Abstract
CTLA-4 is critical in maintaining self-tolerance but the mechanisms of its actions have been controversial. Here, we examined the antigen-specificity of tissue-infiltrating CD4+ T cells in CTLA-4−/− mice and determined the in vivo cellular targets of CTLA-4. Tissue-infiltrating CTLA-4−/− T cells exhibited TCR-dependent homing to their tissues of origin, suggesting reactivity against tissue-specific antigens. We identified the pancreas-specific enzyme Pdia2 as an autoantigen in CTLA-4−/− mice. CTLA-4 expressed either on Pdia2-specific effector cells, or on Tregs, was sufficient to control tissue destruction mediated by Pdia2-specific T cells. These results demonstrate that both cell-intrinsic and cell non-autonomous action of CTLA-4 operate in the context of regulation of an authentic self-antigen for which CTLA-4 is required to maintain tolerance.
Introduction
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a structural homologue of CD28 and a negative regulator required for T cell homeostasis and tolerance1,2. CTLA-4−/− mice develop a fatal lymphoproliferative disorder characterized by expansion of CD4+ T cells into multiple non-lymphoid tissues3–5. While the importance of CTLA-4 is clear, several aspects of its mechanism in maintaining self-tolerance are poorly understood6. Specifically, it is not clear if specific self-antigens are involved in the expansion of CD4+ T cells or in which lymphocyte populations CTLA-4 must be expressed to prevent the fatal lymphoproliferative disorder.
CTLA-4 might regulate T cell activation by several mechanisms. CTLA-4 could exert cell-intrinsic inhibitory actions by competing with CD28 for their shared ligands, B7-1 and B7-27,8, or by delivering inhibitory signals that induce cell cycle arrest and prevent IL-2 production9–11, or by limiting T cell dwell time with antigen presenting cells (APCs)12. In addition, experiments with mixed bone-marrow (BM) chimeras showed that CTLA-4−/− T cells can be controlled by wild-type BM-derived cells13, suggesting a cell-extrinsic, dominant action of CTLA-4 in promoting tolerance similar to that exerted by Tregs. Indeed, a recent study demonstrated that CTLA-4-expressing Tregs can regulate CTLA-4−/− T cells in vivo14. Although CTLA-4 is not required for all normal Treg activities in vitro15,16, it appears essential for Treg function in vivo, since Treg-specific deletion of CTLA-4 caused spontaneous development of systemic lymphoproliferation and fatal disease17. Some evidence suggests that CTLA-4 expression on Tregs acts to decrease CD80 and CD86 expression on dendritic cells17,18 but it is unclear whether the in vivo stimulatory activity of APCs is affected by CTLA-4. Thus, the cellular site of action of CTLA-4 in controlling tolerance requires further examination.
In addition, the antigen-specificity of expanding CD4+ T cells in CTLA-4−/− mice has not been examined. Specifically, it is unclear whether these T cells are reactive generally to self-MHC, reactive to ubiquitous antigens, or reactive to multiple tissue-specific antigens. Some relationship to antigen specificity has been suggested from the observation that introducing rearranged αβ-TCR transgenes onto the CTLA-4−/− background eliminates the fatal lymphoproliferation19–21. However, no direct evidence has supported the interpretation that CTLA-4−/− mice generate an antigen-specific autoimmune disease. For example, spectratype analysis of the TCRβ CDR3 from T cells expanding in CTLA-4−/− mice revealed a diverse and unbiased repertoire, and was interpreted as antigen-independent T cell activation22. Further, no reports have yet directly analyzed the antigen specificity by cloning of CD4+ T cells from CTLA-4−/− mice. However, resolving the nature of the repertoire of T cells expanding in CTLA-4−/− mice is important for two reasons. First, mutations in CTLA-4 are associated with several autoimmune diseases, including hypothyroidism and type 1 diabetes23. Second, anti-CTLA-4 treatment is a potential immunotherapeutic approach in treatment of cancer24, so it is important to determine the potential for activating antigen-specific self-reactive T cells.
To address these issues, we used a similar approach as used in analyzing the repertoire of Treg cells25–27. By crossing the DO11.10 TCRβ transgene28 onto CTLA-4−/− background to restrict TCRβ-specificity, the lethal multi-organ lymphoproliferative expansion of CD4+ T cells in CTLA-4−/− mice was maintained but the mice showed a slightly reduced rate of lethality. In analyzing the specificity of the lymphoproliferative CD4+ T cells, we show for the first time that CTLA-4−/− T cells infiltrating into peripheral non-lymphoid tissues are composed of separate populations of different tissue-specific T cells. We identified one autoantigen recognized by these tissue-infiltrating CTLA-4−/− T cells, isolated a specific TCRαβ reactive to this antigen, and examined the in vivo behavior of T cell with this specificity. Our results show that CTLA-4 expressed on the antigen-specific effector T cells greatly diminishes their pathogenicity in vivo, but that CTLA-4 expression by Tregs is sufficient to control the accumulation of self-reactive tissue-specific effector T cells in target tissues, indicating both cell-intrinsic and cell non-autonomous actions of CTLA-4.
Results
A fixed TCRβ repertoire does not eliminate lethal lymphoproliferation in CTLA-4−/− mice
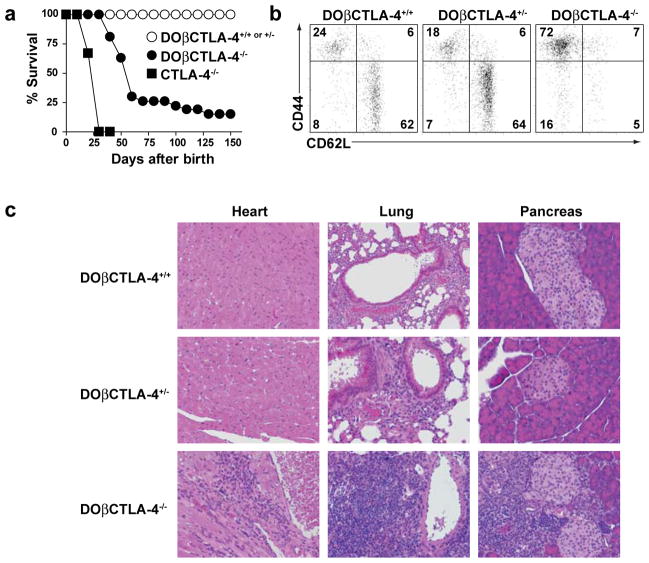
We compared CTLA-4−/− mice with CTLA-4−/− mice expressing the DO11.10 TCRβ chain (DOβ) (Fig. 1a). CTLA-4−/− mice died uniformly by 4 weeks of age, while DOβCTLA-4−/− mice showed a somewhat slower rate of lethality, with 75% of mice dying by 7 weeks of age, and 10% of mice still surviving by 10 weeks (Fig. 1a). Thus, fixing the TCRβ chain prolongs the course of disease but does not eliminate expansion of CD4+ T cells seen in CTLA-4−/− mice, allowing us to better examine their characteristics and tissue-specificity. DOβCD4+ T cells in CTLA-4−/−mice showed an activated phenotype (Fig. 1b) similar to previous reports of activation of CD4+ T cells in CTLA-4−/− mice. DOβCTLA-4+/− mice as well as DOβCTLA-4+/+ mice had a normal distribution of naïve and memory T cells, with 70% of CD4+ splenic T cells exhibiting a naïve surface phenotype (Fig. 1b). In contrast, DOβCTLA-4−/− mice had drastically reduced numbers of naïve T cells, with 60% of T cells showing an activated/memory phenotype. Finally, fixation of the TCRβ chain in CTLA-4−/− mice did not alter the multi-organ nature of disease in CTLA-4−/−mice. DOβCTLA-4−/− mice had lymphocytic infiltration in the heart, lung, and pancreas, whereas DOβCTLA-4+/− mice showed normal tissue histology (Fig. 1c).
Figure 1. DOβCTLA-4−/− mice show spontaneous T cell activation and multi-organ infiltration.
(a) Survival curve of DOβCTLA-4+/+ (n>30; ○), DOβCTLA-4+/− (n=30; ○), DOβCTLA-4−/− (n=27; ●), and CTLA-4−/− (n=9; ■) mice. (b) Expression of CD62L and CD44 on splenic CD4+ T cells from 6-wk-old DOβCTLA-4+/+, DOβCTLA-4+/− and DOβCTLA-4−/− mice. Data shown are representative of 5 mice in each group. (c) H&E-stained sections of heart, lung, and pancreas from 12-wk-old DOβCTLA-4+/+, DOβCTLA-4+/− and DOβCTLA-4−/− mice (Original magnification, x200). Data shown are representative of 3 mice in each group.
Tissue-infiltrating T cells from CTLA-4−/− mice show tissue-specific homing
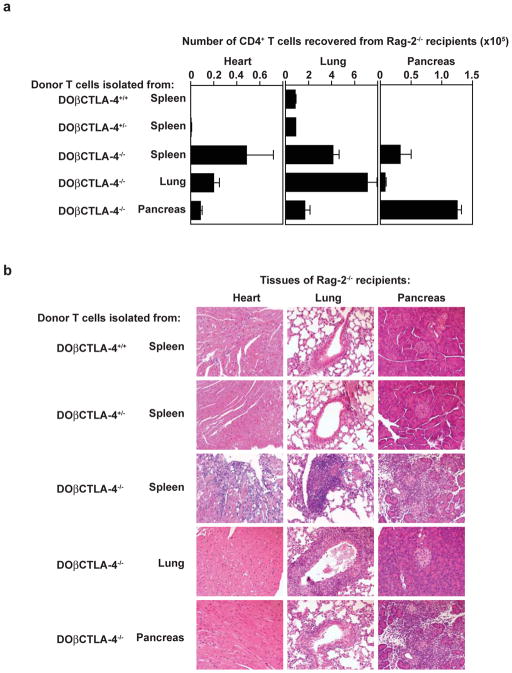
To date, tissue-infiltrating T cells from CTLA-4−/− mice have not been cloned, nor has their antigen-specificity been characterized29,30. We used DOβCTLA-4−/− mice as donors for T cells isolated from various tissues and first set out to examine their pattern of migration and expansion in Rag-2−/− recipient mice. Splenic CD4+ T cells isolated from DOβCTLA-4−/− mice, but not DOβCTLA-4+/+ or DOβCTLA-4+/− mice, showed robust expansion in vivo, and migrated into multiple organs, including the pancreas, lung, and heart (Fig. 2a). In contrast, T cells isolated from peripheral organs of DOβCTLA-4−/− mice homed selectively back to their organ of origin (Fig. 2a). For example, CD4+ T cells originally isolated from lungs of DOβCTLA-4−/−mice showed a greater expansion within lungs of recipient Rag-2−/− mice compared to the pancreas or heart. Most dramatically, CD4+ T cells isolated from the pancreas of DOβCTLA-4−/−mice showed a highly selective expansion within pancreas of recipient Rag-2−/− mice, and not the lung or heart of recipient Rag-2−/− mice.
Figure 2. Tissue-infiltrating T cells from DO β CTLA-4−/− mice cause tissue-specific inflammation.
CD4+ T cells (1×105 cells) purified from spleen of DOβCTLA-4+/+ or DOβCTLA-4+/− mice, or spleen, lung, or pancreas of DOβCTLA-4−/− mice were transferred into RAG-2−/− mice. Pancreas, lung, and heart from individual recipients were removed 3 weeks after transfer. (a) The number of CD4+ T cell recovered from pancreas, lung, and heart of each RAG-2−/− recipient mice are shown. Each bar shows the mean value for three individual mice with SD. (b) H&E-stained sections are shown of the pancreas, lung and heart from RAG-2−/− recipients that had received CD4+ T cells isolated from either spleen of DOβCTLA-4+/+ or DOβCTLA-4+/− mice, or from spleen, lung, or pancreas of DOβCTLA-4−/− mice as indicated in the figure. The results shown are representative of two independent adoptive transfer experiments.
Furthermore, the selective migration of CD4+ T cells isolated from DOβCTLA-4−/− mice was associated histologically with induction of tissue pathology (Fig. 2b). Specifically, CD4+ T cells isolated from the spleen of DOβCTLA-4−/− mice caused an intense tissue-destructive infiltration in the pancreas, heart, and lung of Rag-2−/− recipients. CD4+ T cells isolated from the lung of DOβCTLA-4−/− mice elicited a strong peribronchial infiltration with perivascular infiltration, and associated epithelial changes in the lung of Rag-2−/− recipients (Fig. 2b), but caused no changes in the pancreas or heart. Finally, CD4+ T cells isolated from the pancreas of DOβCTLA-4−/− mice caused tissue-destructive lesions of the exocrine pancreas in Rag-2−/−recipients, but selectively spared pancreatic islets, lung and heart. Tissue lesions caused by the transfer of DOβCTLA-4−/− T cells was histologically similar to those caused by CTLA-4−/− T cells (Supplementary Fig. 1), suggesting that tissue-specific homing of DOβCTLA-4−/− T cells is not an artifact introduced by transgenic DO11.10 TCRβ chain.
TCR-specificity determines tissue-specific expansion of CTLA-4−/− T cells
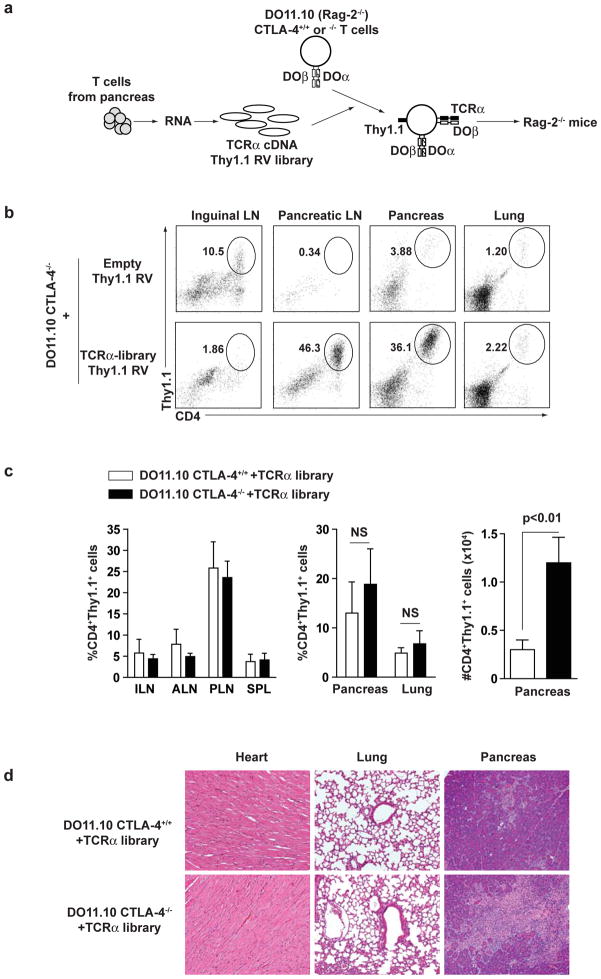
Tissue-specific homing of DOβCTLA-4−/− T cells could either be due to reactivity against tissue-specific antigens or to the selective homing properties imprinted upon tissue entry, for example by selective expression of chemokine receptors. To distinguish these possibilities, we made a TCRα retroviral library from tissue-infiltrating CD4+ T cells of DOβCTLA-4−/− mice and tested the migration of T cells expressing TCRs isolated from tissues. TCRα cDNA made from pancreatic-infiltrating T cells of DOβCTLA-4−/− mice was cloned into a retroviral vector expressing Thy 1.1 as a marker to produce a TCRα library (Fig. 3a). Naïve Rag-2−/− DO11.10 T cells (hereafter referred to as DO11.10 T cells), that either expressed or lacked CTLA-4, were infected with the complete TCRα library or with empty retrovirus (Supplementary Fig. 2), and adoptively transferred into Rag-2−/− mice. Thy1.1+ T cells expressing the TCRα library, but not the empty retrovirus, showed selective accumulation in pancreatic lymph nodes and pancreas, but not in inguinal lymph nodes or lungs (Fig. 3b). Kinetic analysis of infiltration and proliferation of these cells in pancreas and lung demonstrated that library-expressing cells infiltrated into both tissues but proliferated more robustly in pancreas, resulting in greater accumulation of these cells in pancreas (Supplementary Figure 3). The frequency of TCRα library-expressing T cells accumulated in pancreatic lymph nodes and pancreas was unaffected by the presence or absence of CTLA-4 (Fig. 3c left and middle panels). However, when the TCRα library was expressed in CTLA-4−/− T cells, we observed greater accumulation within the pancreatic tissue itself compared to library-derived CTLA-4+/+ T cells (Fig. 3c right panel). Also, library-derived CTLA-4+/+ T cells caused minimal pancreatic disease, while library-derived CTLA-4−/− T cells caused exocrine-specific tissue destruction (Fig. 3d). In summary, TCRα chains derived from pancreatic-infiltrating T cells of CTLA-4−/− mice are sufficient to confer selective pancreatic accumulation thorough tissue-specific expansion of CTLA-4−/− T cells. Furthermore, these antigen-specific T cells cause tissue injury in the absence of CTLA-4.
Figure 3. TCRs derived from pancreas-infiltrating T cells confer selective pancreatic homing.
(a) Scheme of generation of DO11.10 T cells expressing TCRα cDNA RV library. RNA was generated from pancreas-infiltrating CD4+ T cells from DOβCTLA-4−/− mice. TCRα cDNA was synthesized with TCRα-specific primer and ligated with ires-Thy1.1 RV vector to generate TCRα cDNA Thy1.1 RV library. CD4+ T cells from DO11.10 CTLA-4+/+ or DO11.10 CTLA-4−/− mice were infected with TCRα cDNA Thy1.1 RV library. The infected cells (1×106 cells) were transferred to Rag-2−/− mice. (b–d) 3 weeks after transfer, lymphoid and non-lymphoid tissues were harvested from Rag-2−/− mice. (b) The frequency of CD4+Thy1.1+ cells is shown of inguinal LN, pancreatic LN, spleen, pancreas and lung from Rag-2−/− mice transferred with DO11.10 CTLA-4−/− T cells that were infected with empty- or TCRα-library Thy1.1 RV. (c) The frequency or the number of CD4+Thy1.1+ cells is shown of inguinal LN (ILN), axillary LN (ALN), pancreatic LN (PLN), spleen (spleen), pancreas or lung from Rag-2−/− mice transferred with DO11.10 CTLA-4+/+ or DO11.10 CTLA-4−/− T cells that were infected with TCRα Thy1.1 RV library. Data show mean value for three mice with SD. (d) H&E-stained sections of heart, lung, and pancreas from Rag-2−/− mice transferred with DO11.10 CTLA-4+/+ or DO11.10 CTLA-4−/− T cells that were infected with TCRα Thy1.1 RV library. Results represent two independent experiments performed with three mice in each group.
Pdia2 is an autoantigen in CLTA-4−/− mice
CTLA-4−/− mice and DOβCTLA-4−/− mice showed intense lymphocytic infiltration of the exocrine pancreas that largely spared the pancreatic islets (Fig. 1c, and data not shown). When monitored for development of autoimmune diabetes, DOβCTLA-4−/− showed no elevation in blood glucose, but instead showed a significant reduction in blood glucose compared to controls (Supplementary Fig. 4a). In addition, when CD4+ T cells isolated from pancreas of DOβCTLA-4−/− mice were transferred into Rag-2−/− mice, we also observed significant decrease in blood glucose over time compared to mice receiving splenic CD4+ T cells isolated from DOβCTLA-4+/− mice (Supplementary Fig. 4b). These results suggested that CTLA-4−/− T cells may react to a pancreas-specific self antigen selectively expressed in acinar tissue.
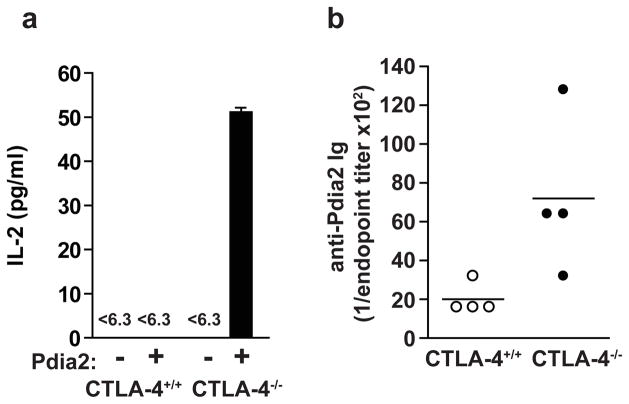
Recent studies suggested a candidate for such a self-antigen31. Non-obese diabetic (NOD) mice destroy β-cells of pancreatic islets, but NOD mice deficient in autoimmune regulator (Aire) react against pancreatic acinar cells and make autoantibodies against protein disulfide isomerase associated 2 (Pdia2), a acinar-specific enzyme31. Since DOβCTLA-4−/− mice showed acinar-restricted autoimmunity, we asked if Pdia2 was an autoantigen in CTLA-4−/− mice. T cells were isolated from the pancreatic lymph nodes of CTLA-4+/+ or CTLA-4−/− mice, and activated in vitro in the presence or absence of Pdia2 protein. T cells from CTLA-4−/− mice, but not CTLA-4+/+ mice, showed a significant response to Pdia2 (Fig. 4a). Furthermore, CTLA-4−/−, but not CTLA-4+/+ mice, showed a humoral response against Pdia2 in serum (Fig. 4b). Therefore, Pdia2 appears to be an authentic autoantigen in CTLA-4−/− mice. We tested the reactivity of CTLA-4−/− T cells against other proteins, such as carbonic anhydrase II, α-amylase, lactoferrin, or carboxypeptidase B, which have been suggested to be associated with autoimmune pancreatitis (ref). However, none of these proteins induced detectable response of CTLA-4−/− T cells (Supplementary Figure 5).
Figure 4. Pdia2 is an autoantigen in CTLA-4−/− mice.
(a) CD4+ T cells (1×105 cells) purified from pancreatic lymph node of 20-day-old CTLA-4+/+ or CTLA-4−/− mice were cultured with (+) or without (−) Pdia2 (10 μM) in the presence of irradiated splenocytes (5×105 cells). The supernatants were harvested 24 h later and IL-2 concentration was determined by ELISA. Results represent three independent experiments. (b) Serum was collected from 20-day-old CTLA-4+/+ (n=4) or CTLA-4−/− (n=4) mice and anti-Pdia2 antibody titers were determined by ELISA.
Isolation of Pdia2-specific TCRs from pancreas-infiltrating CTLA-4−/− T cells
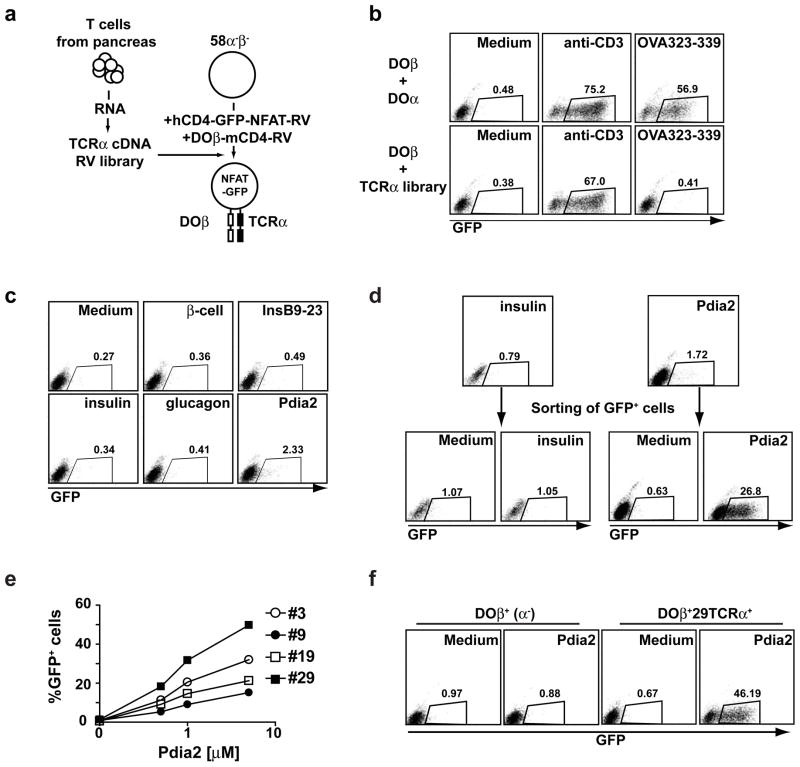
To examine how CTLA-4 regulates autoreactive T cells in vivo, we wished to isolate an authentic autoantigen-specific TCR from CTLA-4−/− mice (Fig. 5a). We modified a T cell hybridoma (58α−β −) to express the DO11.10β chain along with an NFAT-inducible GFP reporter. Pancreas-infiltrating CD4+ T cells were isolated from DOβCTLA-4−/− mice, full length TCRα cDNA generated and cloned into a modified retroviral vector, and expressed in this hybridoma. CD3+ hybridoma cells were then examined for reactivity to various antigens by assaying for the induction of GFP (Fig. 5a). As a control, when the DOα TCR was expressed, hybridoma cells reacted robustly to both to anti-CD3 stimulation and to specific ovalbumin peptide (OVA), but when the TCRα library was tested, cells responded to anti-CD3 only, and not to OVA (Fig. 5b, lower panels).
Figure 5. Isolation of Pdia2-specific TCRs from TCR α-library.
(a) Generation of T cell hybridomas expressing TCRα library. TCRα cDNA generated from pancreas-infiltrating CD4+ T cells in Figure 3 was ligated with retrovirus vector lacking ires-Thy1.1 to generate the TCRα cDNA RV library. 58α−β− hybridomas were infected with NFAT-GFP-hCD4 RV reporter and DOβ-mCD4 RV. Sorted hCD4+mCD4+ cells were infected with the TCRα cDNA library RV. CD3+ cells were sorted to obtain TCRαβ+ cells. (b) TCR-stimulation dependent GFP expression in TCR-reconstituted hybridomas. T cell hybridomas expressing DOβ/DOα or DOβ/TCRα cDNA library were cultured with anti-CD3 (1 μg/ml) or OVA323-339 (0.3 μM) in the presence of irradiated splenocytes. GFP expression was examined after 20 h. The data are representative of three experiments. (c) Identification of Pdia2-reactive population in the TCRα library. T cell hybridomas expressing DOβ/TCRα library were cultured with islet β-cells (1×105 cells), bovine insulin (10 μM), insulin B9-23 peptide (10 μM), porcine glucagon (10 μM), or recombinant Pdia2 (10 μM) in the presence of irradiated splenocytes. GFP expression was examined after 20 h. The data are representative of three experiments. (d) Enrichment of Pdia2-reactive clones. T cell hybridomas expressing DOβ/TCRα library were cultured with Pdia2 or Insulin (10 μM) in the presence of irradiated splenocytes. GFP+ cells were sorted after 20 h. 6 days later, the sorted cells were re-stimulated with Pdia2 or Insulin and GFP expression was examined after 20 h. (e) Generation of Pdia2-specific hybridoma clones. Limiting dilution was performed to generate Pdia2-specific clones. Pdia2-specific GFP expression of 4 representative clones is shown. (f) Pdia2-specific response of DOβ+29TCRα+ hybridomas. TCRα cDNA was isolated from clone #29 (29TCRα). DOβ+ cells expressing NFAT-GFP reporter were infected with retroviral 29TCRα and CD3+ cells (DOβ+29TCRα+) were sorted. DOβ+ cells or DOβ+29TCRα+ cells were cultured with medium alone or 5 μM Pdia2 and GFP expression was examined after 20 h.
First, we tested if the TCRα-hybridoma library contained specificities that react to antigens from the endocrine pancreas. No responses were seen against pancreatic β-cells, insulin, insulin peptide 9–23, or purified glucagon (Fig. 5c), consistent with the lack of diabetes in DOβCTLA-4−/− mice. However, approximately 2% of the TCRα-hybridoma library reacted against Pdia2 antigen. These hybridomas were isolated by sorting GFP+ cells after stimulation with Pdia2, and were found to retain Pdia2-reactivity after expansion (Fig. 5d). In contrast, no enrichment of insulin reactivity was observed after sorting GFP+ cells after stimulation with insulin. When these enriched Pdia2-reactive hybridomas cells were cloned by limiting dilution, they continued to exhibit Pdia2 reactivity in vitro (Fig. 5e).
One such hybridoma, clone 29, was used as a source of an authentic Pdia2-reactive TCRα chain, called 29TCRα (Supplementary Fig. 6). Expression of the 29TCRα chain in the DOβ+ hybridomas regenerated specific Pdia2-reactivity (Fig. 5f), confirming that this combination of TCRβ and TCRα chains specifies a TCR specific for an authentic autoantigen in CTLA-4−/− mice. Expression of the 29TCRα chain into naïve T cells also conferred Pdia2-specific reactivity (Supplemental Fig. 7). Empty Thy1.1 or 29TCRα expressing Thy1.1 retrovirus was infected into CTLA-4+/+ or CTLA-4−/− DO11.10 transgenic T cells. Only the expression of the 29TCRα chain, and not the empty vector, endowed T cells with the ability to undergo proliferation in response to Pdia2. In contrast empty vector and 29TCRα retrovirus left the original reactivity to ovalbumin peptide intact.
Pdia2-specific CTLA-4−/− T cells infiltrate into pancreas
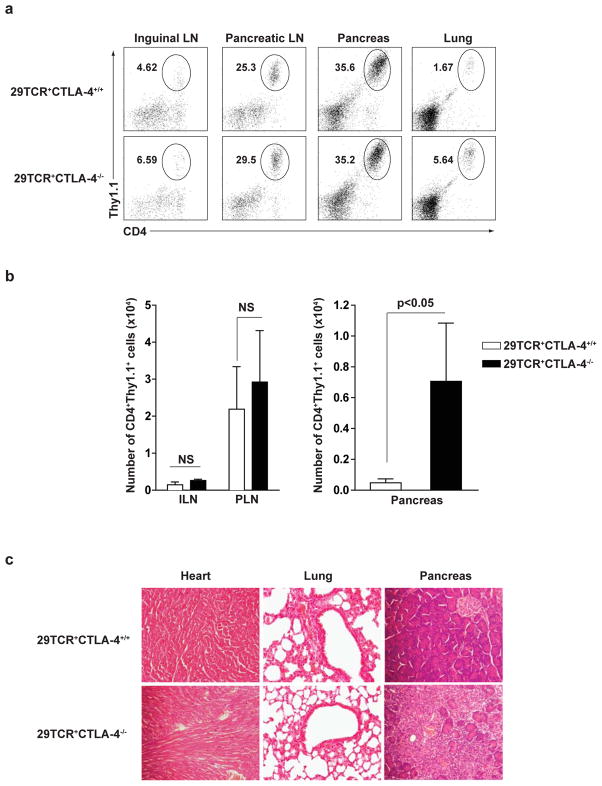
Expression of the 29TCRα chain into naive DO11.10 T cells conferred pancreatic homing as shown by the T cells originally isolated from the pancreas of CTLA-4−/− mice and as shown by T cells expressing the pancreatic TCRα library. Specifically, when the 29TCRα chain was expressed in DO11.10 T cells, and adoptively transferred into Rag-2−/− recipients, there was a significant increase in the accumulation of Thy1.1+ cells to the pancreatic lymph nodes and to pancreas, but not to the inguinal lymph nodes or lung (Fig. 6a), or to the spleen, axillary lymph nodes (not shown). Notably, CTLA-4+/+ and CTLA-4−/− 29TCRα T cells migrate equally into pancreatic lymph nodes (Fig. 6b left panels), but infiltration into the pancreas was greatly effected by the presence of CTLA-4. Approximately 10-fold more T cells infiltrated into pancreas when the 29TCRα chain was expressed in CTLA-4−/− T cells compared to CTLA-4+/+ T cells (Fig. 6b right panels). Again, the pancreatic infiltration was exocrine-specific and was not seen in the heart or lungs (Fig. 6c). Importantly, CTLA-4+/+ T cells expressing 29TCRα did not express Foxp3 in Rag-2−/− recipients (data not shown). These results were confirmed by bone marrow (BM) chimera experiments (Supplementary Figure 8). 29TCRα T cells did not develop into CD4+CD25+ cells in BM chimera irrespective of CTLA-4 expression. Again, CTLA-4+/+29TCRα T cells caused minimal pancreatitis, while CTLA-4−/−29TCRα T cells induced severe pancreatic tissue destruction. Taken together, these results suggest that CTLA-4 on the antigen-specific effector T cells diminished their pathogenicity by inhibiting their infiltration into the target tissues.
Figure 6. Pdia2-specific CTLA-4−/− T cells infiltrate into pancreas.
CD4+ T cells from DO11.10 CTLA-4+/+ or DO11.10 CTLA-4−/− mice were infected with 29TCRα Thy1.1-RV. 1×106 infected cells (29TCR+CTLA-4+/+ or 29TCR+CTLA-4−/−) were transferred to Rag-2−/− mice. 3 weeks after transfer, ILN, PLN, pancreas, and lung were harvested from recipient Rag-2−/− mice. (a) The frequency of CD4+Thy1.1+ cells in ILN, PLN, pancreas, and lung was determined. (b) The number of CD4+Thy1.1+ cells in ILN, PLN, and pancreas was shown. Each bar shows mean value for three mice with SD. (c) H&E-stained sections of heart, lung, and pancreas from the recipient Rag-2−/− mice was shown. Results are representative of two independent experiments performed with three mice in each group.
Treg cells control tissue destruction by CTLA-4−/− Pdia2 specific T cells
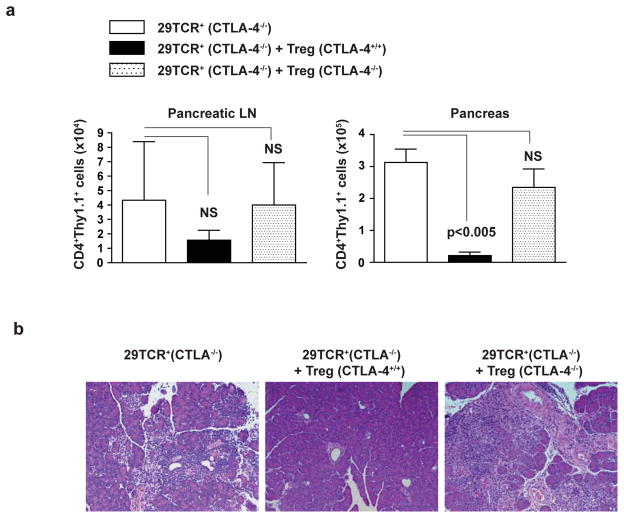
Two recent studies suggested that CTLA-4 expression by Treg cells is required for their suppressive activity17,32. To test if this is true for an authentic self-antigen specific T cells, we transferred CTLA-4−/− 29TCRα DO11.10 cells into Rag-2−/− mice with or without co-transfer of CD4+CD25+ T cells (Treg cells) isolated from CTLA-4+/+ or CTLA-4−/− mice and measured accumulation of Pdia2-specific T cells in the pancreatic lymph nodes and pancreatic tissue. Co-transfer of CTLA-4+/+ Treg cells did not alter the number of Pdia2-specific T cells that accumulated in pancreatic lymph nodes (Fig. 7a). However, co-transfer of CTLA-4+/+ Treg cells significantly reduced the number of Pdia2-specific T cells that infiltrated into the pancreas (Fig. 7a) and was associated with complete protection from destruction of pancreatic tissue elicited by CTLA-4−/− Pdia2-specific T cells (Fig. 7b). On the other hand, co-transfer of CTLA-4−/− Treg cells was completely ineffective at inhibiting infiltration of Pdia2-specific T cells into pancreas and destruction of pancreatic tissue (Fig. 7b). We confirmed in mixed BM chimera experiment that CTLA-4-expressing cells were able to control pancreatic tissue destruction by CTLA-4−/− Pdia2-specific T cells (Supplementary Figure 8). These results demonstrate that autoimmune responses by tissue-specific CTLA-4−/− T cells can be regulated by CTLA-4-expressing Tregs.
Figure 7. CTLA-4-sufficient Tregs inhibit pancreatitis induced by CTLA-4−/− Pdia2-specific T cells.
CD4+ T cells from DO11.10 CTLA-4−/− mice were infected with 29TCRα Thy1.1-RV. The infected cells (0.15×106) were transferred to Rag2−/− mice with or without 0.15×106 CD4+CD62LhiCD25+ cells from CTLA-4+/+ or CTLA-4−/− mice. 3 weeks after transfer, pancreatic LN and pancreas were harvested from the recipient Rag-2−/− mice. (a) The number of CD4+Thy1.1+ cells in pancreatic LN and pancreas was determined. Data show mean value for three mice with SD. (b) H&E-stained section of pancreas from the recipient Rag-2−/− mice was shown. Results represent two independent experiments including three mice in each group.
Discussion
At least two questions regarding CTLA-4 have remained unanswered. What is the specificity of CD4+ T cells expanding in CTLA-4−/− mice? And on what cells does CTLA-4 exert its inhibitory effects? CTLA-4−/− mice were known to develop a rapid multi-organ infiltration of CD4+ T cells, but the antigen-specificity of these T cells has not been examined. Specifically, it was unclear whether these T cells are reactive to self MHC proteins themselves, to ubiquitous antigens presented by MHC, or to particular tissue-specific antigens. Further, various evidence has suggested either a cell-autonomous action for the CTLA-4 cytoplasmic domain33,34 or cell-extrinsic (non cell-autonomous) action mediated by CTLA-4 on other cells expressing CTLA-4 ligands35,36. Resolving these distinctions is important since CTLA-4 polymorphisms have been associated with autoimmune diseases37 and CTLA-4 blockade is an emerging cancer immunotherapy24.
Some evidence has suggested that expanding CTLA-4−/− T cells are not antigen-specific22. TCR spectratyping of peripheral T cells from CTLA-4−/− and Jak3−/− mice indicated that Jak3−/− T cells had a restricted and highly biased TCR repertoire, but that CTLA-4−/− T cells had a diverse and unbiased TCR repertoire22. This was interpreted to suggest that CTLA-4−/− T cells are activated by TCR-MHC interaction involved in peripheral T cell survival or homeostasis38,39, but not necessarily by tissue-specific antigens. However, another study identified restricted TCR spectratypes in splenic CTLA-4−/− T cells40, suggesting that particular combinations of V and J segments analyzed previously were not optimal for CTLA-4−/− TCR repertoire. Neither study examined CTLA-4−/− T cells from tissues other than spleen, and may have missed effects on repertoire bias in tissue-infiltrating T cells.
Introducing the DOβ chain onto the CTLA-4−/− background did not eliminate the lethal multi-organ CD4+ T cell infiltration, but slowed disease enough to examine the specificity of tissue-infiltrating T cells. Unexpectedly, we found that T cells harvested from particular tissues, such as the pancreas, showed selective homing to their tissue of origin upon adoptive transfer into Rag-2−/− hosts. Selective homing alone does not indicate reactivity to tissue-specific antigens, since these T cells could have acquired expression of chemokine (or other) receptors that mediate such behavior. However, a TCRα retroviral library made from pancreas-infiltrating CTLA-4−/− T cells, and expressed in naive DOβ T cells, also conferred pancreas-specific expansion and accumulation, suggesting the initial homing of CTLA-4−/− T cells was TCR-dependent and antigen-specific. These observations are the first to show that tissue-infiltrating CD4+ T cells in CTLA-4−/− mice are reactive to tissue-specific antigens.
We identified Pdia2 as an autoantigen of CTLA-4−/− mice. Pdia2 was originally discovered as an autoantigen recognized by serum from Aire−/− NOD background mice31. Aire−/−NOD mice show exocrine-pancreas specific inflammation, sparing islets and not developing diabetes, similar to our findings DOβCTLA-4−/− mice. How Aire might regulate the emergence of Pdia2 reactive clones is unclear, since Pdia2 expression in thymic epithelial cells was not reduced in Aire−/−NOD mice31. Aire might act to eliminate Pdia2-reactive clones in thymus by influencing the processing and/or presentation of Pdia2, rather than its transcription41. Since thymic selection is normal in CTLA-4−/− mice5, CTLA-4 might be required to regulate Pdia2-specific T cell clones that have escaped from thymic negative selection.
Experiments using ovalbumin-reactive T cells and ovalbumin-transgenic mice42 have suggested the possibility of Treg-dependent and -independent action of CTLA-4. In contrast, our studies examined the in vivo behavior of self-reactive T cells that recognize an authentic self antigen in the setting of CTLA-4 deficiency. By cloning endogenous Pdia2-specific TCRs from pancreas-infiltrating T cells in CTLA-4−/− mice, we found that CTLA-4 expression on Pdia2-specific T cells limited their in vivo pathogenicity by two mechanisms. In the absence of Treg cells, Pdia2-reactive T effector cells specifically accumulated in pancreatic lymph nodes regardless of CTLA-4 expression, but infiltrated into the pancreas only in the absence of CTLA-4. This finding demonstrates a cell-autonomous action of CTLA-4, since CTLA-4 expression by the effector cell was able to limit tissue injury in the absence of Tregs. But in addition, in the presence of Treg cells, CTLA-4−/− Pdia2-specific T cells also were stopped from infiltrating into the pancreas. This result demonstrates a cell-extrinsic regulation of CTLA-4−/− T cells by Treg cells, as suggested by previous studies13,40.
Although CTLA-4−/− mice exhibit increased numbers of Foxp3+ cells32, these Tregs are reportedly non-functional in vivo32. Consistently, conditional deletion of CTLA-4 within Tregs causes fatal systemic lymphproliferation17. However, these findings do not rule out a cell-intrinsic action of CTLA-4 on effector cells. Indeed, CTLA-4 regulates effector T cell activation independently of Treg cells, since CTLA-4 expression in effector cells in Treg-specific CTLA-4 deficient mice can significantly delay fatality of CTLA-4−/− mice17. A recent study in tumor model also demonstrated that CTLA-4 blockade not only on Treg cells but also effector T cells is required for maximal antitumor activity (ref). Our results add to this, since CTLA-4 expression on Pdia2-specific T cells is sufficient to prevent tissue infiltration in the absence of Treg cells.
In summary, we have shown that CTLA-4−/− T cells infiltrating to the peripheral tissues are reactive to tissue-specific self antigens. By examining the behavior of an authentic self-reactive T cell in the presence and absence of CTLA-4, and the presence and absence of Tregs, we have shown that CTLA-4 controls pathogenicity of antigen-specific autoreactive T cells by both cell-autonomous actions in effector T cells and by cell non-autonomous mechanisms in Treg cells.
Methods
Mice
DO11.10 TCR transgenic43, DOβ transgenic28 and CTLA-4−/− mice5 were previously described. CTLA-4−/− mice were backcrossed to BALB/c background. Rag-2−/− (H-2d) and BALB/c mice were purchased from Taconic. DO11.10 mice were bred onto a Rag-2−/− background. All animal studies were approved by the Washington University animal study committee.
Antigens
Islet β-cells were purified from BALB/c pancreas as described previously44. Bovine insulin and porcine glucagon were purchased from Sigma. Pdia2 cDNA was cloned by PCR from BALB/c splenic cDNA, subcloned into the pET28a expression vector (Stratagene), and expressed as a six-histidine fusion protein in E. coli BL21 (DE3)-RIP (Stratagene). The following primers were used for cloning: 5′ Pdia2-BamHI, GCCCAGGGATCCATGGACAAGCAG; and 3′ Pdia2-XhoI, ATTCTCGAGTCCCAATGGCTACAGCTCCTCCT.
T cell preparation and Flow Cytometry
Heart, lung, and pancreas were minced and incubated at 37°C for 30 min with 0.25 mg/ml collagenase B (Roche) and 30 U/ml DNaseI (Sigma). Digested pancreas was further incubated with trypsin/EDTA at 37°C for 10 min. Lymphocytes were collected by percoll gradient purification and CD4+ T cell number was determined by FACS staining with anti-mouse CD4-FITC (Caltag), anti-mouse CD45.2-APC (eBioscience), and anti-mouse Thy1.1-PE (eBioscience). For Ki-67 staining, cells were stained surface markers followed by fixation and permeabilization (eBioscience) and incubated with anti-human Ki-67 (BD Bioscience). CD4+ T cells for adoptive transfer were purified using CD4 MACS microbeads (Miltenyi Biotec). For Treg preparation, CD4+CD62LhiCD25+ T cells were sorted from spleens of BALB/c mice or CTLA-4−/− mice by staining with anti-mouse CD25-PE (BD Biosciences), anti-mouse CD62L (Caltag), and anti-mouse CD4-PE-Cy7 (BD Biosciences). FACS data were collected on a FACSCallibur or FACSCanto II (BD Biosciences) and were analyzed with FlowJo software (Tree Star).
Detection of IL-2
CD4+ T cells (1×105 cells) purified from pancreatic lymph nodes of 20-day-old CTLA-4+/+ or CTLA-4−/− mice and cultured with or without 10 μM Pdia2 in the presence of irradiated BALB/c splenocytes (5×105 cells). The culture supernatants were harvested 24 h later and IL-2 concentration was determined using Mouse IL-2 ELISA set (BD Bioscience).
Determination of Pdia2-specific Ig titers
Serum was collected from 20-day-old CTLA-4+/+ or CTLA-4−/− mice and Pdia2-specific antibody responses were measured by ELISA against plate-coated Pdia2 using serial dilution of serum and HRP-conjugated goat anti-mouse Ig secondary antibodies (Southern Biotech). The pdia2-specific antibody titers are presented as the greatest serum dilution that provided an average optical density exceeding 1.5-fold over the average background optical density at 405 nm.
Adoptive transfer
Purified CD4+ T cells (1×105 cells) from spleen, lung, or pancreas of DOβCTLA-4−/− mice, or DO11.10 T cells (1×106 cells) that were infected with retrovirus were injected i.v. into Rag-2−/−mice. Recipient mice were sacrificed 3 weeks after of the injection. Spleen, lymph nodes, heart, lung, and pancreas were removed to count infiltrating T cells and for histological analysis.
Histology
Mouse organs were fixed with 10% buffered formalin and paraffin embedded. The sections were stained with H&E and examined under a microscope.
TCRα library and retroviral constructs
RNA from pancreas-infiltrating CD4+ T cells in DOβCTLA-4−/− mice was isolated using RNeasy kit (Qiagen). cDNA was synthesized using the SMART cDNA Library Construction kit (Clontech) and a Cα primer specific for the constant region of the TCRα chain (5′-ATCCAGGTGGGATTGTGAATCAGGGCCAAC-3′). Full length TCRα cDNA was amplified with 5′PCR primer and Sfi-Cα PCR primer (5′-TAGGCCGAGGCGGCCAACCAGACCCCAGACAGC-3′). PCR products were digested with SfiI, cloned into the MSCV retrovirus vector (RV) with or without ires-Thy1.1, and transduced into XL-10 Gold (Stratagene) for a library transcript complexity of 2×105. MSCV RV lacking ires-Thy1.1 was generated by removing ires-Thy1.1 cassette from MSCV ires-Thy1.1 RV (a gift from W. Sha) by digestion with EcoRI, followed by blunting with Vent polymerase and religation. The retroviral reporter, human CD4 (hCD4)-pA-GFP-NFAT-RV was constructed by replacing the GFP-IL-2p cassette of RV hCD4-pA-GFP-IL-2p45 with the GFP-NFAT cassette from pKS-NFAT-GFP46 (a gift from M. Iwashima). DOβ-ires-murine CD4 (mCD4)-RV was constructed as follows. First, mCD4 was PCR cloned from splenic cDNA. GFP cDNA in ires-GFP-RV47 was replaced with mCD4 to create ires-mCD4-RV. Then, DOβ was excised from DOβ-ires-GFP-RV48 and subcloned into ires-mCD4-RV. DOα-RV was created by PCR cloning of DOα cDNA using DOα-ires-hCD4-RV48 as the template and ligation of DOα into MSCV RV lacking ires-Thy1.1.
Retrovirus infection
Retroviral vectors were transfected into Phoenix E cells as described previously49 and viral supernatants were collected 2 days later. T cell hybridomas were infected with the viral supernatants in the presence of 2 μg/ml of polybrene by spin infection at 2500 rpm for 30 min. CD4+ T cells purified from DO11.10 mice were activated by plate coated anti-CD3 and anti-CD28 mAb. On days 1 and 2, fresh viral supernatant was added and spun at 2500 rpm for 90 min in the presence of 6 μg/ml of polybrene. Infected CD4+ T cells were harvested on day5 for adoptive transfer.
Bone marrow chimera
BM cells were harvested from DO11.10 or DO11.10 CTLA-4−/− mice 2 days after injection of 150 μg/g 5FU (Sigma). BM cells were cultured for 2 days in the presence of 6 ng/ml murine IL-3, 10 ng/ml murine IL-6, and 100 ng/ml murine stem cell factor. BM cells were then spin-infected with viral supernatants in the presence of 6μg/ml of polybrene for 90 min at 2500 rpm. Recipient Rag-2−/− mice received a total of 480 rads of whole-body radiation and then were injected intravenously with 0.5×106 infected BM cells with or without 0.5×106 BM cells freshly isolated from BALB/c mice. Mice were sacrificed 6 weeks after BM transfer.
Generation of T cell hybridomas and assay
The 58α−β − hybridoma cell line50 was first infected with hCD4-pA-GFP-NFAT-RV and hCD4+ cells were sorted by MoFlo (DakoCytomation) 2 days after infection. The cells were next infected with DOβ-ires-mCD4 RV and mCD4+ cells were sorted. Finally, the cells were infected with a retroviral TCRα library. CD3+ cells were sorted to obtain TCRαβ+ cells. The TCR-reconstituted hybridomas (2.5×104 cells) were cultured with the indicated antigens and irradiated splenocytes (5×105 cells) in 96-well round bottom plates for 20 h. GFP expression on the hybridomas was analyzed by FACS. Retrovirus-specific inserts were recovered from cDNA of hybridomas by PCR as described previously49.
Supplementary Material
Reference List
- 1.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 2.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 3.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 4.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 5.Chambers CA, et al. Thymocyte development is normal in CTLA-4-deficient mice. Proc Natl Acad Sci US A. 1997;94:9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scalapino KJ, Daikh DI. CTLA-4: a key regulatory point in the control of autoimmune disease. Immunological Reviews. 2008;223:143–155. doi: 10.1111/j.1600-065X.2008.00639.x. [DOI] [PubMed] [Google Scholar]
- 7.vanderMerwe PA, et al. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. Journal of Experimental Medicine. 1997;185:393–403. doi: 10.1084/jem.185.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostrov DA, et al. Structure of murine CTLA-4 and its role in modulating T cell responsiveness. Science. 2000;290:816–819. doi: 10.1126/science.290.5492.816. [DOI] [PubMed] [Google Scholar]
- 9.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. Journal of Experimental Medicine. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. Journal of Experimental Medicine. 1998;188:205–210. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider H, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 13.Bachmann MF, et al. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous. Journal of Immunology. 1999;163:1128–1131. [PubMed] [Google Scholar]
- 14.Friedline RH, et al. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. Journal of Experimental Medicine. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Q, et al. Distinct roles of CTLA-4 and TGF-beta in CD4(+)CD25(+) regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka H, et al. CD25(+)CD4(+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. International Immunology. 2005;17:421–427. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 17.Wing K, et al. CTLA-4 control over Foxp3(+) regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 18.Onishi Y, et al. Foxp3(+) natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez VL, et al. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 20.Waterhouse P, et al. Normal thermic selection, normal viability and decreased lymphoproliferation in T cell receptor-transgenic CTLA-4-deficient mice. European Journal of Immunology. 1997;27:1887–1892. doi: 10.1002/eji.1830270811. [DOI] [PubMed] [Google Scholar]
- 21.Chambers CA, Kuhns MS, Allison JP. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4(+) T cell responses. Proc Natl Acad Sci US A. 1999;96:8603–8608. doi: 10.1073/pnas.96.15.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gozalo-Sanmillan S, et al. Cutting edge: Two distinct mechanisms lead to impaired T cell homeostasis in Janus kinase 3-and CTLA-4-deficient mice. Journal of Immunology. 2001;166:727–730. doi: 10.4049/jimmunol.166.2.727. [DOI] [PubMed] [Google Scholar]
- 23.Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 24.Peggs KS, et al. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Current Opinion in Immunology. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh CS, et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh CS, et al. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nature Immunology. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 27.Pacholczyk R, et al. Origin and T cell receptor diversity of Foxo3(+)CD4(+)CD25(+) T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Shinkai Y, et al. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993;259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 29.Genetics of autoimmunity. Novartis Foundation Symposium; Chichester, UK: John Wiley & Sons; 2005. p. 267. [Google Scholar]
- 30.Immunological tolerance. Novartis Foundation Symposium; Chichester, UK: John Wiley & Sons; 1998. p. 215. [Google Scholar]
- 31.Niki S, et al. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. Journal of Clinical Investigation. 2006;116:1292–1301. doi: 10.1172/JCI26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt EM, et al. CTLA-4 Controls Regulatory T Cell Peripheral Homeostasis and Is Required for Suppression of Pancreatic Islet Autoimmunity. Journal of Immunology. 2009;182:274–282. doi: 10.4049/jimmunol.182.1.274. [DOI] [PubMed] [Google Scholar]
- 33.Yin L, Schneider H, Rudd CE. Short cytoplasmic SDYMNM segment of CD28 is sufficient to convert CTLA-4 to a positive signaling receptor. Journal of Leukocyte Biology. 2003;73:178–182. doi: 10.1189/jlb.0702365. [DOI] [PubMed] [Google Scholar]
- 34.Hueber AJ, et al. CTLA-4 lacking the cytoplasmic domain costimulates IL-2 production in T-cell hybridomas. Immunology and Cell Biology. 2006;84:51–58. doi: 10.1111/j.1440-1711.2005.01402.x. [DOI] [PubMed] [Google Scholar]
- 35.Paust S, et al. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci US A. 2004;101:10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 37.Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol Rev. 2005;204:102–115. doi: 10.1111/j.0105-2896.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 38.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 39.Seddon B, et al. Long-term survival but impaired homeostatic proliferation of naive T cells in the absence of p56(lck) Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 40.Tivol EA, Gorski J. Re-establishing peripheral tolerance in the absence of CTLA-4: complementation by wild-type T cells points to an indirect role for CTLA-4. Journal of Immunology. 2002;169:1852–1858. doi: 10.4049/jimmunol.169.4.1852. [DOI] [PubMed] [Google Scholar]
- 41.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Eggena MP, et al. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. Journal of Experimental Medicine. 2004;199:1725–1730. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 44.Shimizu J, Kanagawa O, Unanue ER. Presentation of beta-cell antigens to CD4+ and CD8+ T cells of non-obese diabetic mice. Journal of Immunology. 1993;151:1723–1730. [PubMed] [Google Scholar]
- 45.Zhu H, et al. Unexpected characteristics of the IFN-gamma reporters in nontransformed T cells. Journal of Immunology. 2001;167:855–865. doi: 10.4049/jimmunol.167.2.855. [DOI] [PubMed] [Google Scholar]
- 46.Ohtsuka M, et al. NFAM1, an immunoreceptor tyrosine-based activation motif-bearing molecule that regulates B cell development and signaling. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8126–8131. doi: 10.1073/pnas.0401119101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouyang W, et al. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9:745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 48.Berenson LS, et al. Selective requirement of p38 alpha MAPK in cytokine-dependent, but not antigen receptor-dependent, Th1 responses. Journal of Immunology. 2006;176:4616–4621. doi: 10.4049/jimmunol.176.8.4616. [DOI] [PubMed] [Google Scholar]
- 49.Sedy JR, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 50.Backstrom BT, et al. A motif within the T cell receptor alpha chain constant region connecting peptide domain controls antigen responsiveness. Immunity. 1996;5:437–447. doi: 10.1016/s1074-7613(00)80500-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.