Abstract
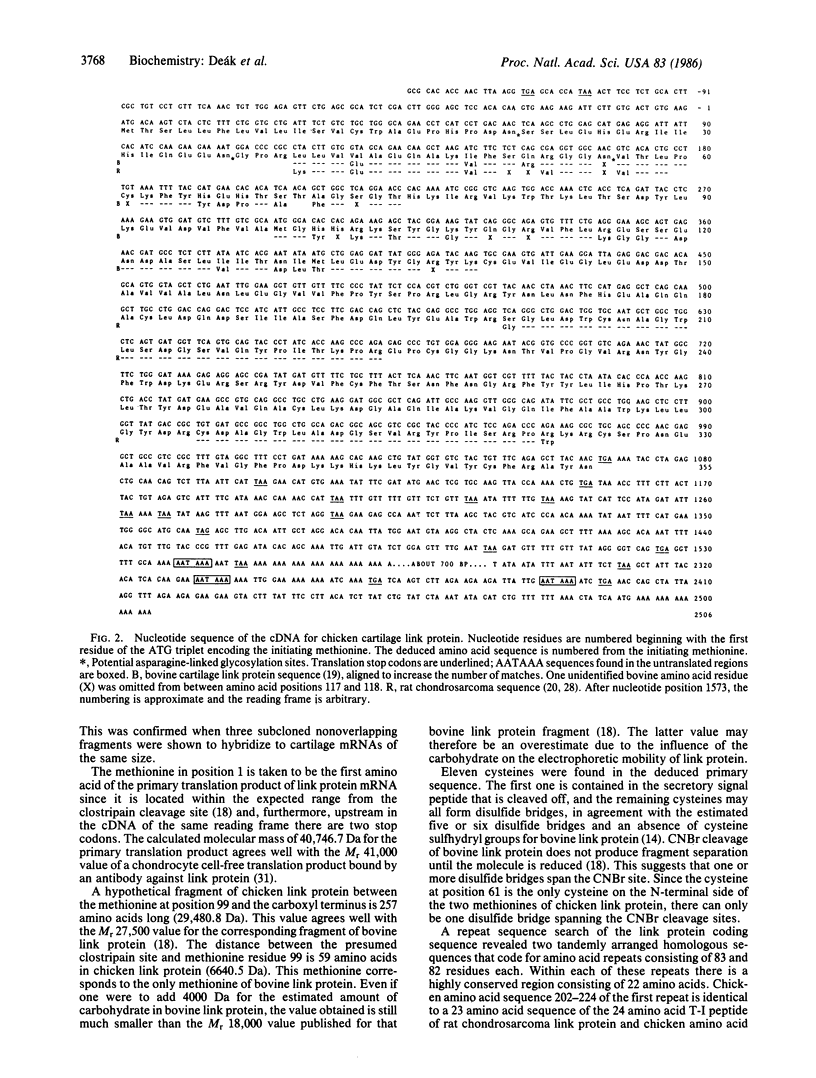
cDNA clones coding for chicken cartilage link protein were isolated and sequenced. The DNA sequence for the entire core polypeptide of the mature link protein and the predicted signal peptide consists of 1065 nucleotides. The deduced primary translation product (355 amino acids) has a molecular mass of 40.7 kDa; the calculated molecular mass of the mature link protein core polypeptide (340 amino acids) is 39.06 kDa. The DNA sequence contains two tandemly arranged repeat sequences that may code for repeated functional domains of link protein involved in binding to hyaluronic acid. The mRNAs for chicken link protein are 6.0, 5.8, and 3.0 kilobase pairs, and the difference between the sizes of the RNA species lies in the 3' untranslated region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. R., Caterson B. The isolation and characterization of the link proteins from proteoglycan aggregates of bovine nasal cartilage. J Biol Chem. 1979 Apr 10;254(7):2387–2393. [PubMed] [Google Scholar]
- Buckwalter J. A., Rosenberg L. C., Tang L. H. The effect of link protein on proteoglycan aggregate structure. An electron microscopic study of the molecular architecture and dimensions of proteoglycan aggregates reassembled from the proteoglycan monomers and link proteins of bovine fetal epiphyseal cartilage. J Biol Chem. 1984 May 10;259(9):5361–5363. [PubMed] [Google Scholar]
- Caputo C. B., MacCallum D. K., Kimura J. H., Schrode J., Hascall V. C. Characterization of fragments produced by clostripain digestion of proteoglycans from the Swarm rat chondrosarcoma. Arch Biochem Biophys. 1980 Oct 1;204(1):220–233. doi: 10.1016/0003-9861(80)90027-2. [DOI] [PubMed] [Google Scholar]
- Caterson B., Baker J. R. The link proteins as specific components of cartilage proteoglycan aggregates in vivo. Associative extraction of proteoglycan aggregate from swarm rat chondrosarcoma. J Biol Chem. 1979 Apr 10;254(7):2394–2399. [PubMed] [Google Scholar]
- Caterson B., Baker J. The interaction of link proteins with proteoglycan monomers in the absence of hyaluronic acid. Biochem Biophys Res Commun. 1978 Feb 14;80(3):496–503. doi: 10.1016/0006-291x(78)91596-6. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Deák F., Argraves W. S., Kiss I., Sparks K. J., Goetinck P. F. Primary structure of the telopeptide and a portion of the helical domain of chicken type II procollagen as determined by DNA sequence analysis. Biochem J. 1985 Jul 1;229(1):189–196. doi: 10.1042/bj2290189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. D. Multiple aggregation factors in cartilage proteoglycan. Biochem J. 1973 Jun;133(2):383–386. doi: 10.1042/bj1330383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. Proteoglycans: their structure, interactions and molecular organization in cartilage. Biochem Soc Trans. 1981 Dec;9(6):489–497. doi: 10.1042/bst0090489. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
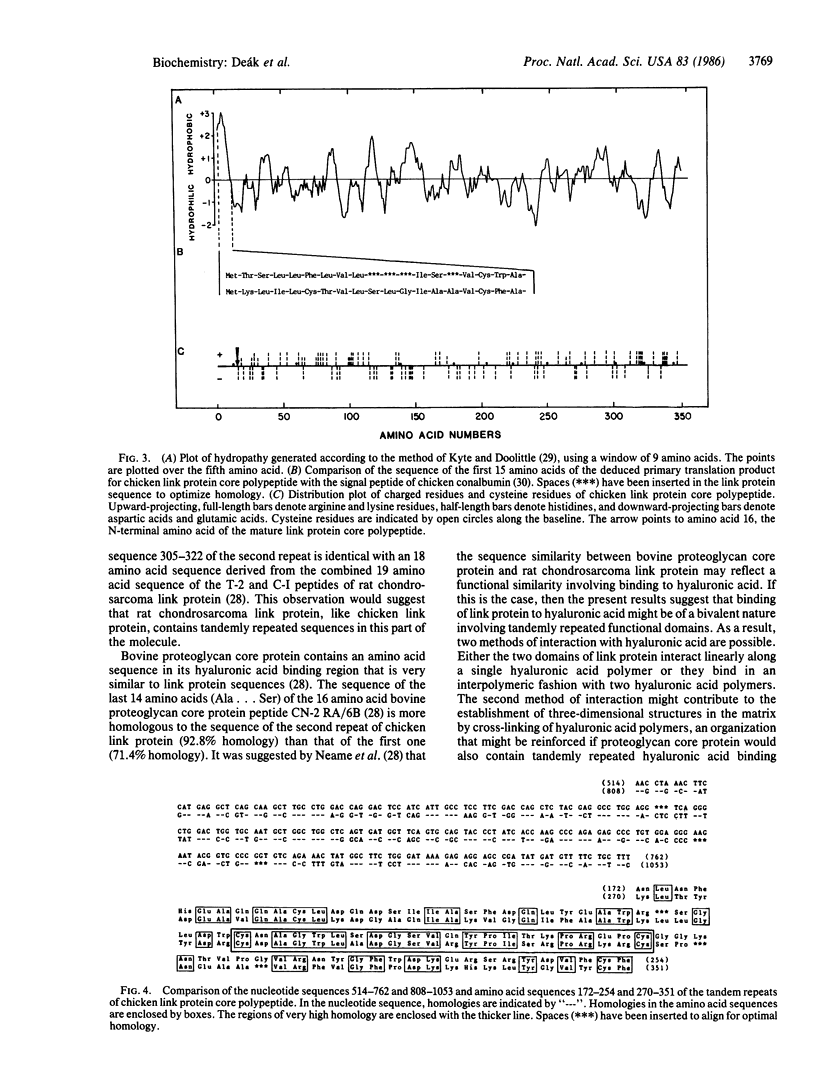
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Le Glédic S., Périn J. P., Bonnet F., Jollès P. Identity of the protein cores of the two link proteins from bovine nasal cartilage proteoglycan complex. Localization of their sugar moieties. J Biol Chem. 1983 Dec 25;258(24):14759–14761. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Velleman S. G., Goetinck P. F. The independent synthesis and secretion of cartilage proteoglycan and link protein by embryonic chicken chondrocytes. J Biol Chem. 1983 Sep 10;258(17):10779–10785. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- Neame P. J., Périn J. P., Bonnet F., Christner J. E., Jollès P., Baker J. R. An amino acid sequence common to both cartilage proteoglycan and link protein. J Biol Chem. 1985 Oct 15;260(23):12402–12404. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Brown M., Dziewiatkowski D. D. The link protein in proteoglycan aggregates from the Swarm rat chondrosarcoma. J Biol Chem. 1977 Sep 25;252(18):6470–6477. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Périn J. P., Bonnet F., Pizon V., Jollès J., Jollès P. Structural data concerning the link proteins from bovine nasal cartilage proteolycan complex. FEBS Lett. 1980 Oct 6;119(2):333–336. doi: 10.1016/0014-5793(80)80283-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Electron microscopic studies of proteoglycan aggregates from bovine articular cartilage. J Biol Chem. 1975 Mar 10;250(5):1877–1883. [PubMed] [Google Scholar]
- Royal P. D., Sparks K. J., Goetinck P. F. Physical and immunochemical characterization of proteoglycans synthesized during chondrogenesis in the chick embryo. J Biol Chem. 1980 Oct 25;255(20):9870–9878. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. H., Rosenberg L., Reiner A., Poole A. R. Proteoglycans from bovine nasal cartilage. Properties of a soluble form of link protein. J Biol Chem. 1979 Oct 25;254(20):10523–10531. [PubMed] [Google Scholar]
- Thibodeau S. N., Lee D. C., Palmiter R. D. Identical precursors for serum transferrin and egg white conalbumin. J Biol Chem. 1978 Jun 10;253(11):3771–3774. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadwell B. V., Mankin D. P., Ho P. K., Mankin H. J. Cell-free synthesis of cartilage proteins: partial identification of proteoglycan core and link proteins. Biochemistry. 1980 May 13;19(10):2269–2275. doi: 10.1021/bi00551a043. [DOI] [PubMed] [Google Scholar]