Abstract
Overwhelming evidence has linked cardiovascular disease and osteoporosis, but the shared root cause of these two diseases of the elderly remains unknown. Low levels of high-density lipoprotein cholesterol (HDL) and bone mineral density (BMD) are risk factors for cardiovascular disease and osteoporosis respectively. A number of correlation studies have attempted to determine if there is a relationship between serum HDL and BMD but these studies are confounded by a number of variables including age, diet, genetic background, gender and hormonal status. Collectively, these data suggest that there is a relationship between these two phenotypes, but that the nature of this relationship is context specific. Studies in mice plainly demonstrate that genetic loci for BMD and HDL co-map and transgenic mouse models have been used to show that a single gene can affect both serum HDL and BMD. Work completed to date has demonstrated that HDL can interact directly with both osteoblasts and osteoclasts, but no direct evidence links bone back to the regulation of HDL levels. Understanding the genetic relationship between BMD and HDL has huge implications for understanding the clinical relationship between CVD and osteoporosis and for the development of safe treatment options for both diseases.
Keywords: High density lipoprotein cholesterol, bone mass, genetics, pleiotropy
1.1 INTRODUCTION
It has long been understood that cardiovascular disease (CVD) and osteoporosis may be linked [1, 2]. As the American population ages, the incidence of both of these diseases is expected to increase [3, 4]. Lifestyle factors such as smoking, lack of exercise, and eating a high fat diet all increase risk for both conditions [5, 6]. Diagnosis of CVD is associated with increased risk of hip fracture [7, 8] and similarly, studies have suggested that low bone mass in women may be an independent predictor of CVD [9]. Women diagnosed with osteoporosis are at approximately a four fold increased risk of suffering a cardiovascular event and this risk is independent of other CVD risk factors. This risk of a cardiovascular event increases with osteoporosis severity, but appears to be independent of the frailty associated morbidities seen with severe osteoporosis [10]. The common root cause of these two diseases is not completely understood.
Serum lipids have long been known to be associated with risk for CVD. Specifically, low levels of high density lipoprotein – cholesterol (HDL) are associated with increased risk of negative cardiac events [11]. While HDL has many functions that are collectively anti-atherogenic, the best-known function of HDL is its role in reverse cholesterol transport. In reverse cholesterol transport, HDL removes cholesterol from the peripheral tissues and transports it to liver. In addition, certain subclasses of HDL have been described as having anti-oxidant and anti-inflammatory properties, both of which likely contribute to the anti-atherogenic effects of HDL [12].
It has been hypothesized that there is relationship between serum HDL levels and bone mineral density (BMD). The aim of this review is to more closely examine the existing evidence supporting or contradicting the hypothesis that these two factors are correlated and/or genetically co-regulated. First, a summary of epidemiological studies investigating the relationship between serum HDL and BMD is presented. A discussion of the covariates that may be influencing the relationship between these two phenotypes and a summary of the data demonstrating the direct interaction between HDL and osteoblasts and osteoclasts follows. Lastly, genetic loci associated with both serum HDL and BMD have been extensively mapped in mouse models and the high degree of co-mapping of loci for these two phenotypes in mice is demonstrated.
2.1 ASSOCIATION BETWEEN HDL AND BONE MINERAL DENSITY
A number of studies have tested for correlation between serum HDL and BMD in human subjects [13–31], as are summarized in Table 1. There is no single consensus that can be reached after reviewing these studies regarding the correlative relationship between HDL and BMD. For a variety reasons, these studies are difficult to compare to one another. First, there are fundamental differences in the compositions of the study cohorts with regards to ethnicity and race of the subjects. It is well understood that BMD is different among various races, with African American's having higher BMD than other racial groups. Ethnicity, a broader term that collectively refers to environmental factors such as cultural practices, diet, activity levels, sunlight exposure etc, as well as genetic factors associated with race may actually have a larger effect on bone than genetics alone [32]. Similarly, HDL is affected by both racial and ethinic differences [33]. The studies presented in Table 1 reflect a larger number of racially and ethnically different populations. A large study of Swedish women (6886 subjects) found a negative correlation between HDL and BMD, but two large studies of Korean women suggest that the correlation is positive, at least in post-menopausal women and three additional large studies found no correlation at all. Thus, ethnic differences may in part account for the lack of consensus findings among these studies. Second, there are significant differences in analysis methods used in the studies in Table 1 as there is no consistent inclusion of covariates in the models. This is discussed further below. Third, many of the studies listed in Table 1 are very small and likely suffer from a lack of power. This raises questions about the validity and interpretability of the findings from these smaller studies. For example, 5 studies have examined HDL and BMD in Korean subjects [13–16, 26]. Two studies, both of which contained over 1000 subjects found a positive relationship between HDL and BMD in post-menopausal women [13, 14]. However no relationship or only a weak negative relationship was found in post-menopausal women in the three much smaller studies [15, 16, 26].
Table 1.
Relationship between serum HDL-C and BMD.
| Study cohort | Relationship between HDL-C and BMD | Ref. |
|---|---|---|
| 1,234 pre- and 931 post-menopausal Korean women | Positive association between HDL-C and lumbar spine BMD in post-menopaual women. | [13] |
|
| ||
| 4,613 pre- and 2,661 post-menopausal Korean women | Positive association between HDL-C and lumbar spine BMD in post-menopaual women. | [14] |
|
| ||
| 295 Korean men and 166 woman | Positive association between HDL-C and BMD in men | [15] |
|
| ||
| 399 post-menopausal Korean women | Weak negative association between HDL-C and lumbar spine and femoral neck BMD | [16] |
|
| ||
| 289 Caucasian Spanish men (64±9 years of age) | No association between HDL-C and lumbar spine and femoral neck BMD Half of subjects had hypercholesterolemia |
[17] |
|
| ||
| 620 men and 635 post-menopausal Dutch women (65–88 years of age) | Both men and women in the highest quartile of HDL-C had significantly lower BMD of the calcaneus compared to the lowest quartile (as measured by quantitative ultrasound) | [18] |
|
| ||
| 107 post-menopausal Turkish women | No difference in serum HDL-C levels in osteoporotic versus non-osteoporotic patients | [19] |
|
| ||
| 224 pre-, 273 post-menopausal Australian women | Negative association between HDL-C and total hip, femoral neck and whole body BMD in pre-menopausal and post-menopausal women on HRT. | [21] |
|
| ||
| 101 osteoporotic and 72 normal post-menopausal Italian women | HDL was significantly higher in osteoporotic women compared to non-osteoporotic controls. | [22] |
|
| ||
| 465 men and 448 women from the United Kingdom | Negative association between HDL-C lumbar spine BMD and total femoral BMD in women and between HDL-C total femoral BMD in men. | [23] |
|
| ||
| 1176 post-menopausal Danish women | No association between HDL-C and BMD of either the lumbar spine or total hip | [24] |
|
| ||
| 7137 men, 4585 pre- and 2248 post-menopausal women from China | No association between HDL-C and whole body BMC in any group | [25] |
|
| ||
| 375 pre- and 355 post-menopausal Korean women | No association between HDL-C and BMD at lumbar spine or hip | [26] |
|
| ||
| 13,592 American men and women | Positive relationship between HDL and BMD in crude analysis. No association between HDL-C and BMD after adjustment for age, sex and menopausal status |
[27] |
|
| ||
| 717 women and 265 men from Italy | Negative associations with HDL and whole body BMD in women (not significant after correction for %Fat, lean mass and age). Negative association between HDL and hip BMD in men, even after correction | [28] |
|
| ||
| 2830 women and 2170 men from Taiwan | Negative association between HDL-C and BMD of the wrist in whole cohort. No association when cohort is divided by sex and menopausal status. | [29] |
|
| ||
| 214 post-menopausal Japanese women | Positive association between HDL-C and BMD of the lumbar spine and radius | [30] |
|
| ||
| 6886 Swedish women | Negative association between HDL-C and BMD of the wrist regardless of hormonal status. | [31] |
BMD and HDL are affected by factors such as age, gender and menopausal status. The vast majority of the subjects included in the studies done to date were over the age 45. None of the studies reported have examined the relationship between these two factors in cohorts of young healthy adults and no studies have looked at longitudinal changes in BMD and HDL. The three largest studies suggest that there is no association between BMD and HDL in men [25, 27, 29], however, the relationship in women is less clear as several studies have suggested that estrogen status affects this relationship. In sum, there does appear to be an association between HDL and BMD, but this relationship is strongly context specific and the existing data is insufficient to determine the specifics of this relationship.
2.2 ESTROGEN, HDL AND BMD
It is well understood that incidence of CVD increases dramatically in women after menopause [34]. Furthermore, BMD begins to fall and bone resorption rates increase in women during the peri-menopausal period [35]. Both the rise in CVD and the decline in BMD at menopause are thought, at least in part, to be due to a loss of estrogen. Estrogen has direct physiological effects on both serum lipid metabolism and on the regulation of bone resorption (reviewed in [34, 36]). Hormone replacement therapy (HRT) is associated with an increase in both HDL and BMD in post-menopausal women [34, 37]. Thus, differences in estrogen status could alter the relationship between BMD and HDL in women.
Of the studies presented in Table 1, six studies included both pre- and post-menopausal women. In two studies, both conducted in Korea, a positive association between HDL and BMD was noted in post-menopausal women, whereas no association between these two factors was observed in pre-menopausal women [13, 14]. In contrast, Cui et al, found no association between HDL and BMD, regardless of menopausal status in a very small study, also of Korean women [26]. Wu et al. found a negative correlation between HDL when considering their entire study cohort consisting of Taiwanese men and women, but this significant association was lost when the study cohort was subdivide based on sex and menopausal status [29]. Hsu et al, found no association between HDL and bone mineral content (BMC) in either pre- or post-menopausal women from China [25], but it must be noted that BMD and BMC, while related, are different phenotypes. Areal BMD, the density measured most commonly in human subjects, is the amount of mineral present in bone corrected for by the projected area or size of bone. Bone mineral content is simply the amount of mineral present in the region of interest. While Hsu et al. did account for subject height in their analysis, skeletal size was not accounted for [25], making it difficult to compare these findings with the studies in which BMD was measured.
Three studies specifically took use of HRT into account in their analysis. In the study by Makovey et al, a negative association between HDL and BMD was noted in both post-menopausal women taking HRT and in pre-menopausal women, whereas no association was found in the post-menopausal women not on HRT [21]. In the study by Lidfelt et al., a negative association between HDL and BMD was noted in pre-menopausal women as well as post-menopausal women, regardless of HRT use [31]. Lastly, in the large study by Solomon et al., after correction for hormonal status, no association was found between serum HDL and BMD [27]. The two large studies of Korean women suggest that in menopausal status affects the relationship between BMD and HDL, at least in certain ethnic backgrounds, however it cannot be determined at this time if it is estrogen per se affects this association.
2.3 GENE BY ENVIRONMENT INTERACTIONS
It has been estimated that up to 85% of the variance in BMD can be explained by heritable factors and the heritability estimates for serum HDL levels range from between 40 and 60% [38, 39]. Genetic differences in the study cohorts are a likely source of the lack of concordance among the studies examining the relationship between HDL and BMD [27]. Furthermore, studies have demonstrated gene-environment interactions for both BMD [40–42] and HDL levels [43–46]. Some key variables which have been implicated in gene-environment interactions, such as smoking, were not accounted for in some of the smaller studies listed in Table 1, but were used in other studies, which may also explain the lack of consensus among these studies [24, 46]. Lastly, some of the studies listed in Table 1 are small, and are therefore too underpowered to account for the combination of genetic and environmental influences on this relationship.
2.4 THE USE OF RODENT MODELS TO BETTER UNDSTAND THE CORRELATION BETWEEN BMD AND HDL
A number of methods have been developed to measure serum and plasma levels of HDL. The gold standard is to use the ultracentrifugation method. This method is very labour intensive and requires large sample sizes, making this method undesirable for use in large high-throughput studies or in studies where there is a limited sample volume. A number of direct methods for measuring HDL have been developed, but not all of these techniques can be used with mouse samples. For example, a number of studies have now suggested that the PEGME method yields unreliable results in samples taken from hyperlipidemic mice [47] whereas HDL measured by the Polymedco/Denka method shows good correlation with measurements made by ultracentrifugation [48]. The Denka method has been reliably used by a variety of investigators to identify genetic loci associated with this phenotype in mice [48].
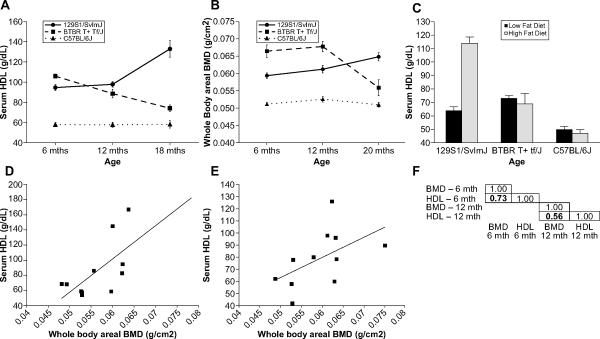
In studies using animal models, environmental variables such as diet can be can be controlled for, and in rodent models, genetic background can be held relatively constant. A large number of strain surveys examining serum lipids and BMD have been conducted in mice and these data can be used to more closely examine the effect of genetic background on the response of HDL and BMD to age and diet. Data for three genetically distinct strains of mice is presented here to highlight the importance of considering age, diet and genetic background. Age does not affect either serum HDL levels or BMD in C57BL/6J mice (Fig 1A and B), the most commonly used strain of laboratory mice (The mouse phenome database project data sets: Yuan3 and Ackert1, [49],). Both serum HDL levels (P>0.001) and BMD (P=0.0017) decrease with age in BTBR T+ Tf/J mice when comparing 6 month old mice to 18 month old mice (Fig 1A and B). However, when comparing 6 month old mice to 12 month old mice of this strain, a significant decrease is observed in HDL (P = 0.003) whereas no difference in BMD is observed (P=0.626), demonstrating that the rate and pattern of age associated changes in these two phenotypes is important to consider. In 129S1/SvImJ mice, both serum HDL and BMD increase with age (P>0.001, Fig 1A and B). Similarly in humans, different ethic populations show different patterns in age related changes in HDL [50–53] further supporting the hypothesis that genetic background affect age related changes in HDL. In a separate strain survey, the impact of dietary fat on serum HDL was examined in a variety of inbred strains (The mouse phenome database project data set: Paigen 2, [49]). Serum HDL increases dramatically in 129S1/SvImJ mice fed a high fat diet (P<0.001) whereas there was no change in this phenotype in either BTBR T+ Tf/J (P=0.624) or C57BL/6J (P=0.47, Fig 1C). While data is not available with regards to the impact of dietary fat intake on BMD in all three of these strains, studies have demonstrated strain specific responses to dietary fat intake with regards to BMD [40]. In summary, in these three strains of mice, three completely different patterns of genetic background by diet and genetic background by age interactions are obvious for HDL. Furthermore, the timing of age related changes in HDL may or may not coincide with age related changes in BMD.
Figure 1. Serum HDL and bone mineral density (BMD) in inbred strains of mice.
Data for a variety of phenotypes in inbred strains of mice can be found in the Mouse Phenome Database [49]. A. Serum HDL data for female C57BL/6J, BTBR T+ Tf/J and 129S1/SvImJ mice was accessed from Project Yuan3. In this longitudinal study, mice were fed a standard low fat diet and serum HDL was measured at 6, 12 and 18 months of age using the Polydmedco/Denka Seiken methodology. B. Whole body areal BMD (aBMD) data from female C57BL/6J, BTBR T+ Tf/J and 129S1/SvImJ mice was accessed from Project Ackert1. In this cross-sectional study, mice were fed a standard low fat diet and aBMD was measured by dual X-ray absorptiometry (DXA) at 6, 12 and 20 months of age. The data in the Yuan3 and Ackert1 projects were collected by the Jackson Aging Centre and the mice were housed under similar environmental conditions. C. Serum HDL data for female C57BL/6J, BTBR T+ Tf/J and 129S1/SvImJ mice was accessed from Project Paigen2. In this study, mice were fed a standard rodent chow up to the age at which baseline HDL was measured (between 7 and 10 weeks of age). The mice were then placed on a high-fat, high-cholesterol atherogenic diet (pga.jax.org/athdiet.html) for 17 weeks and HDL was measured again in the same mice. D. A scatterplot plot comparing HDL versus BMD is presented using strain average data for the female mice from the 6 month old age cohort from the Yuan3 and Ackert1 datasets. Only those strains in which genetic loci for BMD and or HDL had been mapped are presented. E. A scatterplot plot comparing HDL versus BMD is presented for the same strains as in D, but using data from the 12 month old cohort. F. The correlation between the BMD and HDL data from the Yuan3 and Ackert1 datasets for the 6 and 12 months cohorts. Only strains used in genetic mapping studies were used in the correlations.
Both serum HDL and BMD are heritable complex traits, meaning that multiple genes regulate these two phenotypes. A large number of genetic loci have been mapped for both of these phenotypes in mice in a number of independent studies. A more complete description of these genetic mapping efforts is discussed below. The Yuan3 and Ackert1 phenotype data sets contain HDL and BMD phenotype data for many of the strains used to map the genetic loci associated with these two phenotypes. For both of these data sets, the mice were fed the same low fat diet and were raised in the same animal facility. Each mouse of a particular inbred strain can be essentially considered an identical twin of every other mouse of that strain, but each strain of mice is, to a certain degree, genetically distinct from other inbred strains [54]. Thus, there is minimal variation in measures of phenotypes that are largely genetically regulated. By correlating the strain average BMD and HDL data, we can determine if genetic background affects the relationship between these two phenotypes. As is presented in Figure 1D and 1F, at 6 months of age, there is a positive correlation between HDL and BMD when examining the inbred strains used to map genetic loci for these two phenotypes. Similarly, a positive, albeit weaker, correlation between HDL and BMD is observed in when comparing the 12 month old mice of the same strains (Figure 1E and 1F). This suggests that strains of mice trending towards high BMD tend to have higher serum HDL levels. An example strain would be MRL/MpJ (MRL). At 12 months of age, female MRL mice have the highest BMD of the strains examined in the Ackert1 data set and have one of the highest HDL measures in the Yuan3 dataset. Given that lower BMD and lower HDL levels are both associated with increased risk for osteoporotic fracture and CVD respectively, this positive correlation is not unexpected [4, 10, 38]. However, as both of these phenotypes are polygenetic, there will be strains of mice that do not follow the pattern of high BMD being associated with high HDL because of the collection of alleles inherited for both phenotypes. For example, at 6 months of age PWD/PhJ female have high serum HDL, but do not have high BMD. Strain survey data, such as these data sets, are useful for choosing strains for the study of single phenotypes as well as for the study of putatively co-regulated phenotypes. The problem with strain surveys is that there are a limited number of possible allelic combinations available for study. Future studies need to be undertaken in which the relationship between BMD and HDL is examined in genetically diverse mouse strains, such as the collaborative cross [55].
3.1 INTERACTION BETWEEN HDL AND OSTEOBLASTS AND OSTEOCLASTS
Increasingly, it has been appreciated that HDL can act directly on osteoblasts and osteoclasts in bone. Studies using osteoblast-like cells lines have suggest that these cells are able to internalize and degrade certain subclasses of HDL particles. In addition, these cells express scavenger receptor class B type I (SR-B1), scavenger receptor class B type II (SR-BII) and CD36 cell surface receptors, which are involved in the selective uptake of cholesterol esters from HDL in other cell types. It has been demonstrated that osteoblasts are indeed capable of selective uptake of cholesterol esters from HDL, but is unclear if this uptake is mediated by the SR-BI, SR-BII or CD36 receptors [56]. In atherosclerosis, hyperlipidemia is associated with an accumulation of LDL, and the subsequent oxidation of this LDL in the subendothelial matrix of arterial walls. Some studies have suggested that HDL can inhibit this lipid oxidation, but the mechanism is not completely clear (reviewed in [12]). Brodeur and collegues have shown that oxidized LDL can induce apoptosis of osteoblasts and that this effect can be abrogated by the addition of HDL [57]. In osteoclasts, the removal of cholesterol from these cells by HDL particles can induce apoptosis whereas the delivery of cholesterol via low-density lipoprotein cholesterol (LDL) increases osteoclast survival [58]. Scarb1, the gene that codes for both SR-BI and SR-BII, appears to also be expressed in the osteoclast (biogps.gnf.org).
The HDL particle is composed of a variety of proteins, fat-soluble vitamins and steroid esters [59]; many of the components of HDL have been shown to directly affect bone metabolism. Specifically, fatty-acid esters of estrogen are known to be a component of HDL [60]. These steroid esters can be taken up by cells from HDL via SR-BI receptors and converted to free steroids [60, 61]. It is well appreciated that estrogen has direct and important functions in basic bone biology and that decreased levels of estrogen with age are a key component of age related loss of bone mass [36]. It is unclear if HDL associated estrogen esters are involved in bone metabolism. Fat soluble vitamins such as Vitamin E (alpha-tocopherol and delta-tocopherol) [59] and vitamin K (phylloquinone and menaquinone) [62] are also key components of HDL. Application of Vitamin E to calvarial osteoblasts in cultures results in an inhibition of early stage osteoblast maturation [63]. While the epidemiological data gathered thus far has not resolved the relationship between serum levels of Vitamin K and bone mass, in vitro studies have suggested that Vitamin K increases mineralization by the osteoblast and decreases expression by the osteoblast of the pro-osteoclast maturation factor, RANKL [64]. There is insufficient data at this to time to determine if HDL particles regulate bone formation and resorption by the delivery of physiologically relevant compounds to the bone micro-environment.
4.1 PLEIOTROPY
Pleiotropy, by definition, means that one gene affects multiple phenotypes. By extension, it can be assumed that a mutation or polymorphism in a gene with pleiotropic function would be associated with the coincident change in two or more phenotypes [65]. The pleiotropic effect of a gene could be either via direct action on two or more phenotypes, which is true pleiotropy, or indirect in action [66]. In the case of HDL and BMD, a truly pleiotropic gene would in some way directly alter the levels of HDL in the serum by changing production or clearance of HDL and simultaneously affect bone mass. Thus, for a true pleiotropic gene, it can be assumed that HDL particles themselves do not alter the formation or resorption of bone and no hormones or other factors secreted from or released from the bone in any way regulate HDL levels. This seems highly unlikely given that HDL is known to interact directly with both osteoblasts and osteoclasts, as was described in the previous section. An example of indirect model would be one in which some metabolic factor is carried to the bone directly by HDL and taken up by an osteoblast, which causes a change in osteoblast function. In this model, a polymorphism in a gene that regulates serum HDL levels would be observed to change bone mass, but the bone mass is only changed because the levels of HDL are changed. This model appears more plausible based on the available data.
3.2 EVIDENCE FOR SHARED GENETIC REGUALTION OF HDL AND BMD
As has already been described, both HDL and BMD are highly heritable traits. A number of genes have been examined for both HDL and BMD independently. In Table 2, several genes are presented for which a genetic association between serum HDL has been reported in the literature and in separate studies, a role in basic bone biology and or a genetic association with BMD has been reported. This list is not a comprehensive listing of gene associated with BMD and HDL but rather is a listing of genes for which there is multiple lines of evidence supporting the hypothesis that the gene regulates both phenotypes. It is unclear at this time how many, if indeed any, of these genes are truly pleiotropic.
Table 2.
Genes associated with both HDL and BMD.
| Gene symbol | Gene name | Evidence of genetic association | Ref. |
|---|---|---|---|
| ABCG8 | ATP-binding cassette, subfamily G (WHITE), member 8 | Was identified as a candidate gene for a mouse HDL QTL on Chromosome 17. Polymorphisms are associated with serum HDL levels in human subjects. Abcg8−/− mice have higher BMD. |
[85–88] |
|
| |||
| APOE | apolipoprotein E |
Apoe−/− mice have increased BMD. Well-established genetic association with HDL in humans. Inconsistent association between APOE polymorphisms and BMD and fracture in humans. |
[38,79,89–93] |
|
| |||
| ESR1 | estrogen receptor 1 (alpha) | Is one of two receptors for estrogen. Loss of estrogen at menopause is associated with increased osteoclast activity. Ers1 knockout male mice exhibit higher BMD and higher serum HDL levels. |
[36,94–96] |
|
| |||
| GHRH | growth hormone releasing hormone | Signals for release of growth hormone. Growth hormone acts both directly and indirectly on bone. Patients with growth hormone deficiency have low levels of HDL and have low bone mass. Polymorphisms in the GHRH gene are associated with proximal femur and spinal BMD. |
[97–99] |
|
| |||
| IL6 | interleukin 6 |
Il6−/− mice have decreased BMD. Polymorphisms associated with both HDL and BMD in human subjects. |
[100–108] |
|
| |||
| MTHFR | 5,10-methylenetetrahydrofolate reductase | Mutations in this gene causes hyperhomocysteinemia, a risk factor for both CVD and osteoporosis. Polymorphisms in this gene are associated with both HDL and BMD in human subjects. |
[109–113] |
|
| |||
| PON1 | paraoxonase 1 | Numerous studies have linked polymorphisms in this gene with HDL levels. Polymorphism in this gene may be associated with femoral neck and spine BMD in humans. Other studies have suggested no association with BMD, but association with fracture. |
[38,114,115] |
|
| |||
| PPARG | peroxisome proliferator activated receptor gamma |
Pparg+/− mice have higher bone mass. Polymorphisms in PPARG interact with dietary fat to affect BMD in humans. The common C161T and Pro12Ala polymorphisms are associated with HDL levels in humans. |
[40,116–121 ] |
|
| |||
| TNF | tumor necrosis factor |
Tnf−/− mice have increased bone mineral density. Polymorphisms in this gene are associated with both HDL and BMD in human subjects. |
[122–127] |
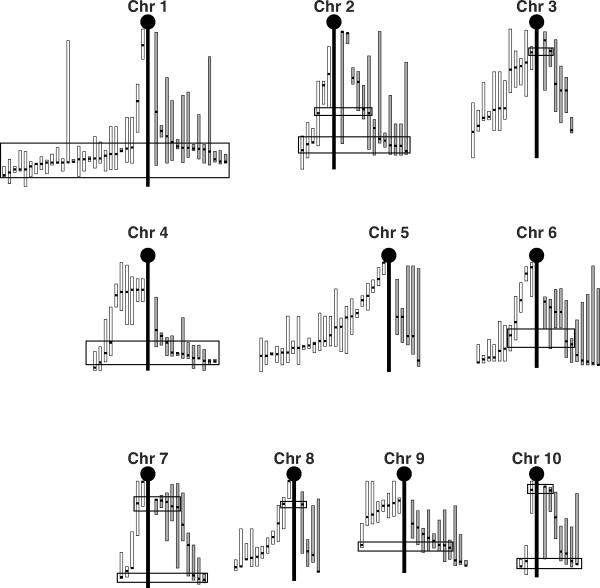
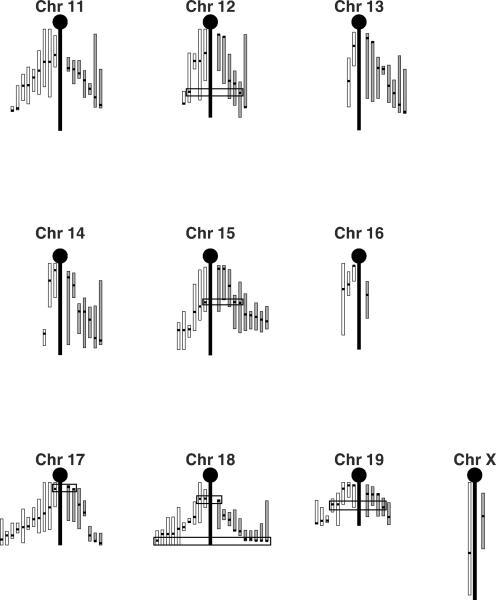
The mouse genome is 95% identical to the human genome, making mice an excellent animal model for the study of human diseases [67, 68]. A quantitative trait locus (QTL) is a region of the genome that is associated with a given quantitative trait such that a gene or genes located at that locus regulate the trait of interest [69]. QTL mapping in mice has been a successful method for determining the mechanisms of genetic regulation for a variety of complex traits. Both BMD and HDL are polygenic traits and a large number of QTLs have been mapped for both phenotypes [70–78]. In Figure 2, the known QTL for these two phenotypes are presented. It can quickly be seen in this figure that there are a number of regions wherein the peak location for QTLs for these two phenotypes co-map. Some of the BMD and HDL QTLs were mapped in the same set of mice. Where the peak location of QTLs for BMD and HDL map very near to each other, it cannot be determined, with the existing data, if the QTLs are co-mapping/ linked QTLs (i.e. QTLs for which the underlying genes map to locations very near to each other) or if these QTLs are indeed caused by pleiotropic genes. It is highly unlikely that these QTLs are mapping together purely by chance. It is much more likely that there are at least some loci wherein the underlying gene co-regulates both phenotypes either directly or indirectly. Preliminary studies suggested that Apoe may be just such a gene. The Apoe−/− mice have increased BMD and increased bone formation, but have decreased serum HDL levels [79, 80]. However, studies in humans have shown that, while polymorphism in APOE are associated with changes in serum lipids, they are not similarly associated with changes in BMD when the two phenotypes were examined in the same cohort. The authors concluded from their data that the association between serum lipids and osteoporosis was indirect in nature [24]. It is also apparent from this figure that there are loci that are private to HDL and loci private to BMD. This could explain why strains like PWD/PhJ do not follow high BMD equals high HDL relationship seen in other strains as, in any given inbred strain of mice, the final the phenotype observed for any complex polygenic trait will reflect the sum of all of the alleles inherited for that trait. The existence of private loci does not negate the possibility that there is a genetic relationship between HDL and BMD, but rather emphasizes that both BMD and HDL are regulated by multiple mechanisms and that only some of these mechanisms are shared between BMD and HDL.
Figure 2. Quantitative trait loci (QTL) for HDL and BMD.
The literature was searched for the known HDL and BMD QTL mapped in mice [70–78]. In total 155 BMD QTL and 175 HDL QTL were identified. These QTL are plotted as follows: The central vertical black bar represents the chromosomal backbone. The HDL QTL are drawn on the left of the chromosome (white bars) and the BMD QTL are drawn on the right (grey bars). For each QTL, the peak location is indicated by a horizontal thick black tick mark and the vertical white or grey bars represent the 95% confidence intervals (CI). The CI are presented to scale using the literature listed CI when available. When no CI was listed, a conservative 20 cM interval was drawn to each side of the peak location, or to the top or bottom of the chromosome if that was closer. Black rectangles highlight locations of concordant peak locations when comparing the HDL and BMD QTL. For chromosomes for which there is little agreement among the crosses with regards to peak location for HDL (i.e. Chromosome 5), no attempt to identify concordant BMD-HDL QTL peaks was made.
5.1 CONCLUSIONS AND FUTURE DIRECTIONS
As summarized, a number of correlation studies have attempted to determine if there is a relationship between serum HDL and BMD. The human studies serve to emphasize that this relationship is confounded by a number of variables including age, diet, genetic background, gender and hormonal status. Collectively, this data suggests that there is a relationship between these two phenotypes, but that the nature of this relationship is context specific. Most of the study cohorts examined to date consisted of postmenopausal women. We have very little knowledge about the relationship between HDL and BMD in younger, healthy adults. Furthermore, we have insufficient data about the importance of co-morbidities and environmental factors such as diet in human and how they influence the relationship between BMD and HDL. While it is temping to suggest that bigger studies or studies of selected cohorts are needed, the specifics with regards to who to study and what to consider in the analysis models remain unclear.
Studies in mice, in which environment, genetics and age can be controlled, are proving to be very helpful to identify how HDL and BMD are related. The examination of inbred strains of mice clearly demonstrates that dietary fat affects HDL in a strain specific manner. Using mice to determine which co-factors should be included in a candidate gene association study has previously been successfully used to find genes associated with BMD [40] and animal models will be very helpful to identify the environmental factors that influence the relationship between BMD and HDL. Studies in mice plainly demonstrate that genetic loci for BMD and HDL co-map and transgenic mouse models have been used to show that a single gene can affect both serum HDL and BMD. A number of centers worldwide are currently involved in broadly phenotyping the mice generated by International Knockout Mouse Consortium and plans are underway to establish additional phenotyping centers. Already, exciting unknown gene functions and novel disease models have been identified as part of this endeavor. Undoubtedly, this effort will identify new genes associated with both bone mass and with serum lipids, which will increase our understanding of the mechanisms by which these two phenotypes are associated. Numerous candidate genes for HDL and BMD have been identified in genome wide association studies (GWAS) of human subjects [39, 70, 81, 82]. While there is a well-recognized need to study pleiotropy at the genome wide association level, the statistical methods and analysis tools for this type of work are still in development [83, 84].
In sum, there is sufficient evidence to conclude that BMD and HDL are genetically linked, but additional studies in human subjects will be complicated due to the large number of factors that affect this relationship and new tools will have to be developed for these studies. Work completed to date has demonstrated that HDL can interact directly with both osteoblasts and osteoclasts, but no direct evidence links bone back to the regulation of HDL levels. Understanding the genetic relationship between BMD and HDL has huge implications for understanding the clinical relationship between CVD and osteoporosis and for the development of safe treatment options for both diseases.
ACKNOWLEDGEMENTS
This publication was made possible by grant number AR060234 from the National Institution of Health (NIH): National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). The author would like to thank Mr. J. Hammer for his assistance in preparation of figures.
REFERENCES
- [1].Elkeles A. Studies on osteoporosis. Br Med J. 1968;4:582. doi: 10.1136/bmj.4.5630.582-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderson JB, Barnett E, Nordin BE. The Relation between Osteoporosis and Aortic Calcification. Br J Radiol. 1964;37:910–2. doi: 10.1259/0007-1285-37-444-910. [DOI] [PubMed] [Google Scholar]
- [3].Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the Future of Cardiovascular Disease in the United States: A Policy Statement From the American Heart Association. Circulation. 2011 doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- [4].Services USDoHaH . Bone Health and Osteoporosis: A Report of the Surgeon General. U.S. Department of Health and Human Services, Office of the Surgeon General; Rockville, MD: 2004. [PubMed] [Google Scholar]
- [5].Qi L, Shen H, Ordovas JM. Hearts and bones. Nutr Metab Cardiovasc Dis. 2003;13:165–74. doi: 10.1016/s0939-4753(03)80176-x. [DOI] [PubMed] [Google Scholar]
- [6].Martinez-Ramirez MJ, Palma S, Martinez-Gonzalez MA, Delgado-Martinez AD, de la Fuente C, Delgado-Rodriguez M. Dietary fat intake and the risk of osteoporotic fractures in the elderly. Eur J Clin Nutr. 2007;61:1114–20. doi: 10.1038/sj.ejcn.1602624. [DOI] [PubMed] [Google Scholar]
- [7].Sennerby U, Farahmand B, Ahlbom A, Ljunghall S, Michaelsson K. Cardiovascular diseases and future risk of hip fracture in women. Osteoporos Int. 2007;18:1355–62. doi: 10.1007/s00198-007-0386-0. [DOI] [PubMed] [Google Scholar]
- [8].Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaelsson K. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666–73. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- [9].Marcovitz PA, Tran HH, Franklin BA, O'Neill WW, Yerkey M, Boura J, Kleerekoper M, Dickinson CZ. Usefulness of bone mineral density to predict significant coronary artery disease. Am J Cardiol. 2005;96:1059–63. doi: 10.1016/j.amjcard.2005.06.034. [DOI] [PubMed] [Google Scholar]
- [10].Tanko LB, Christiansen C, Cox DA, Geiger MJ, McNabb MA, Cummings SR. Relationship between osteoporosis and cardiovascular disease in postmenopausal women. J Bone Miner Res. 2005;20:1912–20. doi: 10.1359/JBMR.050711. [DOI] [PubMed] [Google Scholar]
- [11].Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–14. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- [12].Rye KA, Bursill CA, Lambert G, Tabet F, Barter PJ. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2009;50(Suppl):S195–200. doi: 10.1194/jlr.R800034-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jeon YK, Lee JG, Kim SS, Kim BH, Kim SJ, Kim YK, Kim IJ. Association between bone mineral density and metabolic syndrome in pre- and postmenopausal women. Endocr J. 2011 doi: 10.1507/endocrj.k10e-297. [DOI] [PubMed] [Google Scholar]
- [14].Jeong IK, Cho SW, Kim SW, Choi HJ, Park KS, Kim SY, Lee HK, Cho SH, Oh BH, Shin CS. Lipid profiles and bone mineral density in pre- and postmenopausal women in Korea. Calcif Tissue Int. 2010;87:507–12. doi: 10.1007/s00223-010-9427-3. [DOI] [PubMed] [Google Scholar]
- [15].Choi HS, Kim KJ, Kim KM, Hur NW, Rhee Y, Han DS, Lee EJ, Lim SK. Relationship between visceral adiposity and bone mineral density in Korean adults. Calcif Tissue Int. 2010;87:218–25. doi: 10.1007/s00223-010-9398-4. [DOI] [PubMed] [Google Scholar]
- [16].Park KK, Kim SJ, Moon ES. Association between bone mineral density and metabolic syndrome in postmenopausal Korean women. Gynecol Obstet Invest. 2010;69:145–52. doi: 10.1159/000264665. [DOI] [PubMed] [Google Scholar]
- [17].Hernandez JL, Olmos JM, Ramos C, Martinez J, de Juan J, Valero C, Nan D, Gonzalez-Macias J. Serum lipids and bone metabolism in Spanish men: the Camargo cohort study. Endocr J. 2010;57:51–60. doi: 10.1507/endocrj.k09e-228. [DOI] [PubMed] [Google Scholar]
- [18].Buizert PJ, van Schoor NM, Lips P, Deeg DJ, Eekhoff EM. Lipid levels: a link between cardiovascular disease and osteoporosis? J Bone Miner Res. 2009;24:1103–9. doi: 10.1359/jbmr.081262. [DOI] [PubMed] [Google Scholar]
- [19].Sivas F, Alemdaroglu E, Elverici E, Kulug T, Ozoran K. Serum lipid profile: its relationship with osteoporotic vertebrae fractures and bone mineral density in Turkish postmenopausal women. Rheumatol Int. 2009;29:885–90. doi: 10.1007/s00296-008-0784-4. [DOI] [PubMed] [Google Scholar]
- [20].Sanlier N. The effect of body composition on blood lipids, leptin, bone mineral density, and nutrition in females. Saudi Med J. 2008;29:1636–42. [PubMed] [Google Scholar]
- [21].Makovey J, Chen JS, Hayward C, Williams FM, Sambrook PN. Association between serum cholesterol and bone mineral density. Bone. 2009;44:208–13. doi: 10.1016/j.bone.2008.09.020. [DOI] [PubMed] [Google Scholar]
- [22].D'Amelio P, Di Bella S, Tamone C, Ravazzoli MG, Cristofaro MA, Di Stefano M, Isaia G. HDL cholesterol and bone mineral density in normal-weight postmenopausal women: is there any possible association? Panminerva Med. 2008;50:89–96. [PubMed] [Google Scholar]
- [23].Dennison EM, Syddall HE, Aihie Sayer A, Martin HJ, Cooper C. Lipid profile, obesity and bone mineral density: the Hertfordshire Cohort Study. QJM. 2007;100:297–303. doi: 10.1093/qjmed/hcm023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tanko LB. Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int. 2007;18:505–12. doi: 10.1007/s00198-006-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hsu YH, Venners SA, Terwedow HA, Feng Y, Niu T, Li Z, Laird N, Brain JD, Cummings SR, Bouxsein ML, Rosen CJ, Xu X. Relation of body composition, fat mass, and serum lipids to osteoporotic fractures and bone mineral density in Chinese men and women. Am J Clin Nutr. 2006;83:146–54. doi: 10.1093/ajcn/83.1.146. [DOI] [PubMed] [Google Scholar]
- [26].Cui LH, Shin MH, Chung EK, Lee YH, Kweon SS, Park KS, Choi JS. Association between bone mineral densities and serum lipid profiles of pre- and post-menopausal rural women in South Korea. Osteoporos Int. 2005;16:1975–81. doi: 10.1007/s00198-005-1977-2. [DOI] [PubMed] [Google Scholar]
- [27].Solomon DH, Avorn J, Canning CF, Wang PS. Lipid levels and bone mineral density. Am J Med. 2005;118:1414. doi: 10.1016/j.amjmed.2005.07.031. [DOI] [PubMed] [Google Scholar]
- [28].Adami S, Braga V, Zamboni M, Gatti D, Rossini M, Bakri J, Battaglia E. Relationship between lipids and bone mass in 2 cohorts of healthy women and men. Calcif Tissue Int. 2004;74:136–42. doi: 10.1007/s00223-003-0050-4. [DOI] [PubMed] [Google Scholar]
- [29].Wu LY, Yang TC, Kuo SW, Hsiao CF, Hung YJ, Hsieh CH, Tseng HC, Hsieh AT, Chen TW, Chang JB, Pei D. Correlation between bone mineral density and plasma lipids in Taiwan. Endocr Res. 2003;29:317–25. doi: 10.1081/erc-120025039. [DOI] [PubMed] [Google Scholar]
- [30].Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K. Plasma lipids and osteoporosis in postmenopausal women. Endocr J. 2002;49:211–7. doi: 10.1507/endocrj.49.211. [DOI] [PubMed] [Google Scholar]
- [31].Lidfeldt J, Holmdahl L, Samsioe G, Nerbrand C, Nyberg P, Schersten B, Agardh CD. The influence of hormonal status and features of the metabolic syndrome on bone density: a population-based study of Swedish women aged 50 to 59 years. The women's health in the Lund area study. Metabolism. 2002;51:267–70. doi: 10.1053/meta.2002.300001. [DOI] [PubMed] [Google Scholar]
- [32].Walker MD, Novotny R, Bilezikian JP, Weaver CM. Race and diet interactions in the acquisition, maintenance, and loss of bone. The Journal of nutrition. 2008;138:1256S–60S. doi: 10.1093/jn/138.6.1256S. [DOI] [PubMed] [Google Scholar]
- [33].Kuller LH. Ethnic differences in atherosclerosis, cardiovascular disease and lipid metabolism. Current opinion in lipidology. 2004;15:109–13. doi: 10.1097/00041433-200404000-00003. [DOI] [PubMed] [Google Scholar]
- [34].Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–7. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- [35].Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, Wark JD. Bone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab. 1996;81:3366–71. doi: 10.1210/jcem.81.9.8784098. [DOI] [PubMed] [Google Scholar]
- [36].Pietschmann P, Rauner M, Sipos W, Kerschan-Schindl K. Osteoporosis: an age-related and gender-specific disease--a mini-review. Gerontology. 2009;55:3–12. doi: 10.1159/000166209. [DOI] [PubMed] [Google Scholar]
- [37].Wells G, Tugwell P, Shea B, Guyatt G, Peterson J, Zytaruk N, Robinson V, Henry D, O'Connell D, Cranney A. Meta-analyses of therapies for postmenopausal osteoporosis. V. Meta-analysis of the efficacy of hormone replacement therapy in treating and preventing osteoporosis in postmenopausal women. Endocr Rev. 2002;23:529–39. doi: 10.1210/er.2001-5002. [DOI] [PubMed] [Google Scholar]
- [38].Weissglas-Volkov D, Pajukanta P. Genetic causes of high and low serum HDL-cholesterol. J Lipid Res. 2010;51:2032–57. doi: 10.1194/jlr.R004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ralston S, de Crombrugghe B. Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev. 2006;20:2492–2506. doi: 10.1101/gad.1449506. [DOI] [PubMed] [Google Scholar]
- [40].Ackert-Bicknell CL, Demissie S, Marin de Evsikova C, Hsu YH, DeMambro VE, Karasik D, Cupples LA, Ordovas JM, Tucker KL, Cho K, Canalis E, Paigen B, Churchill GA, Forejt J, Beamer WG, Ferrari S, Bouxsein ML, Kiel DP, Rosen CJ. PPARG by dietary fat interaction influences bone mass in mice and humans. J Bone Miner Res. 2008;23:1398–408. doi: 10.1359/JBMR.080419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Blanchet C, Giguere Y, Prud'homme D, Dumont M, Rousseau F, Dodin S. Association of physical activity and bone: influence of vitamin D receptor genotype. Med Sci Sports Exerc. 2002;34:24–31. doi: 10.1097/00005768-200201000-00005. [DOI] [PubMed] [Google Scholar]
- [42].Kitamura I, Ando F, Koda M, Okura T, Shimokata H. Effects of the interaction between lean tissue mass and estrogen receptor alpha gene polymorphism on bone mineral density in middle-aged and elderly Japanese. Bone. 2007;40:1623–9. doi: 10.1016/j.bone.2007.02.016. [DOI] [PubMed] [Google Scholar]
- [43].Grarup N, Andreasen CH, Andersen MK, Albrechtsen A, Sandbaek A, Lauritzen T, Borch-Johnsen K, Jorgensen T, Schmitz O, Hansen T, Pedersen O. The -250G>A promoter variant in hepatic lipase associates with elevated fasting serum high-density lipoprotein cholesterol modulated by interaction with physical activity in a study of 16,156 Danish subjects. J Clin Endocrinol Metab. 2008;93:2294–9. doi: 10.1210/jc.2007-2815. [DOI] [PubMed] [Google Scholar]
- [44].Kao WT, Yen YC, Lung FW. The effects of beta2 adrenergic receptor gene polymorphism in lipid profiles. Lipids Health Dis. 2008;7:20. doi: 10.1186/1476-511X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mukherjee M, Shetty KR. Variations in high-density lipoprotein cholesterol in relation to physical activity and Taq 1B polymorphism of the cholesteryl ester transfer protein gene. Clin Genet. 2004;65:412–8. doi: 10.1111/j.0009-9163.2004.0237.x. [DOI] [PubMed] [Google Scholar]
- [46].Senti M, Elosua R, Tomas M, Sala J, Masia R, Ordovas JM, Shen H, Marrugat J. Physical activity modulates the combined effect of a common variant of the lipoprotein lipase gene and smoking on serum triglyceride levels and high-density lipoprotein cholesterol in men. Hum Genet. 2001;109:385–92. doi: 10.1007/s004390100584. [DOI] [PubMed] [Google Scholar]
- [47].Escola-Gil JC, Jorba O, Julve-Gil J, Gonzalez-Sastre F, Ordonez-Llanos J, Blanco-Vaca F. Pitfalls of direct HDL-cholesterol measurements in mouse models of hyperlipidemia and atherosclerosis. Clinical chemistry. 1999;45:1567–9. [PubMed] [Google Scholar]
- [48].Wescott D, Schulingkamp R, Slaughter H, Bounous D. High density lipoprotein (HDL) assay comparison in hamster, mouse, rat and monkey plasma. [Accessed April 15, 2011];Clinical chemistry. 2006 http://www.abstractsonline.com/viewer/?mkey={FFEB1FEA-E733-491D-9BFEDBB1C5939B0A}. [Google Scholar]
- [49].The Mouse Phenome Database. [Accessed April 15, 2011]. [Google Scholar]
- [50].Mahley RW, Palaoglu KE, Atak Z, Dawson-Pepin J, Langlois AM, Cheung V, Onat H, Fulks P, Mahley LL, Vakar F, et al. Turkish Heart Study: lipids, lipoproteins, and apolipoproteins. Journal of lipid research. 1995;36:839–59. [PubMed] [Google Scholar]
- [51].Thelle DS, Forde OH, Arnesen E. Distribution of high-density lipoprotein cholesterol according to age, sex, and ethnic origin: cardiovascular disease study in Finnmark 1977. Journal of epidemiology and community health. 1982;36:243–7. doi: 10.1136/jech.36.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li JZ, Chen ML, Wang S, Dong J, Zeng P, Hou LW. A long-term follow-up study of serum lipid levels and coronary heart disease in the elderly. Chinese medical journal. 2004;117:163–7. [PubMed] [Google Scholar]
- [53].Goh VH, Tong TY, Mok HP, Said B. Differential impact of aging and gender on lipid and lipoprotein profiles in a cohort of healthy Chinese Singaporeans. Asian journal of andrology. 2007;9:787–94. doi: 10.1111/j.1745-7262.2007.00294.x. [DOI] [PubMed] [Google Scholar]
- [54].Petkov PM, Ding Y, Cassell MA, Zhang W, Wagner G, Sargent EE, Asquith S, Crew V, Johnson KA, Robinson P, Scott VE, Wiles MV. An Efficient SNP System for Mouse Genome Scanning and Elucidating Strain Relationships. Genome Res. 2004;14:1806–1811. doi: 10.1101/gr.2825804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chesler E, Miller D, Branstetter L, Galloway L, Jackson B, Philip V, Voy B, Culiat C, Threadgill D, Williams R, Churchill GA, Johnson D, Manly K. The Collaborative Cross at Oak Ridge National Laboratory: developing a powerful resource for systems genetics. Mammalian Genome. 2008;19:382–389. doi: 10.1007/s00335-008-9135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Brodeur MR, Brissette L, Falstrault L, Luangrath V, Moreau R. Scavenger receptor of class B expressed by osteoblastic cells are implicated in the uptake of cholesteryl ester and estradiol from LDL and HDL3. J Bone Miner Res. 2008;23:326–37. doi: 10.1359/jbmr.071022. [DOI] [PubMed] [Google Scholar]
- [57].Brodeur MR, Brissette L, Falstrault L, Moreau R. HDL3 reduces the association and modulates the metabolism of oxidized LDL by osteoblastic cells: a protection against cell death. J Cell Biochem. 2008;105:1374–85. doi: 10.1002/jcb.21938. [DOI] [PubMed] [Google Scholar]
- [58].Luegmayr E, Glantschnig H, Wesolowski GA, Gentile MA, Fisher JE, Rodan GA, Reszka AA. Osteoclast formation, survival and morphology are highly dependent on exogenous cholesterol/lipoproteins. Cell Death Differ. 2004;11(Suppl 1):S108–18. doi: 10.1038/sj.cdd.4401399. [DOI] [PubMed] [Google Scholar]
- [59].Hoofnagle AN, Vaisar T, Mitra P, Chait A. HDL lipids and insulin resistance. Curr Diab Rep. 2010;10:78–86. doi: 10.1007/s11892-009-0085-7. [DOI] [PubMed] [Google Scholar]
- [60].Tikkanen MJ, Vihma V, Jauhiainen M, Hockerstedt A, Helisten H, Kaamanen M. Lipoprotein-associated estrogens. Cardiovasc Res. 2002;56:184–8. doi: 10.1016/s0008-6363(02)00535-7. [DOI] [PubMed] [Google Scholar]
- [61].Badeau RM, Metso J, Tikkanen MJ, Jauhiainen M. High-density lipoprotein-associated 17beta-estradiol fatty acyl ester uptake by Fu5AH hepatoma cells: implications of the roles of scavenger receptor class B, type I and the low-density lipoprotein receptor. Biochim Biophys Acta. 2007;1771:1329–34. doi: 10.1016/j.bbalip.2007.08.008. [DOI] [PubMed] [Google Scholar]
- [62].Erkkila AT, Lichtenstein AH, Dolnikowski GG, Grusak MA, Jalbert SM, Aquino KA, Peterson JW, Booth SL. Plasma transport of vitamin K in men using deuterium-labeled collard greens. Metabolism. 2004;53:215–21. doi: 10.1016/j.metabol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- [63].Soeta S, Higuchi M, Yoshimura I, Itoh R, Kimura N, Aamsaki H. Effects of vitamin E on the osteoblast differentiation. J Vet Med Sci. 2010;72:951–7. doi: 10.1292/jvms.09-0487. [DOI] [PubMed] [Google Scholar]
- [64].Atkins GJ, Welldon KJ, Wijenayaka AR, Bonewald LF, Findlay DM. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by {gamma}-carboxylation-dependent and -independent mechanisms. Am J Physiol Cell Physiol. 2009;297:C1358–67. doi: 10.1152/ajpcell.00216.2009. [DOI] [PubMed] [Google Scholar]
- [65].Wagner GP, Zhang J. The pleiotropic structure of the genotype-phenotype map: the evolvability of complex organisms. Nat Rev Genet. 2011;12:204–13. doi: 10.1038/nrg2949. [DOI] [PubMed] [Google Scholar]
- [66].Karasik D, Kiel DP. Evidence for pleiotropic factors in genetics of the musculoskeletal system. Bone. 2010;46:1226–37. doi: 10.1016/j.bone.2010.01.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Paigen B. Genetics of responsiveness to high-fat and high-cholesterol diets in the mouse. Am J Clin Nutr. 1995;62:458S–462S. doi: 10.1093/ajcn/62.2.458S. [DOI] [PubMed] [Google Scholar]
- [68].Korstanje R, Paigen B. From QTL to gene: the harvest begins. Nat Genet. 2002;31:235–236. doi: 10.1038/ng0702-235. [DOI] [PubMed] [Google Scholar]
- [69].Sen S, Churchill GA. A Statistical Framework for Quantitative Trait Mapping. Genetics. 2001;159:371–387. doi: 10.1093/genetics/159.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ackert-Bicknell CL, Karasik D, Li Q, Smith RV, Hsu YH, Churchill GA, Paigen BJ, Tsaih SW. Mouse BMD quantitative trait loci show improved concordance with human genome-wide association loci when recalculated on a new, common mouse genetic map. J Bone Miner Res. 2010;25:1808–20. doi: 10.1002/jbmr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Su Z, Korstanje R, Tsaih SW, Paigen B. Candidate genes for obesity revealed from a C57BL/6J × 129S1/SvImJ intercross. Int J Obes (Lond) 2008;32:1180–9. doi: 10.1038/ijo.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Su Z, Ishimori N, Chen Y, Leiter EH, Churchill GA, Paigen B, Stylianou IM. Four additional mouse crosses improve the lipid QTL landscape and identify Lipg as a QTL gene. J Lipid Res. 2009;50:2083–94. doi: 10.1194/jlr.M900076-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stylianou IM, Tsaih SW, DiPetrillo K, Ishimori N, Li R, Paigen B, Churchill G. Complex genetic architecture revealed by analysis of high-density lipoprotein cholesterol in chromosome substitution strains and F2 crosses. Genetics. 2006;174:999–1007. doi: 10.1534/genetics.106.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wergedal JE, Ackert-Bicknell CL, Beamer WG, Mohan S, Baylink DJ, Srivastava AK. Mapping genetic loci that regulate lipid levels in a NZB/B1NJxRF/J intercross and a combined intercross involving NZB/B1NJ, RF/J, MRL/MpJ, and SJL/J mouse strains. J Lipid Res. 2007;48:1724–34. doi: 10.1194/jlr.M700015-JLR200. [DOI] [PubMed] [Google Scholar]
- [75].Brockmann GA, Karatayli E, Neuschl C, Stylianou IM, Aksu S, Ludwig A, Renne U, Haley CS, Knott S. Genetic control of lipids in the mouse cross DU6i × DBA/2. Mamm Genome. 2007;18:757–66. doi: 10.1007/s00335-007-9068-7. [DOI] [PubMed] [Google Scholar]
- [76].Wang SS, Shi W, Wang X, Velky L, Greenlee S, Wang MT, Drake TA, Lusis AJ. Mapping, genetic isolation, and characterization of genetic loci that determine resistance to atherosclerosis in C3H mice. Arterioscler Thromb Vasc Biol. 2007;27:2671–6. doi: 10.1161/ATVBAHA.107.148106. [DOI] [PubMed] [Google Scholar]
- [77].Stylianou IM, Langley SR, Walsh K, Chen Y, Revenu C, Paigen B. Differences in DBA/1J and DBA/2J reveal lipid QTL genes. J Lipid Res. 2008;49:2402–13. doi: 10.1194/jlr.M800244-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wang X, Paigen B. Genetics of variation in HDL cholesterol in humans and mice. Circ Res. 2005;96:27–42. doi: 10.1161/01.RES.0000151332.39871.13. [DOI] [PubMed] [Google Scholar]
- [79].Schilling AF, Schinke T, Munch C, Gebauer M, Niemeier A, Priemel M, Streichert T, Rueger JM, Amling M. Increased bone formation in mice lacking apolipoprotein E. J Bone Miner Res. 2005;20:274–82. doi: 10.1359/JBMR.041101. [DOI] [PubMed] [Google Scholar]
- [80].Xiao Q, Danton MJ, Witte DP, Kowala MC, Valentine MT, Bugge TH, Degen JL. Plasminogen deficiency accelerates vessel wall disease in mice predisposed to atherosclerosis. Proc Natl Acad Sci U S A. 1997;94:10335–40. doi: 10.1073/pnas.94.19.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Boes E, Coassin S, Kollerits B, Heid IM, Kronenberg F. Genetic-epidemiological evidence on genes associated with HDL cholesterol levels: a systematic in-depth review. Experimental gerontology. 2009;44:136–60. doi: 10.1016/j.exger.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ma L, Yang J, Runesha HB, Tanaka T, Ferrucci L, Bandinelli S, Da Y. Genome-wide association analysis of total cholesterol and high-density lipoprotein cholesterol levels using the Framingham heart study data. BMC medical genetics. 2010;11:55. doi: 10.1186/1471-2350-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Thomas D. Methods for investigating gene-environment interactions in candidate pathway and genome-wide association studies. Annual review of public health. 2010;31:21–36. doi: 10.1146/annurev.publhealth.012809.103619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Stearns FW. One hundred years of pleiotropy: a retrospective. Genetics. 2010;186:767–73. doi: 10.1534/genetics.110.122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Wittenburg H, Lyons MA, Li R, Kurtz U, Wang X, Mossner J, Churchill GA, Carey MC, Paigen B. QTL mapping for genetic determinants of lipoprotein cholesterol levels in combined crosses of inbred mouse strains. J Lipid Res. 2006;47:1780–90. doi: 10.1194/jlr.M500544-JLR200. [DOI] [PubMed] [Google Scholar]
- [86].Guan Y, Ackert-Bicknell CL, Kell B, Troyanskaya OG, Hibbs MA. Functional genomics complements quantitative genetics in identifying disease-gene associations. PLoS Comput Biol. 2010;6:e1000991. doi: 10.1371/journal.pcbi.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Junyent M, Tucker KL, Smith CE, Lane JM, Mattei J, Lai CQ, Parnell LD, Ordovas JM. The effects of ABCG5/G8 polymorphisms on HDL-cholesterol concentrations depend on ABCA1 genetic variants in the Boston Puerto Rican Health Study. Nutr Metab Cardiovasc Dis. 2010;20:558–66. doi: 10.1016/j.numecd.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Junyent M, Tucker KL, Smith CE, Garcia-Rios A, Mattei J, Lai CQ, Parnell LD, Ordovas JM. The effects of ABCG5/G8 polymorphisms on plasma HDL cholesterol concentrations depend on smoking habit in the Boston Puerto Rican Health Study. J Lipid Res. 2009;50:565–73. doi: 10.1194/jlr.P800041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Long JR, Liu PY, Liu YJ, Lu Y, Shen H, Zhao LJ, Xiong DH, Deng HW. APOE haplotypes influence bone mineral density in Caucasian males but not females. Calcif Tissue Int. 2004;75:299–304. doi: 10.1007/s00223-004-0034-z. [DOI] [PubMed] [Google Scholar]
- [90].Peter I, Crosier MD, Yoshida M, Booth SL, Cupples LA, Dawson-Hughes B, Karasik D, Kiel DP, Ordovas JM, Trikalinos TA. Associations of APOE gene polymorphisms with bone mineral density and fracture risk: a meta-analysis. Osteoporos Int. 2010 doi: 10.1007/s00198-010-1311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Schoofs MW, van der Klift M, Hofman A, van Duijn CM, Stricker BH, Pols HA, Uitterlinden AG. ApoE gene polymorphisms, BMD, and fracture risk in elderly men and women: the Rotterdam study. J Bone Miner Res. 2004;19:1490–6. doi: 10.1359/JBMR.040605. [DOI] [PubMed] [Google Scholar]
- [92].Singh M, Singh P, Singh S, Juneja PK, Kaur T. A susceptible haplotype within APOE gene influences BMD and intensifies the osteoporosis risk in postmenopausal women of Northwest India. Maturitas. 2010;67:239–44. doi: 10.1016/j.maturitas.2010.06.017. [DOI] [PubMed] [Google Scholar]
- [93].Tong TY, Yong RY, Goh VH, Liang S, Chong AP, Mok HP, Yong EL, Yap EP, Moochhala S. Association between an intronic apolipoprotein E polymorphism and bone mineral density in Singaporean Chinese females. Bone. 2010;47:503–10. doi: 10.1016/j.bone.2010.05.028. [DOI] [PubMed] [Google Scholar]
- [94].Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–34. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].McCauley LK, Tozum TF, Kozloff KM, Koh-Paige AJ, Chen C, Demashkieh M, Cronovich H, Richard V, Keller ET, Rosol TJ, Goldstein SA. Transgenic models of metabolic bone disease: impact of estrogen receptor deficiency on skeletal metabolism. Connect Tissue Res. 2003;44(Suppl 1):250–63. [PubMed] [Google Scholar]
- [96].Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, Lindberg MK, Warner M, Angelin B, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun. 2000;278:640–5. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- [97].Abdul Shakoor SK, Shalet SM. Effects of GH replacement on metabolism and physical performance in GH deficient adults. J Endocrinol Invest. 2003;26:911–8. doi: 10.1007/BF03345243. [DOI] [PubMed] [Google Scholar]
- [98].Dennison EM, Syddall HE, Jameson KA, Sayer AA, Gaunt TR, Rodriguez S, Day IN, Cooper C, Lips MA. A study of relationships between single nucleotide polymorphisms from the growth hormone-insulin-like growth factor axis and bone mass: the Hertfordshire cohort study. J Rheumatol. 2009;36:1520–6. doi: 10.3899/jrheum.081061. [DOI] [PubMed] [Google Scholar]
- [99].Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–59. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bustamante M, Nogues X, Mellibovsky L, Agueda L, Jurado S, Caceres E, Blanch J, Carreras R, Diez-Perez A, Grinberg D, Balcells S. Polymorphisms in the interleukin-6 receptor gene are associated with bone mineral density and body mass index in Spanish postmenopausal women. Eur J Endocrinol. 2007;157:677–84. doi: 10.1530/EJE-07-0389. [DOI] [PubMed] [Google Scholar]
- [101].Yang TL, Shen H, Xiong DH, Xiao P, Guo Y, Guo YF, Liu YZ, Recker RR, Deng HW. Epistatic interactions between genomic regions containing the COL1A1 gene and genes regulating osteoclast differentiation may influence femoral neck bone mineral density. Ann Hum Genet. 2007;71:152–9. doi: 10.1111/j.1469-1809.2006.00313.x. [DOI] [PubMed] [Google Scholar]
- [102].Huang QY, Shen H, Deng HY, Conway T, Davies KM, Li JL, Recker RR, Deng HW. Linkage and association of the CA repeat polymorphism of the IL6 gene, obesity-related phenotypes, and bone mineral density (BMD) in two independent Caucasian populations. J Hum Genet. 2003;48:430–7. doi: 10.1007/s10038-003-0053-z. [DOI] [PubMed] [Google Scholar]
- [103].Chung HW, Seo JS, Hur SE, Kim HL, Kim JY, Jung JH, Kim LH, Park BL, Shin HD. Association of interleukin-6 promoter variant with bone mineral density in premenopausal women. J Hum Genet. 2003;48:243–8. doi: 10.1007/s10038-003-0020-8. [DOI] [PubMed] [Google Scholar]
- [104].Ota N, Nakajima T, Nakazawa I, Suzuki T, Hosoi T, Orimo H, Inoue S, Shirai Y, Emi M. A nucleotide variant in the promoter region of the interleukin-6 gene associated with decreased bone mineral density. J Hum Genet. 2001;46:267–72. doi: 10.1007/s100380170077. [DOI] [PubMed] [Google Scholar]
- [105].Deng FY, Long JR, Lei SF, Li MX, Deng HW. Potential effect of inter-genic action on peak bone mass (PBM) in Chinese females. Yi Chuan Xue Bao. 2005;32:1003–10. [PubMed] [Google Scholar]
- [106].Hulkkonen J, Lehtimaki T, Mononen N, Juonala M, Hutri-Kahonen N, Taittonen L, Marniemi J, Nieminen T, Viikari J, Raitakari O, Kahonen M. Polymorphism in the IL6 promoter region is associated with the risk factors and markers of subclinical atherosclerosis in men: The Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2009;203:454–8. doi: 10.1016/j.atherosclerosis.2008.07.014. [DOI] [PubMed] [Google Scholar]
- [107].Halverstadt A, Phares DA, Roth S, Ferrell RE, Goldberg AP, Hagberg JM. Interleukin-6 genotype is associated with high-density lipoprotein cholesterol responses to exercise training. Biochim Biophys Acta. 2005;1734:143–51. doi: 10.1016/j.bbalip.2005.03.003. [DOI] [PubMed] [Google Scholar]
- [108].Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko Camacho N, Bostrom MP. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone. 2007;41:928–36. doi: 10.1016/j.bone.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kiel DP, Demissie S, Dupuis J, Lunetta KL, Murabito JM, Karasik D. Genome-wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S14. doi: 10.1186/1471-2350-8-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].McLean RR, Karasik D, Selhub J, Tucker KL, Ordovas JM, Russo GT, Cupples LA, Jacques PF, Kiel DP. Association of a common polymorphism in the methylenetetrahydrofolate reductase (MTHFR) gene with bone phenotypes depends on plasma folate status. J Bone Miner Res. 2004;19:410–8. doi: 10.1359/JBMR.0301261. [DOI] [PubMed] [Google Scholar]
- [111].Real JT, Martinez-Hervas S, Garcia-Garcia AB, Chaves FJ, Civera M, Ascaso JF, Carmena R. Association of C677T polymorphism in MTHFR gene, high homocysteine and low HDL cholesterol plasma values in heterozygous familial hypercholesterolemia. J Atheroscler Thromb. 2009;16:815–20. doi: 10.5551/jat.2196. [DOI] [PubMed] [Google Scholar]
- [112].Petramala L, Acca M, Francucci CM, D'Erasmo E. Hyperhomocysteinemia: a biochemical link between bone and cardiovascular system diseases? J Endocrinol Invest. 2009;32:10–4. [PubMed] [Google Scholar]
- [113].Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–43. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- [114].Kim BJ, Kim SY, Cho YS, Han BG, Park EK, Lee SH, Kim HY, Kim GS, Lee JY, Koh JM. Association of Paraoxonase 1 (PON1) polymorphisms with osteoporotic fracture risk in postmenopausal Korean women. Experimental & molecular medicine. 2011;43:71–81. doi: 10.3858/emm.2011.43.2.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yamada Y, Ando F, Niino N, Miki T, Shimokata H. Association of polymorphisms of paraoxonase 1 and 2 genes, alone or in combination, with bone mineral density in community-dwelling Japanese. J Hum Genet. 2003;48:469–75. doi: 10.1007/s10038-003-0063-x. [DOI] [PubMed] [Google Scholar]
- [116].Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung U-i, Kubota N, Terauchi Y, Harada Y, Azuma Y, Nakamura K, Kadowaki T, Kawaguchi H. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J of Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Brand-Herrmann SM, Kuznetsova T, Wiechert A, Stolarz K, Tikhonoff V, Schmidt-Petersen K, Telgmann R, Casiglia E, Wang JG, Thijs L, Staessen JA, Brand E. Alcohol intake modulates the genetic association between HDL cholesterol and the PPARgamma2 Pro12Ala polymorphism. J Lipid Res. 2005;46:913–9. doi: 10.1194/jlr.M400405-JLR200. [DOI] [PubMed] [Google Scholar]
- [118].Ereqat S, Nasereddin A, Azmi K, Abdeen Z, Amin R. Impact of the Pro12Ala Polymorphism of the PPAR-Gamma 2 Gene on Metabolic and Clinical Characteristics in the Palestinian Type 2 Diabetic Patients. PPAR Res. 2009;2009:874126. doi: 10.1155/2009/874126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rhee EJ, Oh KW, Lee WY, Kim SY, Oh ES, Baek KH, Kang MI, Kim SW. Effects of two common polymorphisms of peroxisome proliferator-activated receptor-gamma gene on metabolic syndrome. Arch Med Res. 2006;37:86–94. doi: 10.1016/j.arcmed.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [120].Tai ES, Corella D, Deurenberg-Yap M, Adiconis X, Chew SK, Tan CE, Ordovas JM. Differential effects of the C1431T and Pro12Ala PPARgamma gene variants on plasma lipids and diabetes risk in an Asian population. J Lipid Res. 2004;45:674–85. doi: 10.1194/jlr.M300363-JLR200. [DOI] [PubMed] [Google Scholar]
- [121].Yang LL, Hua Q, Liu RK, Yang Z. Association between two common polymorphisms of PPARgamma gene and metabolic syndrome families in a Chinese population. Arch Med Res. 2009;40:89–96. doi: 10.1016/j.arcmed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [122].Furuta I, Kobayashi N, Fujino T, Kobamatsu Y, Shirogane T, Yaegashi M, Sakuragi N, Cho K, Yamada H, Okuyama K, Minakami H. Bone mineral density of the lumbar spine is associated with TNF gene polymorphisms in early postmenopausal Japanese women. Calcif Tissue Int. 2004;74:509–15. doi: 10.1007/s00223-003-0105-6. [DOI] [PubMed] [Google Scholar]
- [123].Fontova R, Gutierrez C, Vendrell J, Broch M, Vendrell I, Simon I, Fernandez-Real JM, Richart C. Bone mineral mass is associated with interleukin 1 receptor autoantigen and TNF-alpha gene polymorphisms in post-menopausal Mediterranean women. J Endocrinol Invest. 2002;25:684–90. doi: 10.1007/BF03345101. [DOI] [PubMed] [Google Scholar]
- [124].Wennberg P, Nordstrom P, Lorentzon R, Lerner UH, Lorentzon M. TNF-alpha gene polymorphism and plasma TNF-alpha levels are related to lumbar spine bone area in healthy female Caucasian adolescents. Eur J Endocrinol. 2002;146:629–34. doi: 10.1530/eje.0.1460629. [DOI] [PubMed] [Google Scholar]
- [125].Fontaine-Bisson B, El-Sohemy A. Genetic polymorphisms of tumor necrosis factor-alpha modify the association between dietary polyunsaturated fatty acids and plasma high-density lipoprotein-cholesterol concentrations in a population of young adults. J Nutrigenet Nutrigenomics. 2008;1:215–23. doi: 10.1159/000149825. [DOI] [PubMed] [Google Scholar]
- [126].Parra-Rojas I, Ruiz-Madrigal B, Martinez-Lopez E, Panduro A. Influence of the - 308 TNF-alpha and -174 IL-6 polymorphisms on lipid profile in Mexican subjects. Hereditas. 2006;143:167–72. doi: 10.1111/j.2006.0018-0661.01936.x. [DOI] [PubMed] [Google Scholar]
- [127].Li Y, Li A, Strait K, Zhang H, Nanes MS, Weitzmann MN. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J Bone Miner Res. 2007;22:646–55. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]