes + PHA; WJCs (2×103) + lymphocytes + PHA; unstimulated lymphocytes (negative control). Three ratios of WJCs to lymphocytes were used: 1:10, 1:20, and 1:50. Each trial was repeated in triplicate. A CCK-8 kit was used to assess the immunomodulatory impact of WJCs on mouse splenocytes and human peripheral blood lymphocytes after PHA stimulation. The procedure was carried out according to the manufacturer’s protocol as mentioned above. The inhibitory effect of WJCs on lymphocyte proliferation was evaluated by comparing the optical density (OD) in wells co-cultured on inactivated WJCs with the OD of lymphocytes grown alone.
2.5. Indirect co-culture assay
P3 WJCs were cultured at 37°C with 5% CO2 until 70% confluence; then the medium was exchanged to low-serum medium for 48 h. After the incubation period, WJC supernatants were collected. Lymphocytes were suspended at a concentration of 1×106/ml in RPMI-1640 medium supplemented with 10% FBS (control), RPMI-1640 medium supplemented with 50% WJC culture supernatant and 10% FBS, or WJC culture supernatant supplemented with 10% FBS. PHA (5 μl at 1 μg/μl) was then added into each well to stimulate lymphocyte proliferation. The protocol was performed as described above.
2.6. ELISA array
After being trypsinized, P3 WJCs were adjusted to 5×103/ml and plated (1 ml) in 24-well plates. After the WJCs reached 70% confluence, 10 μl of mitomycin-C (1 μg/μl) were added into each well. After 1 h at 37°C and 5% CO2, the medium was removed and cells were washed twice with PBS. Then 2×105 human peripheral blood lymphocytes in 1 ml of lymphocyte medium were added and co-cultured in the presence of 10 μl of PHA for 72 h at 37°C and 5% CO2. The groups were the following: WJCs (1×104) + lymphocytes + PHA; lymphocytes + PHA; WJCs. The supernatants of each group were collected and evaluated by ELISA detection kit (RD, USA) for TGF-β1 and IFN-γ. P3 WJCs (5×103) were plated in 24-well plates and treated with 10 μl of mitomycin-C (1 μg/μl) for 1 h until they reached 70% confluence. The medium was then removed, and the cells were washed twice with PBS. Human peripheral blood lymphocytes (2×105 in 1 ml of lymphocyte medium) were added and co-cultured for 48 h at 37°C and 5% CO2. Then, the non-adherent cells were harvested, washed extensively, and stimulated with10 μl of PHA (1 μg/μl) for another 24 h. The level of IFN-γ was measured with the ELISA detection kit.
2.7. Statistical analysis
Data are presented as means ± standard deviation. The results were analyzed with the statistical package SPSS 13.0 (SPSS Inc., Chicago, IL). The statistical significance was assessed by paired Student’s t-test and ANOVA; p value < 0.05 was considered statistically significant.
3. Results
3.1. Morphology, surface molecules, and proliferation activity observed in WJCs
Three to five days after the Wharton’s jelly was placed in culture, adherent single-spindled or triangular cells became visible around the tissue. After 2 weeks, some adherent cells had dissociated around the adherent tissue pieces and were visible under the inverted microscope. Cells gradually multiplied and grew into a radial-like array around the adherent tissue pieces (Fig. 1a). After the WJCs were passaged, they showed strong proliferative ability. They adhered rapidly and expanded without visible changes in either the growth pattern or morphology, which was primarily spindle-shaped or triangular. The cells reached confluence after approximately 5 days, at which time they became homogeneous fibroblastoid with smooth borders and a whirlpool-like or parallel array (Fig. 1b-d).
Fig. 1.
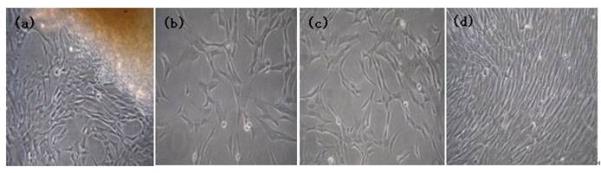
The morphology of human Wharton’s jelly mesenchymal stem cells. (a) After the Wharton’s jelly tissue pieces were plated and cultured for 2 weeks, some fibroblastoid cells dissociated from the tissue and adhered to the culture dish. (b) After 1 day in culture, passage-3 Wharton’s jelly mesenchymal stem cells appeared to be mostly spindle-shaped or triangular. (c) After 3 days in culture, the morphology changed to homogeneous fibroblastoid cells with a smooth border. (d) At 5 days in culture, the cells reached 100% confluence in a whirlpool-like or parallel array.
Flow cytometry analysis showed that the WJCs expressed elevated levels of matrix marker CD44 and integrin marker CD29 and a low level of HLA-ABC (MHC-I). The cells did not express hematopoietic lineage markers (CD34, CD45) or HLA-DR (MHC- II), which are closely related to graft-versus-host disease (Fig. 2).
Fig. 2.
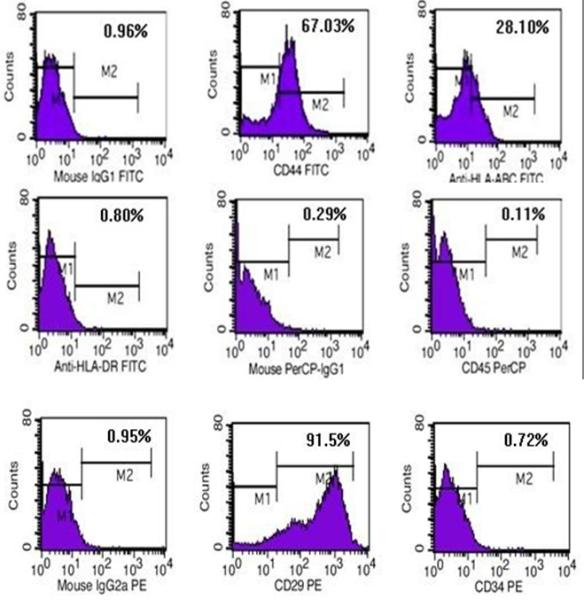
Phenotype of the human Wharton’s jelly mesenchymal stem cells. Cells were cultured for three passages, harvested, and labeled with mouse anti-human monoclonal antibodies CD29, CD44, CD34, CD45, major histocompatibility complex HLA-ABC, and HLA-DR and analyzed by flow cytometry. n=3.
The cell growth curve showed that P1, P3, and P5 possessed good proliferation activity. WJCs rapidly adhered and expanded in a short time after subculture. After 3 days, WJCs entered into the logarithmic growth phase. After 5 days, the proliferative activity decreased, and WJCs entered the stagnate phase. P1, P3, and P5 WJCs exhibited no significant differences in proliferation capability (p>0.05, n=5; Fig. 3).
Fig. 3.
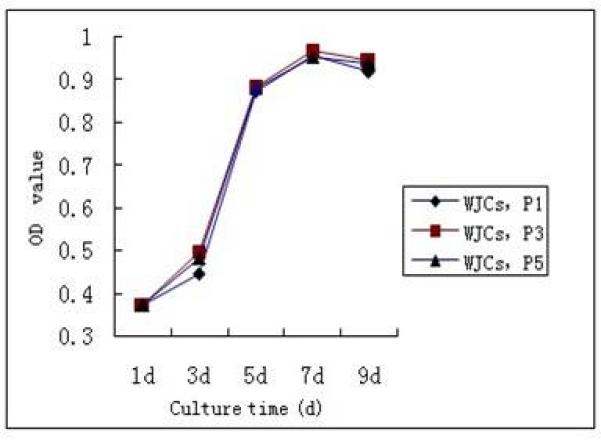
Growth curve of human Wharton’s jelly mesenchymal stem cells (WJCs). Proliferation of human WJCs was measured by the Cell Counting Kit-8 method at passages 1, 3, and 5 (P1, P3, P5). After 3 days in culture, the cells entered the logarithmic growth phase. Five days later, the proliferation activity decreased, and the cells entered into the stagnate phase. n=5. OD, optical density.
3.2. Immunomodulatory effect of WJCs
To investigate the mechanism of immunosuppression mediated by WJCs, we co-cultured PHA-induced mouse splenocytes and human peripheral blood lymphocytes with different concentrations of WJC culture supernatant. The supernatant dose dependently suppressed splenocyte and lymphocyte proliferation, even in the absence of cell-to-cell contact. Splenocyte proliferation was reduced to 23.1±5.2% of control when grown in 50% WJC culture medium (p<0.05, n=5) and to 7.4±1.1% of control when grown in 100% WJC culture medium (p<0.001, n=5). Likewise, the proliferation of human peripheral blood lymphocytes was reduced to 27.3±6.1% of control when grown in 50% WJC culture medium (p<0.05, n=5) and to 8.3±1.5% of control when grown in 100% WJC culture medium ((p<0.001, n=5).
PHA-induced proliferation of mouse splenocytes and human peripheral blood lymphocytes was significantly suppressed by co-culture with different numbers of mitotically inactivated WJCs. The average suppression of lymphocytes after 3 days was 49.9±12.5% with 1×104 WJCs, 47.3±10.8% with 5×103 WJCs, and 55.1±20.7% with 2×103 WJCs (p<0.01 each, n=5); the average suppression of splenocytes was 38.3±7.9% with 1×104 WJCs, 43.1±9.5% with 5×103 WJCs, and 44.9±13.4% with 2×103 WJCs (p<0.01 each, n=5). The inhibitory effect of WJCs was slightly less on splenocytes than on lymphocytes (p>0.05, n=5). Dose-dependent effects were not observed. WJCs added directly to the lymphocyte co-cultures had a stronger inhibitory effect (p<0.01, n=5). The results imply that the inhibitory effect of WJCs is mediated by both cell contact and soluble factors.
3.3. ELISA array
TGF-β1 was 834±152 pg/ml in the medium of WJCs cultured alone, 583±93 pg/ml in the medium of WJC–human peripheral blood lymphocyte co-cultures, and 1725±307 pg/ml in the medium of human peripheral blood lymphocyte cultured alone. The results indicate that WJCs significantly reduce TGF-β1 secretion by human peripheral blood lymphocytes (p<0.01, n=3). IFN-γ secretion by human peripheral blood lymphocytes was 294±44 pg/ml in the absence of WJCs but decreased to 115±16 pg/ml when co-cultured with WJCs (p<0.01, n=3). IFN-γ was absent from the medium of WJCs cultured alone; thus, WJCs do not secrete IFN-γ in an autocrine fashion. These results indicate that the immunomodulatory effect of WJCs may be associated with inhibition of TGF-β1 and IFN-γ secretion by human peripheral blood lymphocytes.
4. Discussion
MSCs can be isolated from many human tissues, such as amniotic fluid, bone marrow, muscle, blood, and umbilical cord blood (28-30). However, the collection of these tissue-derived MSCs is restricted because of limited stem cell numbers and culture techniques. These unfavorable conditions necessitate the identification of alternative sources of MSCs. Wharton’s jelly is an ideal source because its collection is not painful and is associated with limited ethical controversy. WJCs can be used in autologous cell therapy, and they lower the risk of viral contamination.
Within Wharton’s jelly, MSCs have been isolated from three relatively indistinct regions--the perivascular zone, the intervascular zone, and the subamnion (31-33). Two main methods are used to isolate MSCs—enzymatic digestion and tissue culture. For enzymatic digestion, trypsin and collagenase are used to digest the umbilical cord after the membrane and veins have been removed. Although cells obtained by enzymatic digestion have a short primary culture growth cycle, trypsin and collagenase may damage the WJCs and reduce the number that can be harvested. In addition, compared with tissue culture, enzymatic digestion is more expensive, more time consuming, and more difficult to control; it also carries a higher risk for contamination.
In our study, we isolated WJCs successfully by the tissue culture method. We were able to isolate 1×107 WJCs (per centimeter of human umbilical cord length) after primary culture and could expand them approximately 300 fold until the fifth passage. The cell growth curve showed that WJCs had good differentiation capability and that they could rapidly attach and proliferate in vitro by serial passages every 5 days without visible changes in either growth patterns or morphology.
The morphology of MSCs from Wharton’s jelly resembles that of BMSCs. Remarkably, the WJCs did not exhibit contact-inhibited cell growth and continued to grow in multiple layers, even after reaching 100% confluence. Furthermore, the surface marker expression of WJCs was almost the same as that of BMSCs. Analysis of cell surface markers on WJCs showed a lack of hematopoietic markers, such as CD34 and CD45, and high expression of CD44 and CD29. The similarity to BMSCs indicates that WJCs may have biological characteristics similar to those of BMSCs.
Studies have reported that WJCs can be differentiated into many different cell types in vitro, including bone, cartilage, fat, and neural cells (33-35). This capacity suggests that WJCs are potentially useful for tissue engineering and could be used as an important source of MSCs for cell therapy in the future. Recent reports from several laboratories indicate that WJCs have therapeutic potential for neurodegenerative disease, stroke, photoreceptor degeneration, and breast cancer (36-40). Additional investigation is needed to assess WJC responsiveness toward cardiomyogenic, endothelial, hepatic, neuronal, and pancreatic differentiation.
WJCs can be a suitable cell source for tissue engineering and optimal for use in cellular therapy if they are tolerated by a host’s immune system. Research has indicated that WJCs are unable to stimulate the proliferation of allogeneic human peripheral blood lymphocytes in vitro (11). In our study, WJCs expressed low amounts of HLA-ABC (MHC-I) and did not express HLA-DR (MHC-II), findings consistent with what has been reported previously (41). Our results indicate that strict matching may not be required before MSC transplantation and that allogeneic or xenogeneic cell transplantation may be achievable. This possibility has been supported by Medicetty et al. (42), who reported that pig WJCs underwent a moderate expansion following transplantation into immune-competent rat brain and were not acutely rejected.
The immunosuppressive properties of WJCs are broad, no matter whether the stimulation is specific or nonspecific. Our data showed that WJCs significantly inhibited the proliferation of both mouse splenocytes and human peripheral blood lymphocytes induced by PHA in vitro, specifically in allogeneic and xenogeneic formats (43). These immunosuppressive characteristics are consistent with those of BMSCs. In addition, we found that the immunosuppressive effect of WJCs on lymphocytes was dependent on cell number, a trait that might be related to laboratory conditions and the absolute number of cells per unit area. Studies have shown that MSCs have immunomodulatory effects not only on T lymphocytes but also on B lymphocytes, DC, and NK (19-22). These immunomodulatory effects occur primarily through inhibiting the proliferation and cytokine secretion of immune cells or changing immune cell subtypes.
Although the immunomodulatory effect of MSCs has been confirmed by many experiments (10, 23), the specific mechanism of immune suppression is still controversial. Some scholars believe that intercellular contact plays an important role in the immune suppression process [58]. Others think that the effect is produced by soluble factors, such as hepatocyte growth factor, IL-10, and TGF-β1 (10, 23, 42, 43). We found that the proliferation of homologous and heterologous lymphocytes was inhibited by both WJCs and the medium in which the WJCs were grown. Although the effect of the medium alone was smaller than that achieved in the presence of WJCs, the results suggest that suppression is induced by both soluble factors and direct cell contact.
The cytokines involved in the immunomodulatory process have not yet been determined. Nasef et al. (23) reported that MSCs appear to induce T-cell tolerance by two distinct mechanisms. The first mechanism is that the tolerogenic genes, such as IDO, LIF, and HLA-G, are induced without cell-to-cell contact. The second mechanism is cell contact-dependent through modulating IL-10 and TGF-β gene expression. These two mechanisms probably play separate roles in MSC-induced tolerance in allogeneic hematopoietic stem cell transplantation.
The soluble factors involved in MSC immune regulation require additional research. Based on the finding that adding TGF-β1 neutralizing antibody to BMSC-inhibited T lymphocytes can partially restore proliferation, TGF-β1 appears to act as a negative immune regulator that can inhibit the activation and proliferation of T lymphocytes (44). Our results showed that both WJCs and human peripheral blood lymphocytes secrete TGF-β1. However, when the two cell types were co-cultured, the level of TGF-β1 secreted by the lymphocytes decreased substantially, a finding consistent with those of previous studies (10, 45, 46). IFN-γ modulates cell-mediated immunity and immune rejection. Our results showed that, although WJCs did not secrete IFN-γ, they significantly reduced IFN-γ secretion by human peripheral blood lymphocytes, suggesting that WJCs might reduce the incidence of immune rejection by this mechanism.
In summary, we have shown that isolation of MSCs from human Wharton’s jelly is feasible and that WJCs share morphological, immunogenic, and immunoregulatory characteristics with BMSCs. The immunomodulatory effects of MSCs are likely mediated by both direct cell-to-cell interactions and soluble factors such as TGF-β1 and IFN-γ. Multiple cytokines may be involved in the immunomodulation, but they have yet to be identified. Additional in vivo studies are required to fully evaluate the immunomodulatory functions of WJCs. Elucidating the immunoregulatory mechanisms of WJCs will greatly improve the prospects for the use of WJC transplantation to treat immune system-related diseases in the future.
The immunomodulatory effect of human WJCs on lymphocytes was examined.
Both WJCs and WJC culture supernatant inhibited the proliferation of lymphocytes.
WJCs suppressed secretion of TGF-β1 and IFN-γ by human peripheral blood lymphocytes.
The WJC immunomodulatory effect may be mediated by cell contact and soluble factors.
Acknowledgements
The authors wish to acknowledge the contribution of all the students and fellows who participated in various aspects of this work over the past three years. This research was supported by: National Science Foundation of China (81071008, 81171177), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA01030300). 211 Project-phase III of Zhengzhou University-the basic and clinical research of stem cells, the Excellent Youth Foundation of He’nan Scientific Committee (114100510005), the Young Excellent Teachers in University Funded Projects of Henan Province; Bureau of Science and Technology Development Project from Henan Province (200902310250); NIH K01AG031926. We thank Claire Levine for assistance with the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chen MY, Lie PC, Li ZL, Wei X. Endothelial differentiation of Wharton’s jelly-derived mesenchymal stem cells in comparison with bone marrow-derived mesenchymal stem cells. Exp Hematol. 2009;37:629–40. doi: 10.1016/j.exphem.2009.02.003. [DOI] [PubMed] [Google Scholar]
- [2].Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp Hematol. 2000;28:382–90. doi: 10.1016/s0301-472x(00)00134-x. [DOI] [PubMed] [Google Scholar]
- [3].Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113(Pt 7):1161–6. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- [4].Lou J, Xu F, Merkel K, Manske P. Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17:43–50. doi: 10.1002/jor.1100170108. [DOI] [PubMed] [Google Scholar]
- [5].Mbalaviele G, Jaiswal N, Meng A, Cheng L, Van Den Bos C, Thiede M. Human mesenchymal stem cells promote human osteoclast differentiation from CD34+ bone marrow hematopoietic progenitors. Endocrinology. 1999;140:3736–43. doi: 10.1210/endo.140.8.6880. [DOI] [PubMed] [Google Scholar]
- [6].Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- [7].Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [8].Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–90. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- [9].Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–26. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- [10].Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- [11].Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865–74. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- [12].Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- [13].Hematti P. Role of mesenchymal stromal cells in solid organ transplantation. Transplant Rev (Orlando) 2008;22:262–73. doi: 10.1016/j.trre.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- [15].Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–91. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- [16].Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- [17].Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noel D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- [18].Karussis D, Kassis I, Kurkalli BG, Slavin S. Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci. 2008;265:131–5. doi: 10.1016/j.jns.2007.05.005. [DOI] [PubMed] [Google Scholar]
- [19].Magatti M, De Munari S, Vertua E, Gibelli L, Wengler GS, Parolini O. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells. 2008;26:182–92. doi: 10.1634/stemcells.2007-0491. [DOI] [PubMed] [Google Scholar]
- [20].Asari S, Itakura S, Ferreri K, Liu CP, Kuroda Y, Kandeel F, Mullen Y. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol. 2009;37:604–15. doi: 10.1016/j.exphem.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–33. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- [22].English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–8. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- [23].Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebe L, Zhang Y, Gorin NC, Thierry D, Fouillard L. Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expr. 2007;13:217–26. doi: 10.3727/000000006780666957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- [25].Huber S, Schrader J, Fritz G, Presser K, Schmitt S, Waisman A, Luth S, Blessing M, Herkel J, Schramm C. P38 MAP kinase signaling is required for the conversion of CD4+CD25- T cells into iTreg. PLoS One. 2008;3:e3302. doi: 10.1371/journal.pone.0003302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou X, Zhu X, Lu C, Liang W, Liao L, Zenke M, Zhao RC. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46–57. doi: 10.1182/blood-2008-04-154138. [DOI] [PubMed] [Google Scholar]
- [27].Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- [28].Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- [30].Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999;65:22–6. [PubMed] [Google Scholar]
- [31].Peng HQ, Levitin-Smith M, Rochelson B, Kahn E. Umbilical cord stricture and overcoiling are common causes of fetal demise. Pediatr Dev Pathol. 2006;9:14–9. doi: 10.2350/05-05-0051.1. [DOI] [PubMed] [Google Scholar]
- [32].Trevisanuto D, Doglioni N, Zanardo V, Chiarelli S. Overcoiling of the umbilical cord. J Pediatr. 2007;150:112. doi: 10.1016/j.jpeds.2006.11.042. [DOI] [PubMed] [Google Scholar]
- [33].Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220–9. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- [34].Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319–31. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- [35].Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–7. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- [36].Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24:781–92. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- [37].Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115–24. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- [38].Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauve Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin FY, Gosiewska A, Mistry SK. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602–11. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- [39].Jomura S, Uy M, Mitchell K, Dallasen R, Bode CJ, Xu Y. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25:98–106. doi: 10.1634/stemcells.2006-0055. [DOI] [PubMed] [Google Scholar]
- [40].Rachakatla RS, Marini F, Weiss ML, Tamura M, Troyer D. Development of human umbilical cord matrix stem cell-based gene therapy for experimental lung tumors. Cancer Gene Ther. 2007;14:828–35. doi: 10.1038/sj.cgt.7701077. [DOI] [PubMed] [Google Scholar]
- [41].Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–32. doi: 10.1111/j.1365-2567.2008.02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Medicetty S, Bledsoe AR, Fahrenholtz CB, Troyer D, Weiss ML. Transplantation of pig stem cells into rat brain: proliferation during the first 8 weeks. Exp Neurol. 2004;190:32–41. doi: 10.1016/j.expneurol.2004.06.023. [DOI] [PubMed] [Google Scholar]
- [43].Bian L, Guo ZK, Wang HX, Wang JS, Wang H, Li QF, Yang YF, Xiao FJ, Wu CT, Wang LS. In vitro and in vivo immunosuppressive characteristics of hepatocyte growth factor-modified murine mesenchymal stem cells. In Vivo. 2009;23:21–7. [PubMed] [Google Scholar]
- [44].Bommireddy R, Saxena V, Ormsby I, Yin M, Boivin GP, Babcock GF, Singh RR, Doetschman T. TGF-beta 1 regulates lymphocyte homeostasis by preventing activation and subsequent apoptosis of peripheral lymphocytes. J Immunol. 2003;170:4612–22. doi: 10.4049/jimmunol.170.9.4612. [DOI] [PubMed] [Google Scholar]
- [45].Xu G, Zhang Y, Zhang L, Ren G, Shi Y. The role of IL-6 in inhibition of lymphocyte apoptosis by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;361:745–50. doi: 10.1016/j.bbrc.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]


