Abstract
Autism spectrum disorders (ASDs) are characterized by impairments in social behaviors that are sometimes coupled to specialized cognitive abilities. A small percentage of ASD patients carry mutations in genes encoding neuroligins, which are postsynaptic cell adhesion molecules. Here we introduce one of these mutations into mice – the R451C-substitution in neuroligin-3. R451C-mutant mice showed impaired social interactions but enhanced spatial learning abilities. Unexpectedly, these behavioral changes were accompanied by an increase in inhibitory synaptic transmission, with no apparent effect on excitatory synapses. Deletion of neuroligin-3, in contrast, did not cause such changes, indicating that the R451C-substitution represents a gain-of-function mutation. These data suggest that increased inhibitory synaptic transmission may contribute to human ASDs and that the R451C KI mice may be a useful model for studying autism-related behaviors.
Autism is a widespread cognitive disorder characterized by impairments in social interactions, including verbal communication and social play, and can be accompanied by stereotyped patterns of behavior (1–3). Autism is a heterogeneous condition, prompting the designation of "autism spectrum disorders" (ASDs). Individuals with ASDs occasionally show enhanced cognitive abilities (the ‘autistic savant syndrome’ [4]). At the other end of the spectrum, ASDs are often associated with mental retardation, and the symptoms of ASDs are part of several neurological diseases, such as fragile X- and Rett-syndrome (5–7). Genetics strongly contributes to ASDs (1,2), and a small number of cases with idiopathic ASD are associated with mutations in a single gene, including genes encoding neuroligins and their associated proteins (8).
Neuroligins are a family of postsynaptic cell-adhesion molecules that are ligands (or receptors, depending on the perspective) for neurexins, another class of synaptic cell-adhesion molecules (9,10). Humans express five neuroligins, including neuroligin-3, an X-chromosomal gene that undergoes regular X-inactivation, and neuroligin-4 and -5, which are encoded by a pair of pseudoautosomal genes on the X- and Y-chromosomes (11). Mice express close homologs to human neuroligin-1, -2, and -3 (9), and a fourth isoform that appears to be more distantly related to other neuroligins (GenBank Acc.# EF692521; 11). Neuroligin-1 and -2 are differentially localized to excitatory or inhibitory synapses (12–14). Overexpression of neuroligins in transfected neurons increases synapse numbers and the frequency of spontaneous synaptic events (15–20). Consistent with their localizations, overexpression of neuroligin-1 enhances only excitatory synaptic transmission, whereas overexpression of neuroligin-2 enhances only inhibitory synaptic transmission, respectively (20). Deletion of neuroligin-1 or -2 in mice causes corresponding selective decreases in excitatory or inhibitory synaptic transmission, respectively, but no significant synapse loss, while neuroligin-3 has not been examined (11,21).
Missense and non-sense mutations in neuroligin-3 and -4 have been identified in a subset of human patients with ASDs (22–24). One of these mutations, the R451C-substitution in neuroligin-3, alters a conserved residue in the extracellular esterase-homology domain of neuroligin-3 (22). In transfected neurons, the R451C-substituion causes partial retention of neuroligin-3 in the endoplasmic reticulum, but does not abolish its ability to promote synapse formation (20,25,26). In addition, an internal deletion in the gene encoding neurexin-1 that interacts with neuroligins was connected to ASDs (27), and three different non-sense mutations in Shank3, an intracellular binding partner for neuroligins, were also found in patients with ASDs (28). Thus, in rare instances mutations in three gene families that encode neuroligins or their interacting proteins are associated with familial idiopathic ASDs.
An increase in inhibitory synapse markers in R451C-mutant mice
Autism is thought to arise from functional changes in neural circuitry and to be associated with an imbalance between excitatory and inhibitory synaptic transmission, but the mechanisms involved are unknown (29). To investigate possible mechanisms, we introduced the R451C-substitution into the endogenous neuroligin-3 gene in mice by gene targeting, generating R451C knockin (KI) mice (Fig. S1, 30). Moreover, to test whether the R451C-substitution represents a gain- or a loss-of-function change, we also analyzed neuroligin-3 knockout (KO) mice (Fig. S1). Since the neuroligin-3 gene is X-chromosomal, analyses were performed on male offspring derived from matings of a heterozygous female with a wild-type male mouse. Neuroligin-3 R451C KI and neuroligin-3 KO mice were viable and fertile, and exhibited no obvious abnormalities, morbidity or premature mortality (Fig. S2 and 11).
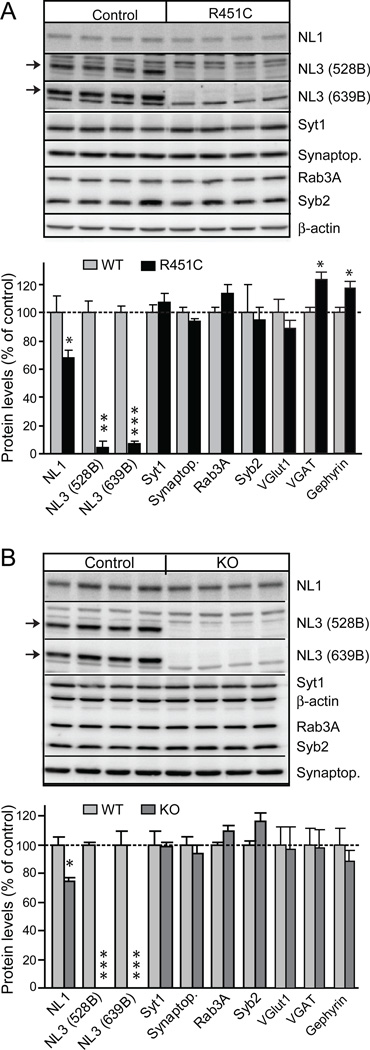
We first analyzed the levels of neuroligin-3 and of other synaptic proteins in neuroligin-3 R451C KI and KO mice. The R451C-substitution caused a decrease in neuroligin-3 levels of ~90% in forebrain as measured by quantitative immunoblotting with two different antibodies, whereas the KO caused a complete loss of neuroligin-3 (Fig. 1). In addition, we observed a small decrease in neuroligin-1 in the KI and the KO mice, and a significant increase in the levels of two markers for inhibitory synapses (the vesicular GABA-transporter VGAT and the postsynaptic protein gephyrin) in the R451C KI mice, whereas no change in VGAT levels were detected in the KO mice (Fig. 1). No significant change in the levels of other proteins examined were observed, in particular no change in the levels of the vesicular glutamate transporter or other proteins characteristic of excitatory synapses (Figs. 1, S3, and S4; 30). These data suggest that the neuroligin-3 R451C KI and KO did not cause a global change in the molecular composition of the brain, except for a small increase in inhibitory markers in the KI but not in the KO mice.
Figure 1.
Generation and characterization of neuroligin-3 R451C KI and neuroligin-3 KO mice. (A and B) Representative immunoblots and summary graphs of protein levels in the brains of neuroligin-3 R451C KI mice (A) and neuroligin-3 KO mice (B). Selected synaptic proteins (NL1, neuroligin-1; NL3, neuroligin-3; Synaptop., synaptophysin; Syt1, synaptotagmin-1; Syb2, synaptobrevin-2) were analyzed by quantitative immunoblotting; two different neuroligin-3 antibodies were used (528B and 639B; arrows point to neuroligin-3 band; data shown are means ± SEMs; n=4 littermate pairs; * = p<0.05; ** = p<0.01; *** = p<0.005 by Student's t-test).
The decreased levels of R451C-mutant neuroligin-3 could be due to a destabilization of the mutant protein. Alternatively, since the construction of the R451C knockin mice involved the introduction of loxP and frt recombination sites into the neuroligin-3 gene intron, it is possible that the genetic manipulation may have impaired expression of neuroligin-3. To differentiate between these two possibilities, we measured the mRNA levels of neuroligin-3 in wild-type and R451C KI mice using quantitative RT-PCR, but detected no decrease of the neuroligin-3 mRNA in the mutant mice (Fig. S5). These data indicate that the R451C substitution destabilizes neuroligin-3, consistent with the retention of R451C-mutant neuroligin-3 in the endoplasmic reticulum observed in transfection studies (20, 25).
We next examined the R451C-mutant brains morphologically, but failed to detect a major change in brain architecture (Fig. S6). We then measured the intensity of synapse staining using antibodies to synaptic vesicle proteins. We stained cryostat sections from somatosensory cortex and the CA1 and CA3 regions of the hippocampus and from the somatosensory cortex with antibodies to synaptophysin, a general marker of all synapses, and to the vesicular glutamate transporter VGlut1 and the vesicular GABA transporter VGAT, markers of excitatory and inhibitory synapses, respectively. The staining patterns observed were characteristic for the excitatory and inhibitory synapses in these brain regions, but the intensity for VGAT staining appeared to be brighter in R451C KI mice than in control or in KO mice (Figs. 2A and S7–S8). To test this, we used image analysis that estimates the number and size of puncta labeled with the various antibodies above the same threshold value applied to all images (Figs. 2B–2E and S7 and S8). We observed a dramatic increase in the number of VGAT-positive puncta in the R451C KI mice in all three brain regions analyzed (50–80% increase). In contrast, the number of VGlut1- or synaptophysin-positive puncta above threshold was unchanged, and the average size of the puncta was also not altered for any of the three antibodies (Figs. 2B–2E and S7 and S8; 30). Moreover, no increase in the density of VGAT-positive puncta was detected in the neuroligin-3 KO mice (Figs. 2B–2E).
Figure 2.
The R451C-substitution in neuroligin-3 increases the synaptic signal of the vesicular GABA transporter (VGAT) but not the number of inhibitory synapses. (A) Representative confocal immunofluorescence images of sections of the CA1 region of the hippocampus from littermate wild-type and R451C knockin mice double-labeled with antibodies to the vesicular glutamate-transporter VGlut1 (red; top panels) and the vesicular GABA-transporter VGAT (green; middle panels); the bottom panel depicts the merged image (for representative images from other brain areas or sections stained with other antibodies, see Figs. S6–S8). Scale bars in bottom panels that apply to all panels in a column. (B-E) Quantitation of the density of synaptic puncta that are above threshold by image analysis of confocal immunofluorescence sections from the CA1 and CA3 region of the hippocampus (B-D) and from the somatosensory cortex (E) from wild-type and R451C KI mice (top) or neuroligin-3 KO mice (bottom). Quantitations are from sections stained with antibodies to synaptophysin (B; labels all synapses) or to VGAT and VGlut (C-E; label only inhibitory or excitatory synapses, respectively). (F) Representative electron micrographs from layer 2/3 of the somatosensory cortex of wild-type (a) and R451C KI (b) mice (black arrowheads = asymmetric synapses; white arrowheads = symmetric synapses). (G-J) Quantitation of the density of asymmetric (excitatory) and symmetric (inhibitory) synapses (G), of the ratio of asymmetric to symmetric (H) synapses, and of the width of a synapse (J) and its synaptic vesicle numbers (K) in electron micrographs from 3 pairs of littermate wild-type and R451C KI mice (data shown in B-E and G-J are means ± SEMs; n=3 littermate pairs; * = p<0.05; ** = p<0.01; *** = p<0.005 by Student's t-test).
The increased number of VGAT-positive puncta above threshold in the R451C KI mice could be due to an increase in inhibitory synapse numbers, or to a shift in the distribution of VGAT, such that more synapses contain a high concentration of the transporter. To differentiate between these two possibilities, we examined the number and structure of synapses in layer 2/3 of the somatosensory cortex by electron microscopy, but detected no major ultrastructural change (Figs. 2F–2J). Thus, the R451C-substitution does not increase synapse formation, but appears to act at a step downstream of synapse formation to increase the average VGAT signal per synapse. This conclusion is also consistent with the fact that neuroligin deletions in general have not been found to alter synapse numbers, but instead selectively impair synaptic strength (11, 21).
Inhibitory synaptic strength is increased in neuroligin-3 R451C KI but not KO mice
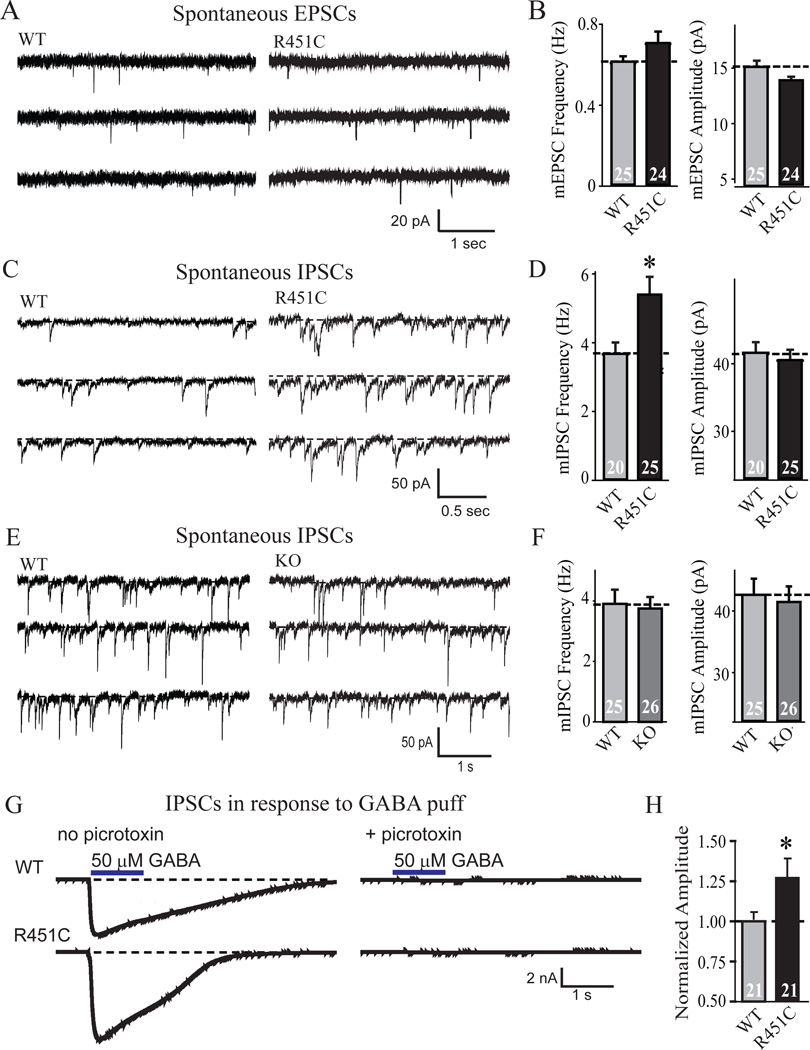
We measured synaptic function in the R451C KI mice using whole-cell recordings in layer 2/3 of the somatosensory (barrel) cortex in acute slices. Examination of spontaneous synaptic 'mini' events (Figs. 3A–3D) uncovered no significant change in the frequency or size of excitatory events, but detected a ~50% increase in the frequency of spontaneous inhibitory events. No change in the amplitude of spontaneous inhibitory events was detected. To determine whether the increased frequency of spontaneous inhibitory synaptic events is due to the loss of neuroligin-3 induced by the R451C-substitution (Fig. 1), or reflects a specific action of the mutant protein, we measured the frequency and size of spontaneous inhibitory mini events in neuroligin-3 KO mice (Fig. 3E). Strikingly, neuroligin-3 KO mice exhibited no increase in the frequency of spontaneous mini inhibitory events (Fig. 3F).
Figure 3.
Neuroligin-3 R451C-knockin but not neuroligin-3 KO mice exhibit increased spontaneous inhibitory synaptic transmission. Recordings were performed in whole-cell patch-clamp mode in pyramidal neurons in layer 2/3 of the somatosensory cortex in acute slices. (A-F) Representative traces (A, C and E) and summary graphs of the amplitudes and frequency (B, D and F) of spontaneous miniature excitatory postsynaptic currents (mEPSCs; A and B) and inhibitory postsynaptic currents (mIPSCs; C and D) from R451C KI (A-D) or KO mice (E and F). (G and H) Representative traces (G) and summary graph for response to a locally applied GABA puff (50 µM injected at 5 psi for 1 s) in layer 2/3 of the somatosensory cortex. In G, responses are also shown in the presence of 50 µM picrotoxin to document their inhibitory nature (data shown are means ± SEMs; n=3 littermate pairs; total number of cells recorded are indicated within bars; * = p<0.05; ** = p<0.01; *** = p<0.005 by Student's t-test; all electrophysiological parameters are listed in Table S1).
The selective increase of inhibitory spontaneous events agrees with the increase in the levels of inhibitory synaptic proteins (Fig. 1) and the number of inhibitory synapses with VGAT signals above threshold (Fig. 2), suggesting that inhibitory synaptic transmission may be enhanced by the R451C-substitution. Also consistent with this hypothesis, the amplitude of the response to exogenous GABA puffed onto neurons in layer 2/3 of the somatosensory cortex was significantly increased (Figs. 3G and 3H).
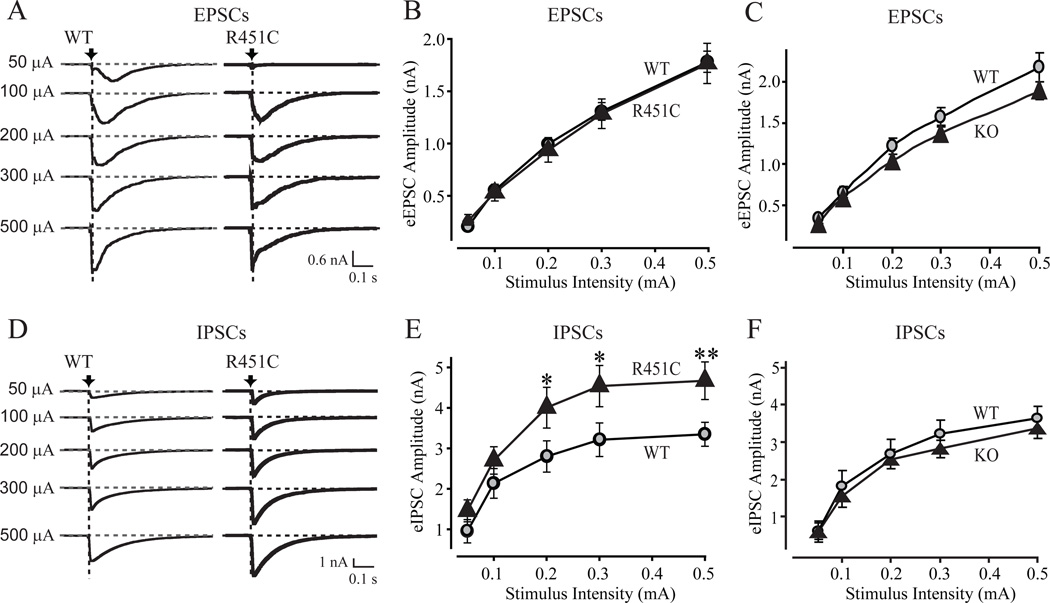
We next investigated synaptic strength by measuring input/output curves of evoked synaptic responses. We detected no difference in excitatory responses between wild-type and R451C KI mice, but observed a significant increase in inhibitory responses (~50%; Figs. 4A, 4B, 4D, and 4E). Measurements in neuroligin-3 KO mice, by contrast, uncovered no change in inhibitory responses, but detected a small, although insignificant, decrease in excitatory responses (Figs. 4C, 4F, and S9). This result confirms the finding made in the spontaneous synaptic measurements that the neuroligin-3 R451C KI but not the KO causes a selective increase in inhibitory synaptic transmission. Consistent with the postsynaptic localization of neuroligins (12), we found that the short-term synaptic plasticity properties of inhibitory synapses in neuroligin-3 R451C KI or KO brains did not exhibit a major change (Figs. S10–S12).
Figure 4.
Selective increase in inhibitory synaptic strength in neuroligin-3 R451C KI but not in neuroligin-3 KO mice. Representative traces (A and D) and summary graphs (C, D, E and F) of synaptic responses induced with increasing stimulus intensities applied with a local microelectrode in acute slices of the somatosensory cortex from littermate pairs of R451C KI mice (A, B, D, and E) or KO mice (C and F). Recordings were obtained in the whole-cell mode in layer 2/3. EPSCs (A-C) and IPSCs (D-F) were analyzed separately after pharmacological isolation. In A and D, arrows and vertical dashed lines indicate peaks measured for determining evoked response amplitude. Dotted horizontal lines represent baselines. All data were recorded in acute slices from littermate R451C-mutant and wild-type mice (data shown are means ± SEMs; n=4 or 3 littermate pairs for EPSCs (KI or KO), and 5 or 3 littermate pairs for IPSCs (KI or KO); * = p<0.05; ** = p<0.01 by t-test). For representative traces from the KO mice, see Fig. S9; for short-term plasticity measurements in KI and KO mice, see Figs. S10–13.
Neuroligin-3 R451C KI mice exhibit impaired social behaviors but enhanced spatial learning abilities
To determine whether the changes in synaptic transmission in R451C KI mice produce behavioral impairments, we first tested the mice for global behavioral changes, but detected no changes in locomotor activity, motor coordination, and anxiety-related behaviors in R451C KI mice using a series of tests (dark/light box, open field, novel home cage activity, rotarod, open field arena, and elevated plus maze; Fig. S13).
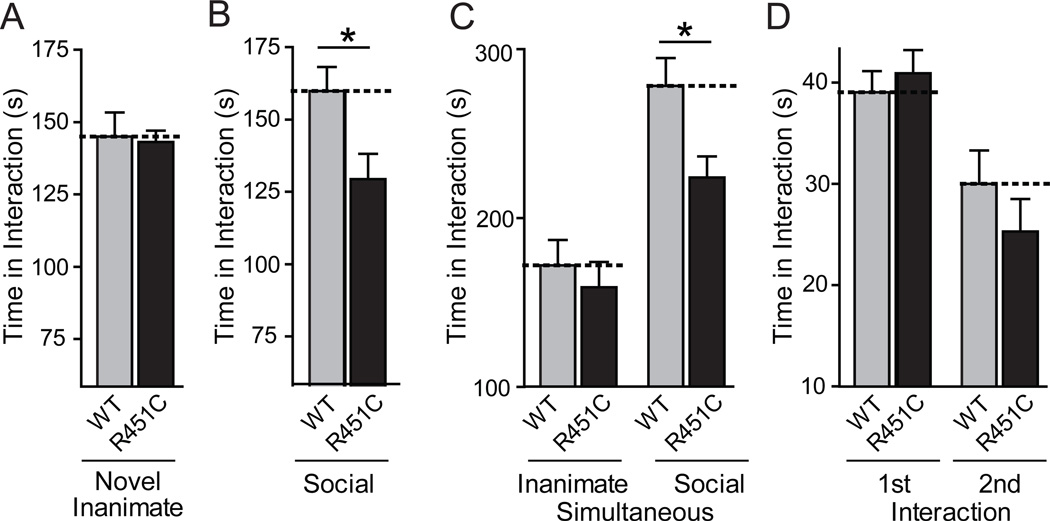
We next investigated whether R451C KI mice display abnormal social behaviors. Indeed, R451C KI mice showed no change in the time of interaction with a novel inanimate object, but a small but significant decrease in interaction with a novel caged adult target mouse compared to wild-type littermate controls, indicating a social interaction deficit (Figs. 5A and 5B). Similarly, in a test for social versus inanimate preference, R451C KI mice spent significantly less time interacting with a social target than the wild-type littermate controls (Fig. 5C). In agreement with a selective effect on social behavior, R451C KI mice spent the same amount of time interacting with an inanimate target as controls during this task. However, when placed into a neutral home cage with a freely moving conspecific juvenile target mouse for 2 min, R451C-mutant and wild-type littermate control mice interacted similarly with the target mouse, presumably because in this test the target mouse initiates the interaction as much as the test mouse, potentially masking social interaction deficits of the test mouse deficit (Fig. 5D). When re-exposed to the same juvenile three days later, both the control and the R451C KI mice exhibited a significant decrease in social interaction compared to the initial interaction, demonstrating that the mutant mice recognize the familiar juvenile mouse and are capable of social learning.
Figure 5.
Impaired social interaction behaviors in neuroligin-3 R451C KI mice. (A) Interacting time of individual wild-type and R451C KI mice exposed to a novel inanimate object in an unfamiliar cage (5 min). (B) Interacting time of mice that are exposed to an unfamiliar immobilized target mouse in a now familiar cage (5 min; procedure immediately follows A). (C) Interacting time of mice that are exposed simultaneously to a novel inanimate object and a novel, caged target mouse. (D) Social learning measured by monitoring the time of direct interactions of wild-type and R451C KI mice with the same freely moving juvenile target mouse on day 1 (1st Interaction) and day 4 (2nd Interaction for social learning). All data shown are means ± SEMs; n=19 male littermate pairs; only statistically significant differences between wild-type and R451C KI mice are specifically identified in the figure (* = p<0.05; ** = p<0.01; *** = p<0.001 by t-test or two-way ANOVA); a detailed statistical analysis for all parameters is provided in Table S2 (30).
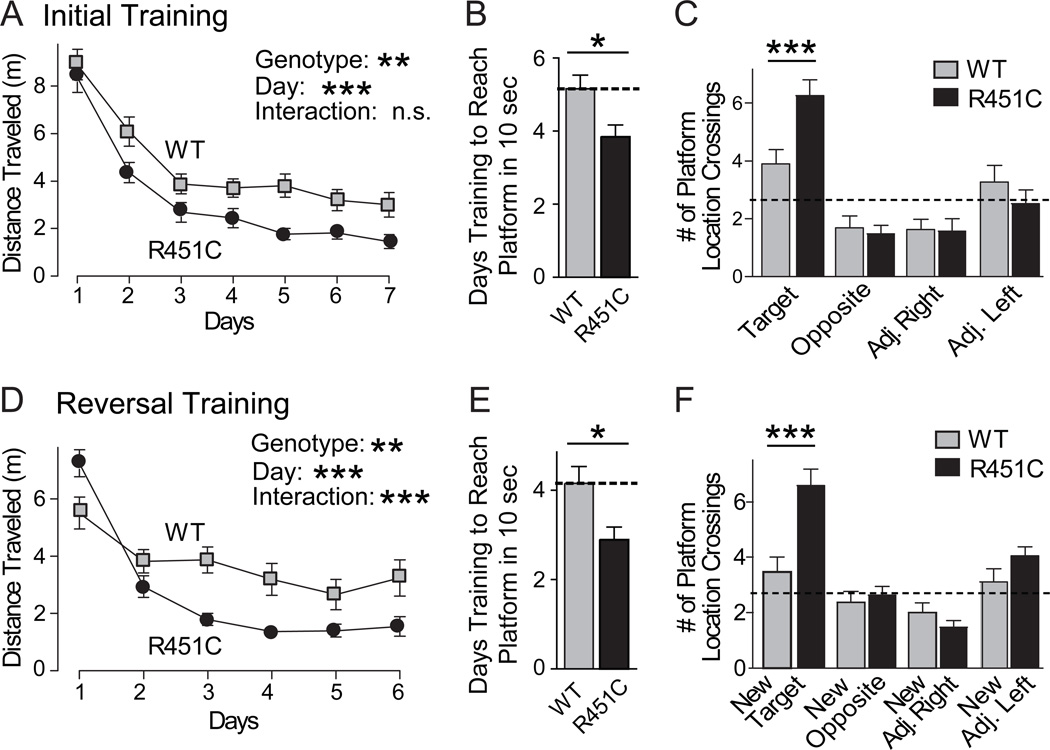
Individuals with ASDs exhibit impaired social abilities, but can display normal or, rarely, even enhanced spatial cognitive abilities (1–4). To examine whether the selective decrease in social interactions in R451C KI mice is associated with a gain or a loss of other cognitive abilities, we tested spatial learning and memory in R451C KI mice using the Morris water maze. R451C KI mice learned to locate and mount a visible platform as well as wild-type littermate control mice, indicating that basic neurological functions required for swimming, vision, etc. were intact. When the platform was hidden, the R451C KI mice exhibited a significantly enhanced ability to locate the platform (Fig. 6A), and required fewer days of training to learn the location of the platform (Fig. 6B). During the probe trial 24 hours after the 7th day of training, both wild-type and R451C KI mice displayed a significant preference for the target versus the opposite quadrant, but the R451C KI mice crossed the precise former location of the target platform almost twice as often as their wild-type littermate controls (Figs. 6B and S14).
Figure 6.
Neuroligin-3 R451C KI mice exhibit enhanced spatial learning. (A) Morris water maze analysis of spatial learning in R451C KI and littermate wild-type control mice during the initial 7 days of training as measured by the distance traveled to reach a submerged platform. (B) Number of days of initial training required to reach the submerged platform in an average of 10 sec or less. (C) Number of crossings over the previous location of the target platform and over corresponding locations in the other 3 quadrants measured on day 8 after removal of the platform (probe trial). (D) Reversal learning experiment in which on day 9 after the probe trial the platform was moved to the opposite quadrant, and the learning of the new location of the platform by the mice was monitored. Learning is measured as distance traveled prior to mounting the newly localized target platform as a function of days of training. (E) Number of days of reversal training required to reach the submerged platform in an average of 10 sec or less. (F) Probe trial after reversal learning uncovers a large increase in learning abilities of the R451C KI mice. Only statistically significant differences between wild-type and R451C KI mice are identified in the panels (* = p<0.05; ** = p<0.01; *** = p<0.001; in A and D, Genotype = main effect of genotype, Day = main effect of day of training; Interaction = interaction between genotype and day). All data shown are means ± SEMs; n=19 male littermate pairs; see Fig. S14 Table S2 for all statistical comparisons.
To ensure that an increase in number of target location crossings in the R451C KI mice on probe trial was due to enhanced spatial memory rather than perseveration, we reversed the location of the platform and re-trained the same cohort of mice (so-called reversal training). Again, R451C KI mice exhibited a significantly enhanced learning curve during (Fig. 6D), and required fewer days of training to learn the location of the platform (Fig. 6E). Twenty-four hours after the final reversal training day, R451C KI mice displayed enhanced spatial memory during the probe trial. R451C KI mice showed a significant preference for the new target quadrant and spent significantly more time in the target quadrant than wild-type littermate control mice (Fig. 6F). Similarly, R451C KI mice crossed the new target location more often than control mice and exhibited a significant preference for the target location over all other locations, unlike wild-type mice (Figs. 6F and S14), suggesting that they have an increased ability for spatial learning and memory.
Summary
The phenotype of the neuroligin-3 R451C KI mice suggests that this mouse may pave the way for a mechanistic analysis of the pathogenesis of idiopathic ASDs, and provide a possible model system to search for more effective treatments for ASDs. We found that the R451C-substitution increases inhibitory synaptic transmission without affecting excitatory synaptic transmission, and simultaneously impairs social behaviors while selectively enhancing spatial learning abilities. These findings are surprising because ASDs were thought to be associated with a loss of inhibitory drive (29,31), although a Rett-syndrome mouse model also exhibits an increase in synaptic inhibitory drive (32). The R451C-substitution increases inhibitory synapse markers, spontaneous inhibitory event frequency, and the size of inhibitory synaptic responses, but does not change short-term synaptic plasticity of inhibitory synapses (Figs. 1–3 and Figs. S7 and S8), suggesting that the mutation enhances inhibitory synaptic transmission without changing the release probability of these synapses. Thus, our results not only validate the hypothesis that neuroligins act at synapses to specify synaptic properties (20, 33), but also indicate that interfering with the function of a neuroligin alters the excitatory/inhibitory balance in vivo. Moreover, if the mouse model mimics the situation in humans with ASDs, it may be possible to ameliorate autism-related behavioral abnormalities using attenuation of inhibitory synaptic transmission.
How does the R451C mutation increase inhibitory synaptic transmission? A facile explanation would have been that the destabilization of neuroligin-3 by the R451C substitution (25) and the resulting loss of neuroligin-3 protein produces a loss-of-function of neuroligin-3 which then causes the phenotype. However, our analysis of the neuroligin-3 KO mice rules out this explanation. Even though only ~10% of the neuroligin-3 protein remains in the R451C KI mice, this remaining neuroligin-3 protein produces increased inhibitory synaptic transmission whereas the complete neuroligin-3 KO exerts no such effect. Thus, the R451C-substitution likely acts as a gain-of-function mutation, a hypothesis that also explains why no loss-of-function neuroligin-3 mutation was found in humans with ASDs, whereas several loss-of-function mutations were found in neuroligin-4 in such individuals (22–24).
Our data strongly support the notion that a change in the inhibitory/excitatory balance contributes to the pathogenesis of ASDs. Such a change may alter oscillatory rhythms in brain (34, 35). Given the relatively focused nature of behavioral abnormalities in the R451C KI mice and in some humans with idiopathic ASDs, it is likely that this change is not global, but selectively affects only a subset of the many classes of inhibitory interneurons in the forebrain (reviewed in 36, 37), a question that can now be addressed with the R451C KI mice.
Supplementary Material
Acknowledgments
We thank I. Kornblum, J. Mitchell, L. Fan, J. Cormier, and A. Roth for technical support. Supported by grants from the National Institute of Mental Health (R37 MH52804-08 to T.C.S. & K08 MH065975-04 to C.M.P.).
REFERENCES and NOTES
- 1.Geschwind DH, Levitt P. Curr Opin Neurobiol. 2007;17:103. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Persico AM, Bourgeron Bourgeron T. Trends Neurosci. 2006;29:349. doi: 10.1016/j.tins.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 2002 [ISBN 0890420254] [Google Scholar]
- 4.O'Connor N, Hermelin B. Br J Psychol. 1989;80:97. doi: 10.1111/j.2044-8295.1989.tb02305.x. [DOI] [PubMed] [Google Scholar]
- 5.Moretti P, Zoghbi HY. Curr Opin Genet Dev. 2006;16:276. doi: 10.1016/j.gde.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Belmonte MK, Bourgeron T. Nat Neurosci. 2006;9:1221. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- 7.Moldin SO, Rubenstein JL, Hyman SE. J Neurosci. 2006;26:6893. doi: 10.1523/JNEUROSCI.1944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garber K. Science. 2007;317:190. doi: 10.1126/science.317.5835.190. [DOI] [PubMed] [Google Scholar]
- 9.Ichtchenko K, Nguyen T, Südhof TC. J. Biol. Chem. 1996;271:2676. doi: 10.1074/jbc.271.5.2676. [DOI] [PubMed] [Google Scholar]
- 10.Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC. Science. 1992;257:50. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- 11.Varoqueaux F, et al. Neuron. 2006;51:741. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Song J-Y, Ichtchenko K, Südhof TC, Brose N. Proc. Natl Acad. Sci., USA. 1999;96:1100. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Cell. 2004;119:1013. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varoqueaux F, Jamain S, Brose N. Eur J Cell Biol. 2004;83:449. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 15.Prange O, Wong TP, Gerrow K K, Wang YT, El-Husseini A. Proc Natl Acad Sci U S A. 2004;101:13915. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boucard A, Chubykin AA, Comoletti D, Taylor P, Südhof TC. Neuron. 2005;20:229. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Nam CI, Chen L. L. Proc Natl Acad Sci U S A. 2005;102:6137. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Futai K, Kim MJ, Hashikawa T, Scheiffele P, Sheng M, Hayashi Y. Nat Neurosci. 2007;10:186. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chih B, Afridi SK, Clark L, Scheiffele P. Hum Mol Genet. 2004;13:1471. doi: 10.1093/hmg/ddh158. [DOI] [PubMed] [Google Scholar]
- 20.Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. J Biol Chem. 2005;280:17312. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- 21.Chubykin AA, et al. Neuron. 2007;54:919. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamain S, et al. Nat. Genet. 2003;34:27. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laumonnier F, et al. Am. J. Hum. Genet. 2004;74:552. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan J, et al. Mol Psychiatry. 2005;10:329. doi: 10.1038/sj.mp.4001629. [DOI] [PubMed] [Google Scholar]
- 25.Comoletti D, et al. J Neurosci. 2004;24:4889. doi: 10.1523/JNEUROSCI.0468-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chubykin AA, et al. J. Biol. Chem. 2005;280:22365. doi: 10.1074/jbc.M410723200. [DOI] [PubMed] [Google Scholar]
- 27.Szatmari P, et al. Nat Genet. 2007;39:319. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durand CM, et al. Nat Genet. 2007;39:25. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubenstein JL, Merzenich MM. Genes Brain Behav. 2003;2:255. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.For Materials and methods, additional figures S1 to S14, and tables S1 and S2, see Supporting Online Materials (SOMs).
- 31.Hussman JP. J Autism Dev Disord. 2001;31:247. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- 32.Dani VS, et al. Proc Natl Acad Sci USA. 2005;102:12560. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichtchenko, et al. Cell. 1995;81:435. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 34.Uhlhaas PJ, Singer W. Neuron. 2006;52:155. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Buzsaki G, Draguhn A. Science. 2004;304:1926. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 36.Silberberg G, Grillner S, LeBeau FE, Maex R, Markram H. Trends Neruosci. 2005;28:541. doi: 10.1016/j.tins.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Somogyi P, Klausberger T. J Physiol. 2005;562:9. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.