Abstract
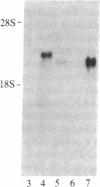
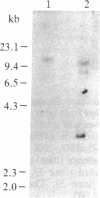
The expression of human placental-type alkaline phosphatase (ALPase) in the placenta and in three choriocarcinoma cell lines was examined by translation in vitro and RNA blot analysis using a cDNA for placental ALPase. Placental RNA directed the synthesis of two polypeptides that could be immunoprecipitated with antiserum to placental ALPase. Translation of RNA from the choriocarcinoma cell lines, with or without sodium butyrate treatment, yielded a single immunoprecipitable product of molecular weight intermediate between those of the products from the placenta mRNA. Two cDNA clones for placental ALPase were isolated by antibody screening of a placental cDNA library constructed in lambda gt11. The overlapping cDNAs include 462 nucleotides of coding sequence. RNA blot analysis has confirmed that induction of placental-type ALPase levels during placental development is accompanied by an increase in steady-state placental ALPase mRNA concentrations. Examination of the mRNAs revealed a placental ALPase mRNA of 3.0 kilobases (kb) and a distinct choriocarcinoma placental-type ALPase mRNA of 2.6 kb, implying that transformation of normal to malignant trophoblast is associated with the expression of a distinct placental-type ALPase gene transcript and its protein product.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bielinska M., Grant G. A., Boime I. Processing of placental peptide hormones synthesized in lysates containing membranes derived from tunicamycin-treated ascites tumor cells. J Biol Chem. 1978 Oct 25;253(20):7117–7119. [PubMed] [Google Scholar]
- Billadello J. J., Roman D. G., Grace A. M., Sobel B. E., Strauss A. W. The nature of post-translational formation of MM creatine kinase isoforms. J Biol Chem. 1985 Dec 5;260(28):14988–14992. [PubMed] [Google Scholar]
- Boime I., Szczesna E., Smith D. Membrane-dependent cleavage of the human placental lactogen precursor to its native form in ascites cell-free extracts. Eur J Biochem. 1977 Mar 1;73(2):515–520. doi: 10.1111/j.1432-1033.1977.tb11345.x. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Wada H. G., Sussman H. H. The plasma membrane of human placenta. Isolation of microvillus membrane and characterization of protein and glycoprotein subunits. J Biol Chem. 1976 Jul 10;251(13):4139–4146. [PubMed] [Google Scholar]
- Darnell R. B. Independent regulation by sodium butyrate of gonadotropin alpha gene expression and cell cycle progression in HeLa cells. Mol Cell Biol. 1984 May;4(5):829–839. doi: 10.1128/mcb.4.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Edlow J. B., Ota T., Relacion J. R., Kohler P. O., Robinson J. C. Enzymes of normal and malignant trophoblast: phosphoglucose isomerase, phosphoglucomutase, hexokinase, lactate dehydrogenase, and alkaline phosphatase. Am J Obstet Gynecol. 1975 Mar 1;121(5):674–681. doi: 10.1016/0002-9378(75)90472-x. [DOI] [PubMed] [Google Scholar]
- Ezra E., Blacher R., Udenfriend S. Purification and partial sequencing of human placental alkaline phosphatase. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1076–1083. doi: 10.1016/s0006-291x(83)80252-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fishman L., Miyayama H., Driscoll S. G., Fishman W. H. Developmental phase-specific alkaline phosphatase isoenzymes of human placenta and their occurrence in human cancer. Cancer Res. 1976 Jul;36(7 Pt 1):2268–2273. [PubMed] [Google Scholar]
- Fishman W. H., Inglis N. I., Stolbach L. L., Krant M. J. A serum alkaline phosphatase isoenzyme of human neoplastic cell origin. Cancer Res. 1968 Jan;28(1):150–154. [PubMed] [Google Scholar]
- Fishman W. H. Perspectives on alkaline phosphatase isoenzymes. Am J Med. 1974 May;56(5):617–650. doi: 10.1016/0002-9343(74)90631-7. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Sussman H. H. Structual comparison of ectopic and normal placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2936–2940. doi: 10.1073/pnas.70.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Ito F., Chou J. Y. Biosynthesis and processing of placental alkaline phosphatase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):611–618. doi: 10.1016/0006-291x(83)90350-9. [DOI] [PubMed] [Google Scholar]
- Ito F., Chou J. Y. Induction of placental alkaline phosphatase biosynthesis by sodium butyrate. J Biol Chem. 1984 Feb 25;259(4):2526–2530. [PubMed] [Google Scholar]
- Jemmerson R., Shah N., Fishman W. H. Evidence for homology of normal and neoplastic human placental alkaline phosphatases as determined by monoclonal antibodies to the cancer-associated enzyme. Cancer Res. 1985 Jul;45(7):3268–3273. [PubMed] [Google Scholar]
- Millán J. L., Stigbrand T. Antigenic determinants of human placental and testicular placental-like alkaline phosphatases as mapped by monoclonal antibodies. Eur J Biochem. 1983 Oct 17;136(1):1–7. doi: 10.1111/j.1432-1033.1983.tb07697.x. [DOI] [PubMed] [Google Scholar]
- Millán J. L., Stigbrand T., Ruoslahti E., Fishman W. H. Characterization and use of an allotype-specific monoclonal antibody to placental alkaline phosphatase in the study of cancer-related phosphatase polymorphism. Cancer Res. 1982 Jun;42(6):2444–2449. [PubMed] [Google Scholar]
- Nakayama T., Yoshida M., Kitamura M. L-leucine sensitive, heat-stable alkaline-phosphatase isoenzyme detected in a patient with pleuritis carcinomatosa. Clin Chim Acta. 1970 Nov;30(2):546–548. doi: 10.1016/0009-8981(70)90152-x. [DOI] [PubMed] [Google Scholar]
- Neuwald P. D., Anderson C., Salivar W. O., Aldenderfer P. H., Dermody W. C., Weintraub B. D., Rosen S. W., Nelson-Rees W. A., Ruddon R. W. Expression of oncodevelopmental gene products by human tumor cells in culture. J Natl Cancer Inst. 1980 Mar;64(3):447–459. [PubMed] [Google Scholar]
- Neuwald P. D., Brooks M. Altered form of placental alkaline phosphatase produced by JAR choriocarcinoma cells in culture. Cancer Res. 1981 May;41(5):1682–1689. [PubMed] [Google Scholar]
- PIERCE G. B., Jr, MIDGLEY A. R., Jr THE ORIGIN AND FUNCTION OF HUMAN SYNCYTIOTROPHOBLASTIC GIANT CELLS. Am J Pathol. 1963 Aug;43:153–173. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Sakiyama T., Robinson J. C., Chou J. Y. Characterization of alkaline phosphatases from human first trimester placentas. J Biol Chem. 1979 Feb 10;254(3):935–938. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeg K. V., Jr, Azizkhan J. C., Stromberg K. Characteristics of alkaline phosphatase from two continuous lines of human choriocarcinoma cells. Exp Cell Res. 1977 Mar 1;105(1):199–205. doi: 10.1016/0014-4827(77)90166-5. [DOI] [PubMed] [Google Scholar]
- Sussman H. H., Bowman M., Lewis J. L., Jr Placental alkaline phosphatase in maternal serum during normal and abnormal pregnancy. Nature. 1968 Apr 27;218(5139):359–360. doi: 10.1038/218359a0. [DOI] [PubMed] [Google Scholar]
- Sussman N. L., Seetharam S., Blaufuss M. C., Alpers D. H. Translation of rat intestinal RNA yields two alkaline phosphatases. Biochem J. 1986 Mar 15;234(3):563–568. doi: 10.1042/bj2340563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesna E., Boime I. mRNA-dependent synthesis of authentic precursor to human placental lactogen: conversion to its mature hormone form in ascites cell-free extracts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1179–1183. doi: 10.1073/pnas.73.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trépanier J. M., Seargeant L. E., Stinson R. A. Affinity purification and some molecular properties of human liver alkaline phosphatase. Biochem J. 1976 Jun 1;155(3):653–660. doi: 10.1042/bj1550653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]