Abstract
Primary sclerosing cholangitis (PSC) represents a chronic cholestatic liver disease with fibroobliterative sclerosis of intra- and/or extrahepatic bile ducts, eventually leading to biliary cirrhosis.
The association with human leukocyte antigen (HLA) and non-HLA haplotypes and the presence of autoantibodies in sera of PSC patients support a crucial role for immune-mediated mechanisms in the initiation and progression of PSC. The strong clinical association between PSC and inflammatory bowel diseases led to intriguing pathogenetic concepts, in which the inflamed gut with translocation of bacterial products and homing of gut-primed memory T lymphocytes via aberrantly expressed adhesion molecules plays a fundamental role. Genetically or chemically modified bile composition was shown to induce sclerosing cholangitis and liver fibrosis in a number of animal models ("toxic bile concept"). The potential role of vascular injury with ischemia of bile duct epithelium cells in the development of sclerosing cholangitis is supported by animal models of endothelial cell injury showing close morphological similarities with human PSC.
Keywords: Primary sclerosing cholangitis (PSC), Aetiology, Pathogenesis, Immunology, Inflammatory bowel diseases
Introduction
Aetiology and pathogenesis of PSC still pose many unresolved questions and remain a scientific and clinical challenge. The lack of a generally accepted pathogenetic concept might be linked to several critical issues including (i) the lack of an “ideal” animal model for PSC, (ii) the lack of reliable clinical tests allowing early detection and detailed follow-up studies of early PSC, (iii) the fact that bile ducts are difficult to investigate and directly access without invasive tests such as endoscopy or liver biopsy, and (iv) weaknesses of currently used definitions and diagnostic standards for PSC. Consequently PSC probably represents an umbrella term, since besides “classical” PSC various different etiologies may cause secondary sclerosing cholangitis (SSC) or cholangiopathies “mimicking” PSC and which in some cases may have been previously classified as PSC. The diagnosis of PSC requires firm exclusion of secondary causes. Thus, PSC as an own disease entity is as per definition increasingly fragmenting/splitting up into secondary forms and subtypes as identifiable causes emerge; one recent example for this development may be the IgG4-positive forms of cholangitis. However, most likely PSC represents a multifactorial disease, in which the influence of a single mechanism may vary considerably among the different clinical subtypes (e.g. PSC with and without IBD). Evidence supporting a genetic predisposition for PSC is derived from an obvious familial and geographic clustering with high prevalence in Northern countries (e.g. Norway, Sweden) compared to Southern Europe and Asia [1]. This clustering suggests that combined genetic/environmental factors might be required for PSC development (e.g. selen and vitamin D deficiency). Thus, PSC is likely to be caused by interplay of multiple genetic variants and environmental factors [2]. However, these complex interactions are still not entirely understood. Based on its associations with HLA halplotypes, autoimmune diseases and the presence of inflammatory bowel disease (IBD), especially ulcerative colitis (UC) in the majority of PSC patients, immunopathogenetic mechanisms have been implicated in PSC pathogenesis [3]. Although the mechanistic links between PSC and IBD are still not satisfactorily explained, this association of PSC suggests a common pathogenetic agent or common inflammatory pathway in the pathogenesis of both diseases. However, PSC cannot be considered as a classical autoimmune disease, as it occurs with a 2:1 male predominance and lacks characteristic response to immunosuppressants [4]. Thus, PSC has recently been re-classified as an immune-mediated disease comparable to UC. Fig. 1 illustrates human data and clues derived from animal models that are currently implicated in PSC pathogenesis. The aim of this review is to summarize the current knowledge and hypothetical models implicated in the pathogenesis of PSC.
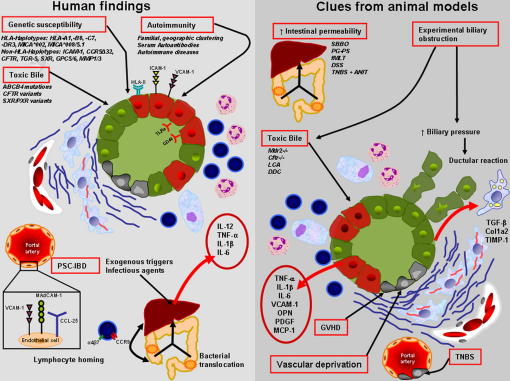
Fig. 1.
Shows a comparative illustration of potential pathogenetic factors in human PSC (left) and clues derived from animal models (right).
Genetic background/susceptibility
Following the identification of certain HLA variants (i.e. HLA-DR3 and HLA-B8) in PSC patients in 1982 [5] the genetic architecture of PSC patients attracted increasing interest. Accordingly, studies on PSC heritability have shown that first-degree relatives of PSC patients have a disease prevalence of 0.7%, representing a nearly 100-fold increased risk of developing PSC compared to the general population [6]. In siblings the prevalence even reaches 1.5% [6,7]. Taken together, these epidemiologic data with the distinct geographic occurrence of PSC (high prevalence in Northern countries compared with Southern Europe and Asia) [1] emphasize the role of genetic factors in PSC pathogenesis. Recently, strong evidence for genetic susceptibility was derived from genome-wide association analyses (GWA) showing strong associations with a subset of HLA and non-HLA genes involved in bile homeostasis and associated with regulatory inflammatory pathways as key components of the genetic architecture in PSC patients (listed in Table 1) [8].
Table 1.
Associations of PSC with HLA and non-HLA haplotypes.
| Haplotype | RR/Impact | Ref. |
|---|---|---|
| HLA associations | ||
| B8-MICA∗008-TNFA∗2-DRB3∗0101-DRB1∗0301-DQB1∗0201 | 2.69 | [16] |
| DRB3∗0101-DRB1∗1301-DQA1∗0103-DQB1∗0603 | 3.8 | [16] |
| MICA∗008-DRB5∗0101-DRB1∗1501-DQA1∗0102-DQB1∗0602 | 1.52 | [16] |
| MICA∗008 homozygosity | 5.01 | [18] |
| DRB4∗0103-DRB1∗0401-DQA1∗03-DQB1∗0302 | 0.26 | [16] |
| DRB4∗0103-DRB1∗0701-DQA1∗0201-DQB1∗0303 | 0.15 | [16] |
| MICA∗002 | 0.12 | [18] |
| Non-HLA associatons | ||
| ICAM-1 | 0.26 | [29] |
| CCR5Δ32 | n.d./protective | [35] |
| 3.17 | [34] | |
| no association | [36] | |
| CFTR | 0.25 | [107] |
| TGR5 | ||
| SNP rs12612347 | 1.26 | [8] |
| SNP rs11554825 | 1.14 | [38] |
| PXR/SXR | n.d./modifier gene | [50] |
| MDR3 | n.d./weak association | [99] |
| GPC5/GPC6 | 0.77 | [8] |
| MMP-1 | n.d./no association | [113] |
| MMP-3 | n.d./no association | [113] |
| MMP3 alelle 5 | 1.78 | [114] |
Abbreviations: CCR5Δ32, chemokine receptor 5 with 32-base pair deletion; CFTR, cystic fibrosis transmembrane conductance regulator; GPC5/6, glypican 5/6; HLA, human leukocyte antigen; ICAM-1, intercellular adhesion molecule-1; MDR, multidrug resistance; MICA, major histocompatibility complex class I chain-related A; MMP, matrix metalloproteinase; n.d., not determined; PXR, pregnane X receptor; SNP, single-nucleotide polymorphism; SXR, steroid and xenobiotic receptor; TGR5, G-protein coupled bile acid receptor-1.
HLA-type genes and PSC
The HLA complex is located on the short arm of chromosome 6, stretches across 7.6 million base pairs (bp) of DNA and contains 252 expressed protein-coding genes, of which ∼30% are related to immunological functions [9]. Whereas HLA class I molecules are expressed on all nucleated cells, present intracellular antigens to CD8+ lymphocytes and serve as ligands for inhibitory killer immunoglobulin-like receptors (KIRs) on natural killer (NK) cells as well as γδ T-lymphocytes, HLA class II molecules are expressed on antigen presenting cells and present exogenous antigens to CD4+ lymphocytes [10]. Early studies on PSC HLA association from Norway and the UK identified HLA-DR3 (DRB1*0301) and HLA-B8 (HLA-B*0801) haplotypes as important susceptibility markers [5,11]. While subsequent studies from Finland [12], Australia [13] and recently from Norway [8] confirmed these data, no associations with any HLA-B haplotype were found in an Italian Study [14] and in Brazil [15] studies. In addition, the HLA-A1 allele [16], the HLA-C7 [17], the major histocompatibility complex class I chain-related A (MICA)*002 and 008/5.1 alleles [18,19] as well as the tumour necrosis factor alpha (TNFα) promoter -308 A allele [14,20] were identified to be associated with PSC susceptibility. Susceptibility alleles encoded in the HLA class II haplotype include DRB1*0301 (DR3) and DRB1*13 (DR6) [21,22]. In a comprehensive study from five different European countries (UK, Italy, Norway, Spain and Sweden), PSC was positively associated with three different HLA class II haplotypes, the DRB1*03, DQA1*0501, DQB1*02, the DRB1*15, DQA1*0102, DQB1*0602 and the DRB1*13, DQA1*0103, DQB1*0603 haplotypes [21]. A negative association was found for the DRB1*04, DQA1*03, DQB1*0302 haplotype. The highest relative risk to develop PSC is conferred by the DRB1*03, DQA1*0501, DQB1*02 homozygous genotype [21]. Approximately half of all PSC patients in Norway and Sweden carry at least one of these haplotypes [23], whereas none of these haplotype associations was detected in PSC patients from Italy and only the DRB1*13, DQA1*0103, DQB1*0603 was present in PSC patients from Brazil [14,15,21,23]. These data underscore the considerable heterogeneity in both HLA classes of different populations and may contribute to the observed differences.
Non-HLA-type genes in PSC
Specific combinations of HLA class I and killer immunoglobulin-like receptor (KIR) alleles have been implicated in autoimmunity, tumour surveillance and viral diseases [23]. KIRs function as NK cell receptors which bind HLA class I molecules which may activate or constitutively inhibit NK cell activity. However, the exact mechanisms how KIR/HLA class I ligand genotypes influence susceptibility to autoimmune diseases are not entirely clear [24]. Imbalance of KIR and/or HLA class I ligands resulting in reduced inhibition or increased activation might be pivotal in PSC pathogenesis [10,25]. Possible interactions between HLA class I alleles and KIR genes were studied in 365 Scandinavian PSC patients and 368 healthy controls [25] showing reduced frequency of ligands, HLA-Bw4 and HLA-C2, for the inhibitory KIRs 3DL1 and 2DL1 in PSC. Consequently an increase in NK cell activity by decreased suppression might be critical for PSC susceptibility. Additional evidence for a central role of HLA-related NK cell activity in PSC was derived from studies on MIC genes, namely MICA and MICB that can directly activate NK cells receptors [18]. A strong protective effect was found for MICA*002 allele in PSC patients from the UK [18]. In a Norwegian study, MICA5.1 and MICB24 alleles were significantly increased in PSC patients [19]. However, when stratified for DR3-positivity, the association of these markers was no longer evident. This means that both MICA5.1 and MICB24 markers are associated with PSC only in the presence of HLA-B8 and -DR3 haplotypes and vice versa. Therefore, the authors suggested an association of the extended B8-MICA5.1-MICB24-DR3 haplotype with PSC [19].
Approximately 80% of PSC patients in Northern Europe have IBD, especially UC [1], suggesting shared inflammatory pathways based on common susceptibility genes. In contrast to PSC, the association between UC and HLA was weak and inconsistent [23]. A direct comparison of Scandinavian PSC patients with Norwegian UC patients showed distinct HLA associations [26]. No significant differences were noted between PSC patients with concurrent UC and PSC patients without IBD, suggesting different HLA associated genetic susceptibility. Furthermore, these data support the assumption of a distinct UC phenotype in PSC patients [26]. Taken together, appropriate interpretations of HLA-associations in PSC seem to be extremely difficult.
Intercellular adhesion molecule-1 (ICAM-1, CD54) gene polymorphisms have been implicated in the susceptibility to various inflammatory diseases including IBD. As adhesion molecule and ligand for lymphocyte function associated-1 (LFA-1), ICAM-1 mediates leukocyte adhesion during immune responses and transendothelial migration of neutrophils and T-cell activation [23]. In PSC, ICAM-1 shows expression on proliferating bile ducts in late stage PSC [27] and serum levels of soluble ICAM-1 are increased [28]. The polymorphism K469E in exon 6 causes a change from glutamic acid to lysine in the Ig-like domain of ICAM-1 on biliary epithelial cells affecting interactions between LFA-1 and B-lymphocytes [29]. However data on the frequency and effects of this polymorphism in PSC patients are conflicting [29,30]. In addition, members of the chemokine family, including the CC-type chemokine receptor 5 (CCR-5), have been related to UC and PSC progress. A 32-base pair deletion (CCR5-∆32) results in a frame shift mutation and encodes a non-functioning receptor [31]. Population-based studies showed conflicting data ranging from a protective to adverse effect of the CCR5∆32 polymorphism on PSC susceptibility [32–35]. The largest study covering 363 Scandinavian PSC patients, however, failed to show any involvement of CCR5-∆32 in either PSC susceptibility or progression [36].
Based on its dual function in biliary bicarbonate secretion and anti-inflammatory effects in macrophage/Kupffer cells [37] the G protein-coupled bile acid receptor 1 (GPBAR1) TGR5 has been implicated in PSC pathogenesis. Recently, significant associations for one exonic single-nucleotide polymorphism of the TGR5 gene were found for both PSC and UC [8,38]. However, the functional role of TGR5 as a potential candidate gene in both diseases conditions is still unclear and requires further mechanistic studies [38]. Interestingly, the expression of TGR5 in colon shares the same right-sided predominance as reported for PSC-UC [39].
The multidrug-resistance gene 1 (MDR1) gene represents an interesting candidate for PSC susceptibility due to its role as membrane transport protein mediating efflux of a wide range of xenobiotics and toxins [40] and MDR1 gene variants may be associated with IBD disease progression and severity [41]. However, no significant associations could be identified in PSC or different IBD studies [42,43].
The steroid and xenobiotic receptor (SXR), and its rodent analogue pregnane X receptor (PXR), is predominantly expressed in liver and intestine [44]. SXR regulates proteins pivotal for drug transport and metabolism, such as cytochrome P450 3A4 (CYP3A4) and drug efflux pumps like MDR1 p-glycoprotein [45]. Moreover, SXR serves as a bile acid receptor and regulator of bile acid and cholesterol homeostasis [46]. It may be worthwhile to study SXR gene polymorphisms in PSC and IBD since the expression of various enzymes and bile acid transporters under control of SXR has shown to be affected [47,48]. However, the reported associations between SXR variants and PSC/IBD are rather weak [49,50].
Pathogenetic clues from the association between IBD and PSC
Despite the description of PSC-UC association more than 40 years ago by Smith and Loe [51] this interesting clinical observation is still not entirely understood. Most important, the activity of IBD does not correlate with the severity of PSC and vice versa [52]. As such, PSC can occur several years after colectomy and conversely IBD may even develop years after liver transplantation [52–54]. In contrast and interestingly enough, comparative data on the impact of pre-transplant colectomy and post-transplant colitis activity on PSC recurrence in the PSC-IBD patients identified the absence of active colitis after liver transplantation as a protective factor against PSC recurrence [55]. The comparison of liver-transplanted and non-transplanted PSC-IBD patients revealed a favourable impact of liver transplantation on clinical and histological IBD activity [56]. These clinical associations between PSC and IBD stimulated several intriguing pathogenetic models.
The leaky gut hypothesis
Translocation of bacteria or bacterial components and products entering the portal-venous system via an increased intestinal permeability resulting from an inflamed gut with concomitant induction of an inflammatory reaction concentrated to portal fields is frequently referred to as the “leaky gut hypothesis” [3]. Bacteria may penetrate the damaged mucosal layer during acute inflammatory episodes, enter the liver and consequently stimulate release of chemokines/cytokines by Kupffer cells and macrophages leading to cholangitis and resulting wound healing process with concentric periductal fibrosis [3, 4]. Experimental evidence derived from animal models suggest that small intestinal bacterial overgrowth and infusion of bacterial antigens into the portal circulation can indeed lead to hepatic inflammation with at least some characteristic features of human PSC (i.e. pericholangitis) [57–59]. Small intestinal bacterial overgrowth in genetically susceptible rats induces macroscopic and microscopic features similar to human PSC [57]. Components of anaerobic bacteria, such as peptidoglycan-polysaccharides, are likely to be mainly responsible for these morphological changes. In addition, administration of a chemotactic peptide produced by E. coli into the colon of rats with acetate-induced colitis induced hepatic lesions reminiscent of PSC [58]. Therefore it was tempting to hypothesize that in genetically susceptible individuals, bacterial antigens function as molecular mimics to trigger the immune response for initiation of PSC. However, studies following that concept revealed no direct clinical evidence for increased portal vein bacteraemia in PSC/IBD patients [60,61]. Moreover, potential technical drawbacks and pitfalls of such studies have to be considered. A Scandinavian investigation on intestinal permeability and small bowel bacterial flora in PSC patients identified bacterial overgrowth in only one out of 22 PSC cases and comparable intestinal permeability in PSC and control patients [62]. Indirect evidence against this concept may also be drawn from negative studies testing antibiotics in PSC patients [63] and more recently a randomized placebo-controlled trial evaluating the effects of metronidazole plus ursodeoxycholic acid on PSC progression [64]. Taken together, these findings at least in part dismiss a major impact for increased intestinal permeability, bacterial overgrowth or translocation in PSC pathogenesis [62]. Nevertheless, translocation of intestinal bacteria/bacterial products may be episodic, hard to detect and still accelerate disease progression [65]. Future studies will also have to determine the role of the gut microbiome in PSC pathogenesis.
Gut lymphocyte homing hypothesis
The observation that PSC frequently runs a course independent of IBD activity led to the idea that CCR9+ α4β7+ memory T lymphocytes primed in the inflamed gut may persist as long-lived memory cells, undergo enterohepatic circulation with the possibility to trigger portal inflammation in PSC via aberrantly expressed adhesion molecules in liver and gut [4,66]. This is currently referred to as the “gut lymphocyte homing hypothesis” [66]. Studies on the expression pattern of adhesion molecules and characterization on the inflammatory infiltrate argue at least in part for such a concept in several points: (i) PSC livers show ectopic expression of the adhesion molecule mucosal addressin cell adhesion molecule-1 (MAdCAM-1) which is normally restricted to the gut [67], (ii) in IBD, the intestinal expression of the vascular adhesion protein-1 (VAP-1) expression is significantly increased [68], and (iii) the inability of liver dentritic cells (DCs) to imprint gut tropism [67]. However, the presence of MAdCAM-1 staining in the portal veins could also be detected in other chronic liver diseases, including autoimmune hepatitis, primary biliary cirrhosis and chronic hepatitis C [69]. Thus, MAdCAM-1 expression might rather be a consequence than a cause of inflammation in PSC. Grant’s hypothesis [66] provides no explanation of PSC cases without IBD or why some IBD patients suffer from PSC while others do not.
Taken together, there is a clear clinical association between PSC and IBD, although the pathogenetic link still remains to be unsatisfactorily explained.
Cellular immune response in PSC
The definition and characterization of the cellular immune response in PSC widely depends on the availability of liver tissue suitable for morphological studies including immunohistochemical methods. As far as studying and interpreting of liver samples in PSC patients is concerned the following general problems have to be taken into consideration: (i) a sampling error due to a large intrahepatic heterogeneity in PSC is most likely, (ii) large ducts are usually not seen and efficiently evident on liver biopsy, (iii) the lack of sufficient numbers of liver tissue samples from early stage patients, and (iv) frequently histology – if performed at all due to its low diagnostic value in PSC - is done in late stage disease with advanced fibrosis or cirrhosis. Thus, liver biopsy may rather reveal the footprints of the disease rather than early primary events of PSC which would most probably offer more clues to pathogenesis.
Early histological changes in PSC include a diffuse mixed inflammatory cell infiltrate of lymphocytes, plasma cells and neutrophils that are most intense around the bile ducts [70]. As the disease advances, the inflammation has a tendency to subside, leaving a combination of portal fibrosis and oedema, focal ductular reaction and progressive reduction in the number of bile ducts. The typical histological picture of end-stage-PSC is composed of fibro-obliterative bile duct lesions, characterized by an “onion-skin” type of periductal fibrosis of medium-sized or larger bile ducts, with degeneration and atrophy of biliary epithelial cells [70]. Notably, since this morphological features can only be considered as characteristic but not PSC-specific, the histological distinction between PSC and SSC may be extremely difficult or even impossible. This probably also reflects the limited reaction pattern of bile ducts.
Based on histological findings, the hepatic innate immune response has been considered to be a primary inciting event in the pathogenesis of PSC. Accordingly, it is tempting to speculate that PSC development might be initiated by exogenous triggers such as bacteria or pathogen-associated molecular patterns (PAMPs) which enter the portal circulation via a permeable intestinal mucosa. Consequently, inflammatory cells, such as macrophages, dentritic cells (DCs), and NK cells are activated through pattern recognition receptors, secrete cytokines and perpetuate inflammatory reaction by activation of NK cells through IL-12 and recruitment of lymphocytes via TNF-α, IL-1β and CXCL8 [23]. Biliary epithelial cells (BECs) have an active role in propagating the proinflammatory and profibrotic response. Under physiological conditions BECs express only HLA class I and not class II molecules. In PSC, aberrant expression of HLA class molecules was observed [4,71]. In addition, BECs can acquire a kind of reactive phenotype with overexpression of adhesion molecules and the ability to produce and secrete proinflammatory and chemotactic cytokines and growth factors, further accelerating the inflammatory process [72].
Adaptive immunity is characterized by responses to specific nonself-processed peptide antigens or auto-antigens presented via MHC class I and II molecules on antigen presenting cells to T-cell receptors (TCR) [3,73]. In PSC, a predominant T cell infiltrate in the portal area can be found. However, the composition of T cells (CD4/CD8 ratio) in PSC patients shows considerable inconsistencies in different studies [74,75] which might reflect the distribution of T cell subsets within the liver, in which CD4 cells are seen more commonly in the portal tracts and CD8 cells predominate in areas of lobular hepatitis [76]. TCRs consist of four disulphide linked polypeptides, namely αβ and γδ, and determine the specificity of T cells [4]. Increased proportions of γδ+ T cells have been found in PSC patients, although their significance in disease development is still unknown [77].
Sclerosing cholangitis of the intra- and extrahepatic bile ducts can be observed in patients with autoimmune pancreatitis (AIP), causing still some diagnostic confusion with PSC due to the lack of clear cut diagnostic criteria. Accordingly, sclerosing cholangitis associated with AIP is now referred to as IgG4-related sclerosing cholangitis (IAC) [78,79]. IAC is characterized by a steroid-responsive multisystem fibroinflammatory disorder in which affected organs have a lymphoplasmocytic infiltrate rich in IgG4-positive cells and has recently become widely recognized as distinct clinical entity from classical PSC [80]. Of interest, 10–15% of PSC patients have been shown to have elevated IgG4 in serum and nearly 50% may show IgG4 positive plasma cells on liver biopsy [81]. The fact that in the majority of these IAC cases corticosteroid therapy significantly improves both the pancreatic and the biliary findings supports the idea that these lesions are not typical PSC [80]. So far, however, it is unclear whether IgG4 may also play a role in the pathobiology of classic PSC.
The hypothesis of autoimmune pathogenesis of PSC is supported by the presence of various autoantibodies, including perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) or nuclear antibodies (ANA) in the sera of PSC patients [82]. More than 80% of PSC patients show atypical anti-neutrophil cytoplasmic antibodies (ANCA) [83]. However, due to the overlap with autoimmune hepatitis [83] and the missing correlation with PSC activity [84], ANCA are of limited clinical value as diagnostic or therapy tailoring tool in PSC patients. In general, two distinct staining patterns of ANCA can be distinguished, the cytoplasmic (c-ANCA) and the perinuclear (p-ANCA) which can be further subdivided into so-called classical p-ANCA or atypical p-ANCA [85]. The latter appear to be specific for PSC with predominant IgG classes of antibodies. The question of the autoantigen to which atypical p-ANCA in PSC are react, remains to be determined. A number of proteins, including azurocidin, bactericidal/permeability increasing protein, cathepsin G, elastase and lactoferrin [86–89] have been suggested as potential candidates. However, only in a minority of PSC patients, reactivity to these antigens has been found [85]. Recently, the tubulin beta isoform 5 present in human neutrophils and the bacterial cell division protein FtsZ that is present in nearly all bacteria of the intestinal microflora, have been identified [85]. According to the authors, this might reflect the basis for molecular mimicry in which autoantibodies triggered by a bacterial infection cross-react and inhibit normal immune cell function [23,85]. Cholangiocytes are suggested to be the primary targets of immune attack, since autoantibodies directed against surface antigens on BECs were found in a significantly higher number of PSC compared with PBC, AIH and control patients [90]. Binding of antibodies to the BEC-antigens initiates ERK1/2 signalling and upregulation of toll-like receptors (TLR), which induces the production of cytokines/chemokines by BECs, leading to recruitment of inflammatory cells and initiation and perpetuation of the inflammatory process [91].
Taken together, the general problem of defining cell response in PSC can be attributed to the difficult detection of early PSC lesions in representative tissues. Novel imaging strategies as well as novel serum markers for inflammatory response in PSC patients are therefore warranted.
Novel clues from animal models
To date, no ideal animal model has been established that shows all characteristic of human PSC [59]. Animal models can be classified into the following groups (summarized in Table 2): (i) chemically-induced cholangitis, (ii) knock-out mouse models, (iii) cholangitis induced by infectious agents, (iv) models of experimental biliary obstruction, (v) models involving enteric bacterial cell-wall components or colitis and (vi) models of primary biliary epithelial and endothelial cell injury [59].
Table 2.
Animal models of sclerosing cholangitis.
| Animal model | Species | Proposed pathophysiological mechanisms of SC | Ref. |
|---|---|---|---|
| Chemically induced cholangitis | |||
| TNBS | Sprague–Dawley, Lewis rats | TNBS haptenization, multifactorial? | [115, 116] |
| ANIT | Sprague–Dawley rats | ANIT-induced BEC-injury? | [117] |
| DDC | Swiss albino mice | porphyrogenic properties of DDC | [108] |
| LCA | Swiss albino mice | physiochemical properties of LCA | [109] |
| Knockout mouse models | |||
| Mdr2−/− | FVB/N | toxic bile | [93] |
| Cftr−/− | C57BL/6J | toxic bile | [94] |
| fch/fch | BALB/c | PP accumulation | [95, 96] |
| Infectious agents | |||
| Cryptosporidium parvum | BALB/c nu/nu, BALB/c SCID, C57BL76-SCID, NIH-III nu/nu CD40−/−, IFNγ−/−, CD154−/−, CD40-CD154−/−, Tnfsf5−/−, Tnfrsf1a−/−, Tnfrsf1b−/−, Tnfrsf1a/1b−/−, Tnfsf5-Tnfrsf1a−/−, Tnfsf5-Tnfrsf1b−/−, Tnfsf5-Tnfrsf1a/1b−/−, CD40-Tnfrsf1a/1b−/− |
dysfunctional T cell response to exogenous trigger | [118–120] |
| Helicobacter hepaticus | A/JCr, C3H/HeNCr, C57BL/6NCr, A/J | unknown | [121] |
| Experimental biliary obstruction | C57BL/6J | toxic bile, ↑ biliary pressure | [122] |
| Models involving enteric bacterial cell-wall components or colitis | |||
| SBBO | Lewis and Wistar rats | [54] | |
| PG-PS | Lewis rats | ↑ intestinal permeability | [58] |
| fMLT | Wistar rats | [58] | |
| DSS | CD-1 mice | [123] | |
| TNBS + ANIT | Spraque-Dawley rats | [124] | |
| Models of biliary epithelial and endothelial cell injury | |||
| Experimental GVHD | BALB/c | BEC injury | [111] |
| TNBS | Lewis rats | endothelial cell injury | [125] |
| Complete hepatic arterial deprivation | Wistar rats | hypoxia, ischemia of BECs | [126] |
Abbreviations: ANIT, alpha-naphthylisothiocyanate; BEC, biliary epithelial cell; Cftr, cystic fibrosis transmembrane conductance regulator; DDC, 3,5-diethoxycarbonyl-1,4-dihydrocollidine; DSS, dextrane sodium sulfate; fch, ferrochelatase; fMLT, N-formyl L-methionine L-leucin L-tyrosine; GVHD, graft-versus-host disease; LCA, lithocholic acid; Mdr2, multidrug resistance protein-2; PG-PS, peptidoglycan-polysaccharide; PP, protoporphyrin; SBBO, small bowel bacterial overgrowth; SCID, severe combined immunodeficiency; SFBL, self-filling blind loop; TNBS, 2,4,5-trinitrobenzene sulfonic acid.
The genetic modification of bile composition was shown to induce sclerosing cholangitis and biliary fibrosis via the development of toxic bile in a number of animal models [92–96]. Mice with targeted disruption of the Mdr2 (Abcb4) gene encoding a canalicular phospholipid flippase spontaneously develop cholangitis and typical onionskin type periductal fibrosis mirroring some of the key features of human PSC [92,93]. The development of these morphological changes is most likely due to defective biliary phospholipid secretion resulting in an increased concentration of free non-micellar bile acid [92]. However, the following limitations of the Mdr2−/− mouse model have to be considered: (i) Mdr2−/− mice do not develop IBD or cholangiocellular carcinoma, (ii) the composition of bile in PSC patients without elevated bilirubin was shown to be normal [97], and (iii) the role of MDR3 variants in the pathogenesis of PSC is still unclear [98]. In a study comparing the genetic variability and haplotype structure of MDR3 in PSC patients with healthy controls, no difference in the total number of MDR3 variants or in the allele frequency was observed [99]. However, one heterozygous MDR3 mutation was shown to be specific for PSC and one MDR3 haplotype was more frequently observed in the PSC group [99]. Although the current data do not support a major role for MDR3 in PSC pathogenesis, gene variants could still play an important role by altering bile composition and thereby the aggressiveness of bile, which could influence the secondary response to any primary (e.g., immune-mediated or ischemic) bile duct injury and consequently disease course [98]. However, certain MDR3 haplotypes could be associated with subphenotypes of the disease [99]. Since PSC includes a heterogeneous group of patients with variable pathogenetic background, MDR3 variations should be investigated in selected PSC subgroups, such as those with small duct PSC [98]. Notably, DSS-induced colitis in heterozygous Mdr2+/− mice as a “second hit” induced only mild portal inflammation but not the full-blown picture of sclerosing cholangitis [100].
Mice harbouring a mutation of exon 10 of the cystic fibrosis (CF) transmembrane conductance regulator (CFTR) gene were shown to develop focal cholangitis and biliary cirrhosis within one year of age [94]. However, other investigators identified a predominant intestinal phenotype in Cftr−/− mice with mild or no pathological changes in other organs [101–104]. This is consistent with lacking association of CFTR variants in PSC patients [105]. Intriguingly, recent studies suggest that loss of CFTR affects BEC innate immunity and causes TLR4–NF–κB-mediated inflammation [106]. Paradoxically, a recent study investigating the influence of CFTR polymorphisms on the development and evolution of PSC identified the 1540G variant and the TG11-T7 haplotypes to be associated with protection from PSC, particularly in subjects without IBD [107].
Further evidence for the important role of the bile composition in the development of sclerosing cholangitis and biliary type of liver fibrosis is derived from animal models with chemically-induced bile composition [108,109]. Feeding of 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) in mice induced characteristic features of sclerosing cholangitis [108]. Although the exact underlying mechanisms of DDC-induced cholangitis are unknown, increased porphyrine secretion and induction of a reactive cholangiocyte phenotype via toxic bile components are implicated in the development of the histological features in this model. A recent elegant study suggests a pivotal role for impaired micelle formation and phospholipid secretion also in this model [110].
Feeding of lithocholic acid (LCA) results in the development of bile infarcts, destructive cholangitis, periductal edema and fibrosis in mice, features that are observed in early-stage PSC [109]. The morphological changes are most likely linked to the physiochemical properties of LCA (i.e. high hydrophobicity and lithogenicity) together with obstruction of bile ducts through LCA precipitates [109]. We think that LCA feeding leads to periductal fibrosis via an efflux of “toxic bile” into the portal field and subsequent activation of BECs and periductal myofibroblasts [109]. Due to the short rapid changes observed in LCA-fed animals, this mouse model is a valid short-term model to study early changes in the development of sclerosing cholangitis. Strong evidence for the potential role of vascular injury with subsequent ischemia of BECs in the development of sclerosing cholangitis is provided by animal models of endothelial cell injury. In a mouse model of experimental graft-versus-host disease (GVHD) severe cholangitis of intra- and extrahepatic bile ducts with consequent periductular fibrosis was induced by injecting spleen and bone marrow cells of congenic B10.D2 mice into sublethally irradiated BALB/c mice [111]. The close morphological similarities between this GVHD-model and human PSC suggests that PSC and GVHD share immunological mechanisms [59]. Notably, loss/obliteration of the peribiliary capillary plexus is also a hallmark of bile duct injury in the Abcb4−/− model.
Taken together, none of these model systems embody all the characteristics which one would arrogate from an ideal PSC animal model but allows studying at least specific aspects of the still enigmatic pathobiology of this devastating disease.
Summary
Despite recent advances in identifying important subgroups of PSC and learning important aspects on their natural history as well as the increasing availability of animal models a clear and conclusive picture in regard to disease initiation and pathogenesis is still lacking. Our research agenda should include (i) to identify and develop novel non-invasive tools for early PSC detection and (ii) to increase the use of tests that are already available (e.g. MRCP). In patients with high suspicion of PSC, such as IBD patients with a cholestatic enzyme pattern, systematically screening and repeated imaging using MRCP should be performed. Partially, the clinical impact of such an approach is already reflected by the changing clinical picture of PSC, since patients are increasingly diagnosed in asymptomatic stages [112]. Careful clinical observational studies of clearly defined patient (sub)populations together with novel animal models should significantly speed up our gain in knowledge on the pathogenesis of PSC.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grant P19118 from the Austrian Science Fund.
References
- 1.Schrumpf E., Boberg K.M. Epidemiology of primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:553–562. doi: 10.1053/bega.2001.0204. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell S.A., Thyssen M., Orchard T.R. Cigarette smoking, appendectomy, and tonsillectomy as risk factors for the development of primary sclerosing cholangitis: a case control study. Gut. 2002;51:567–573. doi: 10.1136/gut.51.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Mahony C.A., Vierling J.M. Etiopathogenesis of primary sclerosing cholangitis. Semin Liver Dis. 2006;26:3–21. doi: 10.1055/s-2006-933559. [DOI] [PubMed] [Google Scholar]
- 4.Chapman R., Cullen S. Etiopathogenesis of primary sclerosing cholangitis. World J Gastroenterol. 2008;14:3350–3359. doi: 10.3748/wjg.14.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrumpf E., Fausa O., Førre O. HLA antigens and immunoregulatory T cells in ulcerative colitis associated with hepatobiliary disease. Scand J Gastroenterol. 1982;17:187–191. doi: 10.3109/00365528209182038. [DOI] [PubMed] [Google Scholar]
- 6.Bergquist A., Lindberg G., Saarinen S. Increased prevalence of primary sclerosing cholangitis among first-degree relatives. J Hepatol. 2005;42:252–256. doi: 10.1016/j.jhep.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist A., Montgomery S.M., Bahmanyar S. Increased risk of primary sclerosing cholangitis and ulcerative colitis in first-degree relatives of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. 2008;6:939–943. doi: 10.1016/j.cgh.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Karlsen T.H., Franke A., Melum E. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138:1102–1111. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Horton R., Wilming L., Rand V. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 10.Parham P.M.H.C. class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 11.Chapman R.W., Varghese Z., Gaul R. Association of primary sclerosing cholangitis with HLA-B8. Gut. 1983;24:38–41. doi: 10.1136/gut.24.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leidenius M.H., Koskimies S.A., Kellokumpu I.H. HLA antigens in ulcerative colitis and primary sclerosing cholangitis. APMIS. 1995;103:519–524. doi: 10.1111/j.1699-0463.1995.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey G.P., Reed W.D., Laurence B.H. Primary sclerosing cholangitis: clinical and immunopathological review of 21 cases. J Gastroenterol Hepatol. 1990;5:135–140. doi: 10.1111/j.1440-1746.1990.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 14.Neri T.M., Cavestro G.M., Seghini P. Novel association of HLA-haplotypes with primary sclerosing cholangitis (PSC) in a southern European population. Dig Liver Dis. 2003;35:571–576. doi: 10.1016/s1590-8658(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 15.Bittencourt P.L., Palacios S.A., Cançado E.L. Susceptibility to primary sclerosing cholangitis in Brazil is associated with HLA-DRB1∗13 but not with tumour necrosis factor alpha -308 promoter polymorphism. Gut. 2002;51:609–610. doi: 10.1136/gut.51.4.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson P.T. Genetics of liver disease: immunogenetics and disease pathogenesis. Gut. 2004;53:599–608. doi: 10.1136/gut.2003.031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moloney M.M., Thomson L.J., Strettell M.J. Human leukocyte antigen-C genes and susceptibility to primary sclerosing cholangitis. Hepatology. 1998;28:660–662. doi: 10.1002/hep.510280309. [DOI] [PubMed] [Google Scholar]
- 18.Norris S., Kondeatis E., Collins R. Mapping MHC-encoded susceptibility and resistance in primary sclerosing cholangitis: the role of MICA polymorphism. Gastroenterology. 2001;120:1475–1482. doi: 10.1053/gast.2001.24041. [DOI] [PubMed] [Google Scholar]
- 19.Wiencke K., Spurkland A., Schrumpf E. Primary sclerosing cholangitis is associated to an extended B8-DR3 haplotype including particular MICA and MICB alleles. Hepatology. 2001;34:625–630. doi: 10.1053/jhep.2001.27543. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell S.A., Grove J., Spurkland A. Association of the tumour necrosis factor alpha -308 but not the interleukin 10 -627 promoter polymorphism with genetic susceptibility to primary sclerosing cholangitis. Gut. 2001;49:288–294. doi: 10.1136/gut.49.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spurkland A., Saarinen S., Boberg K.M. HLA class II haplotypes in primary sclerosing cholangitis patients from five European populations. Tissue Antigens. 1999;53:459–469. doi: 10.1034/j.1399-0039.1999.530502.x. [DOI] [PubMed] [Google Scholar]
- 22.Farrant J.M., Doherty D.G., Donaldson P.T. Amino acid substitutions at position 38 of the DR beta polypeptide confer susceptibility to and protection from primary sclerosing cholangitis. Hepatology. 1992;16:390–395. doi: 10.1002/hep.1840160217. [DOI] [PubMed] [Google Scholar]
- 23.Aron J.H., Bowlus C.L. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol. 2009;31:383–397. doi: 10.1007/s00281-009-0154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pazmany L. Do NK cells regulate human autoimmunity? Cytokine. 2005;32:76–80. doi: 10.1016/j.cyto.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Karlsen T.H., Boberg K.M., Olsson M. Particular genetic variants of ligands for natural killer cell receptors may contribute to the HLA associated risk of primary sclerosing cholangitis. J Hepatol. 2007;46:899–906. doi: 10.1016/j.jhep.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 26.Karlsen T.H., Boberg K.M., Vatn M. Different HLA class II associations in ulcerative colitis patients with and without primary sclerosing cholangitis. Genes Immun. 2007;8:275–278. doi: 10.1038/sj.gene.6364377. [DOI] [PubMed] [Google Scholar]
- 27.Adams D.H., Hubscher S.G., Shaw J. Increased expression of intercellular adhesion molecule 1 on bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 1991;14:426–431. [PubMed] [Google Scholar]
- 28.Polzien F., Ramadori G. Increased intercellular adhesion molecule-1 serum concentration in cholestasis. J Hepatol. 1996;25:877–886. doi: 10.1016/s0168-8278(96)80292-3. [DOI] [PubMed] [Google Scholar]
- 29.Yang X., Cullen S.N., Li J.H. Susceptibility to primary sclerosing cholangitis is associated with polymorphisms of intercellular adhesion molecule-1. J Hepatol. 2004;40:375–379. doi: 10.1016/j.jhep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Bowlus C.L., Karlsen T.H., Broomé U. Analysis of MAdCAM-1 and ICAM-1 polymorphisms in 365 Scandinavian patients with primary sclerosing cholangitis. J Hepatol. 2006;45:704–710. doi: 10.1016/j.jhep.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Venkatesan S., Petrovic A., Locati M. A membrane-proximal basic domain and cysteine cluster in the C-terminal tail of CCR5 constitute a bipartite motif critical for cell surface expression. J Biol Chem. 2001;276:40133–40145. doi: 10.1074/jbc.M105722200. [DOI] [PubMed] [Google Scholar]
- 32.Donaldson P., Agarwal K., Saarinen S. Investigation of CCR5 Delta 32 deletion and FAS/APO-1 (CD95) single nucleotide polymorphisms in primary sclerosing cholangitis (PSC) Hepatology. 2000;32:174A. [Google Scholar]
- 33.Satsangi J., Simmons J., Marshall S. CCR5Δ32 polymorphism in inflammatory bowel disease: further association with ulcerative colitis and with primary sclerosing cholangitis. Gastroenterology. 2000;118:A337. [Google Scholar]
- 34.Eri R., Jonsson J.R., Pandeya N. CCR5-Delta32 mutation is strongly associated with primary sclerosing cholangitis. Genes Immun. 2004;5:444–450. doi: 10.1038/sj.gene.6364113. [DOI] [PubMed] [Google Scholar]
- 35.Henckaerts L., Fevery J., Van Steenbergen W. CC-type chemokine receptor 5-Delta32 mutation protects against primary sclerosing cholangitis. Inflamm Bowel Dis. 2006;12:272–277. doi: 10.1097/01.MIB.0000209790.21737.28. [DOI] [PubMed] [Google Scholar]
- 36.Melum E., Karlsen T.H., Broomé U. The 32-base pair deletion of the chemokine receptor 5 gene (CCR5-Delta32) is not associated with primary sclerosing cholangitis in 363 Scandinavian patients. Tissue Antigens. 2006;68:78–81. doi: 10.1111/j.1399-0039.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 37.Keitel V., Donner M., Winandy S. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 38.Hov J.R., Keitel V., Laerdahl J.K. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS One. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loftus E.V., Jr., Harewood G.C., Loftus C.G. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91–96. doi: 10.1136/gut.2004.046615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trauner M., Boyer J.L. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 41.Ho G.T., Moodie F.M., Satsangi J. Multidrug resistance 1 gene (P-glycoprotein 170): an important determinant in gastrointestinal disease? Gut. 2003;52:759–766. doi: 10.1136/gut.52.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karlsen T.H., Hampe J., Wiencke K. Genetic polymorphisms associated with inflammatory bowel disease do not confer risk for primary sclerosing cholangitis. Am J Gastroenterol. 2007;102:115–121. doi: 10.1111/j.1572-0241.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- 43.Zintzaras E. Is there evidence to claim or deny association between variants of the multidrug resistance gene (MDR1 or ABCB1) and inflammatory bowel disease? Inflamm Bowel Dis. in press. [DOI] [PubMed]
- 44.Orans J., Teotico D.G., Redinbo M.R. The nuclear xenobiotic receptor pregnane X receptor: recent insights and new challenges. Mol Endocrinol. 2005;19:2891–2900. doi: 10.1210/me.2005-0156. [DOI] [PubMed] [Google Scholar]
- 45.Schuetz E., Strom S. Promiscuous regulator of xenobiotic removal. Nat Med. 2001;7:536–537. doi: 10.1038/87856. [DOI] [PubMed] [Google Scholar]
- 46.Parks D.J., Blanchard S.G., Bledsoe R.K. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 47.Langmann T., Moehle C., Mauerer R. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004;127:26–40. doi: 10.1053/j.gastro.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Oswald M., Kullak-Ublick G.A., Paumgartner G. Expression of hepatic transporters OATP-C and MRP2 in primary sclerosing cholangitis. Liver. 2001;21:247–253. doi: 10.1034/j.1600-0676.2001.021004247.x. [DOI] [PubMed] [Google Scholar]
- 49.Glas J., Seiderer J., Fischer D. Pregnane X receptor (PXR/NR1I2) gene haplotypes modulate susceptibility to inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1917–1924. doi: 10.1002/ibd.21562. [DOI] [PubMed] [Google Scholar]
- 50.Karlsen T.H., Lie B.A., Frey Frøslie K. Polymorphisms in the steroid and xenobiotic receptor gene influence survival in primary sclerosing cholangitis. Gastroenterology. 2006;131:781–787. doi: 10.1053/j.gastro.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 51.Smith M.P., Loe R.H. Sclerosing cholangitis; review of recent case reports and associated diseases and four new cases. Am J Surg. 1965;110:239–246. doi: 10.1016/0002-9610(65)90018-8. [DOI] [PubMed] [Google Scholar]
- 52.Fausa O., Schrumpf E., Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991;11:31–39. doi: 10.1055/s-2008-1040420. [DOI] [PubMed] [Google Scholar]
- 53.Gow P.J., Chapman R.W. Liver transplantation for primary sclerosing cholangitis. Liver. 2000;20:97–103. doi: 10.1034/j.1600-0676.2000.020002097.x. [DOI] [PubMed] [Google Scholar]
- 54.Aadland E., Schrumpf E., Fausa O. Primary sclerosing cholangitis: a long-term follow-up study. Scand J Gastroenterol. 1987;22:655–664. doi: 10.3109/00365528709011139. [DOI] [PubMed] [Google Scholar]
- 55.Cholongitas E., Shusang V., Patch D. Pathogenesis of primary sclerosing cholangitis. J Hepatol. 2008;49:863–864. doi: 10.1016/j.jhep.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Jørgensen KK, Grzyb K, Lundin KE, et al. Inflammatory bowel disease in patients with primary sclerosing cholangitis: clinical characterization in liver transplanted and nontransplanted patients. Inflamm Bowel Dis. in press. [DOI] [PubMed]
- 57.Lichtman S.N., Sartor R.B. Hepatobiliary injury associated with experimental small-bowel bacterial overgrowth in rats. Immunol Res. 1991;10:528–531. doi: 10.1007/BF02919752. [DOI] [PubMed] [Google Scholar]
- 58.Yamada S., Ishii M., Liang L.S. Small duct cholangitis induced by N-formyl L-methionine L-leucine L-tyrosine in rats. J Gastroenterol. 1994;29:631–636. doi: 10.1007/BF02365447. [DOI] [PubMed] [Google Scholar]
- 59.Pollheimer MJ, Trauner M, Fickert P. Will we ever model PSC? - "It’s hard to be a PSC model!" Clin Res Hepatol Gastroenterol. in press. [DOI] [PubMed]
- 60.Brooke B.N., Dykes P.W., Walker F.C. A study of liver disorder in ulcerative colitis. Postgrad Med J. 1961;37:245–251. doi: 10.1136/pgmj.37.427.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olsson R., Björnsson E., Bäckman L. Bile duct bacterial isolates in primary sclerosing cholangitis: a study of explanted livers. J Hepatol. 1998;28:426–432. doi: 10.1016/s0168-8278(98)80316-4. [DOI] [PubMed] [Google Scholar]
- 62.Björnsson E., Cederborg A., Akvist A. Intestinal permeability and bacterial growth of the small bowel in patients with primary sclerosing cholangitis. Scand J Gastroenterol. 2005;40:1090–1094. doi: 10.1080/00365520510023288. [DOI] [PubMed] [Google Scholar]
- 63.Mistilis S.P., Skyring A.P., Goulston S.J. Effect of long-term tetracycline therapy, steroid therapy and colectomy in pericholangitis associated with ulcerative colitis. Australas Ann Med. 1965;14:286–294. doi: 10.1111/imj.1965.14.4.286. [DOI] [PubMed] [Google Scholar]
- 64.Färkkilä M., Karvonen A.L., Nurmi H. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology. 2004;40:1379–1386. doi: 10.1002/hep.20457. [DOI] [PubMed] [Google Scholar]
- 65.ter Borg P.C., van Buuren H.R., Depla A.C. Bacterial cholangitis causing secondary sclerosing cholangitis: a case report. BMC Gastroenterol. 2002;2:14. doi: 10.1186/1471-230X-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grant A.J., Lalor P.F., Salmi M. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359:150–157. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- 67.Eksteen B., Mora J.R., Haughton E.L. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009;137:320–329. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eksteen B., Miles A.E., Grant A.J. Lymphocyte homing in the pathogenesis of extra-intestinal manifestations of inflammatory bowel disease. Clin Med. 2004;4:173–180. doi: 10.7861/clinmedicine.4-2-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grant A.J., Lalor P.F., Hübscher S.G. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease) Hepatology. 2001;33:1065–1072. doi: 10.1053/jhep.2001.24231. [DOI] [PubMed] [Google Scholar]
- 70.Portmann B.C., Nakanuma Y. Diseases of the bile ducts. In: Burt A.D., Portmann B.C., Ferrell L.D., editors. MacSween’s pathology of the liver. 5th ed. Elsevier; Philadelphia: 2007. pp. 549–550. [Google Scholar]
- 71.Van den Oord J.J., Sciot R., Desmet V.J. Expression of MHC products by normal and abnormal bile duct epithelium. J Hepatol. 1986;3:310–317. doi: 10.1016/s0168-8278(86)80483-4. [DOI] [PubMed] [Google Scholar]
- 72.Lazaridis K.N., Strazzabosco M., Larusso N.F. The cholangiopathies: disorders biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 73.Delves P.J., Roitt I.M. The immune system. second of two parts. N Engl J Med. 2000;343:108–117. doi: 10.1056/NEJM200007133430207. [DOI] [PubMed] [Google Scholar]
- 74.Bo X., Broome U., Remberger M. Tumour necrosis factor alpha impairs function of liver derived T lymphocytes and natural killer cells in patients with primary sclerosing cholangitis. Gut. 2001;49:131–141. doi: 10.1136/gut.49.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whiteside T.L., Lasky S., Si L. Immunologic analysis of mononuclear cells in liver tissues and blood of patients with primary sclerosing cholangitis. Hepatology. 1985;5:468–474. doi: 10.1002/hep.1840050321. [DOI] [PubMed] [Google Scholar]
- 76.Hashimoto E., Lindor K.D., Homburger H.A. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clin Proc. 1993;68:1049–1055. doi: 10.1016/s0025-6196(12)60897-0. [DOI] [PubMed] [Google Scholar]
- 77.Lindor K.D., Wiesner R.H., Katzmann J.A. Lymphocyte subsets in primary sclerosing cholangitis. Dig Dis Sci. 1987;32:720–725. doi: 10.1007/BF01296138. [DOI] [PubMed] [Google Scholar]
- 78.European Association for the Study of the Liver EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 79.Björnsson E., Chari S., Silveira M. Primary sclerosing cholangitis associated with elevated immunoglobulin G4: clinical characteristics and response to therapy. Am J Ther. 2011;18:198–205. doi: 10.1097/MJT.0b013e3181c9dac6. [DOI] [PubMed] [Google Scholar]
- 80.Mendes F.D., Jorgensen R., Keach J. Elevated serum IgG4 concentration in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2006;101:2070–2075. doi: 10.1111/j.1572-0241.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 81.Ali S., Fischer S.E., Meaney C. High prevalence of IgG4 positive immunohistochemical cholangitis in liver explants from patients with PSC. Hepatology. 2010;52:490A. [Google Scholar]
- 82.Angulo P., Peter J.B., Gershwin M.E. Serum autoantibodies in patients with primary sclerosing cholangitis. J Hepatol. 2000;32:182–187. doi: 10.1016/s0168-8278(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 83.Terjung B., Worman H.J. Anti-neutrophil antibodies in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:629–642. doi: 10.1053/bega.2001.0209. [DOI] [PubMed] [Google Scholar]
- 84.Schwarze C., Terjung B., Lilienweiss P. IgA class antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis and autoimmune hepatitis. Clin Exp Immunol. 2003;133:283–289. doi: 10.1046/j.1365-2249.2003.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Terjung B., Spengler U. Atypical p-ANCA in PSC and AIH: a hint toward a "leaky gut”. Clin Rev Allergy Immunol. 2009;36:40–51. doi: 10.1007/s12016-008-8088-8. [DOI] [PubMed] [Google Scholar]
- 86.Zhao M.H., Lockwood C.M. Azurocidin is a novel antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in systemic vasculitis. Clin Exp Immunol. 1996;103:397–402. doi: 10.1111/j.1365-2249.1996.tb08293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao M.H., Jones S.J., Lockwood C.M. Bactericidal/permeability-increasing protein (BPI) is an important antigen for anti-neutrophil cytoplasmic autoantibodies (ANCA) in vasculitis. Clin Exp Immunol. 1995;99:49–56. doi: 10.1111/j.1365-2249.1995.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Halbwachs-Mecarelli L., Nusbaum P., Noël L.H. Antineutrophil cytoplasmic antibodies (ANCA) directed against cathepsin G in ulcerative colitis, Crohn’s disease and primary sclerosing cholangitis. Clin Exp Immunol. 1992;90:79–84. doi: 10.1111/j.1365-2249.1992.tb05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peen E., Almer S., Bodemar G. Anti-lactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn’s disease. Gut. 1993;34:56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu B., Broome U., Ericzon B.G. High frequency of autoantibodies in patients with primary sclerosing cholangitis that bind biliary epithelial cells and induce expression of CD44 and production of interleukin 6. Gut. 2002;51:120–127. doi: 10.1136/gut.51.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karrar A., Broomé U., Södergren T. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology. 2007;132:1504–1514. doi: 10.1053/j.gastro.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 92.Smit J.J., Schinkel A.H., Oude Elferink R.P. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 93.Fickert P., Fuchsbichler A., Wagner M. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Durie P.R., Kent G., Phillips M.J. Characteristic multiorgan pathology of cystic fibrosis in a long-living cystic fibrosis transmembrane regulator knockout murine model. Am J Pathol. 2004;164:1481–1493. doi: 10.1016/S0002-9440(10)63234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meerman L., Koopen N.R., Bloks V. Biliary fibrosis associated with altered bile composition in a mouse model of erythropoietic protoporphyria. Gastroenterology. 1999;117:696–705. doi: 10.1016/s0016-5085(99)70464-6. [DOI] [PubMed] [Google Scholar]
- 96.Libbrecht L., Meerman L., Kuipers F. Liver pathology and hepatocarcinogenesis in a long-term mouse model of erythropoietic protoporphyria. J Pathol. 2003;199:191–200. doi: 10.1002/path.1257. [DOI] [PubMed] [Google Scholar]
- 97.deVree J.M. University of Amsterdam; 1999. Defects in hepatobiliary transport. genetics and therapy of progressive familial intrahepatic cholestasis type 3. [Google Scholar]
- 98.Trauner M., Fickert P., Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis. 2007;27:77–98. doi: 10.1055/s-2006-960172. [DOI] [PubMed] [Google Scholar]
- 99.Pauli-Magnus C., Kerb R., Fattinger K. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39:779–791. doi: 10.1002/hep.20159. [DOI] [PubMed] [Google Scholar]
- 100.Jahnel J., Fickert P., Langner C. Impact of experimental colitis on hepatobiliary transporter expression and bile duct injury in mice. Liver Int. 2009;29:1316–1325. doi: 10.1111/j.1478-3231.2009.02044.x. [DOI] [PubMed] [Google Scholar]
- 101.Snouwaert J.N., Brigman K.K., Latour A.M. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 102.Dorin J.R., Dickinson P., Alton E.W. Cystic fibrosis in the mouse by targeted insertional mutagenesis. Nature. 1992;359:211–215. doi: 10.1038/359211a0. [DOI] [PubMed] [Google Scholar]
- 103.Colledge W.H., Abella B.S., Southern K.W. Generation and characterization of a delta F508 cystic fibrosis mouse model. Nat Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- 104.Zeiher B.G., Eichwald E., Zabner J. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Girodon E., Sternberg D., Chazouillères O. Cystic fibrosis transmembrane conductance regulator (CFTR) gene defects in patients with primary sclerosing cholangitis. J Hepatol. 2002;37:192–197. doi: 10.1016/s0168-8278(02)00161-7. [DOI] [PubMed] [Google Scholar]
- 106.Fiorotto R., Scirpo R., Trauner M., Fabris L., Hoque R., Spirli C. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-Mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henckaerts L., Jaspers M., Van Steenbergen W. Cystic fibrosis transmembrane conductance regulator gene polymorphisms in patients with primary sclerosing cholangitis. J Hepatol. 2009;50:150–157. doi: 10.1016/j.jhep.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 108.Fickert P., Stöger U., Fuchsbichler A. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fickert P., Fuchsbichler A., Marschall H.U. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol. 2006;168:410–422. doi: 10.2353/ajpath.2006.050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lyoumi S., Abitbol M., Rainteau D., Karim Z., Bernex F., Oustric V. Protoporphyrin retention in hepatocytes and kupffer cells prevents sclerosing cholangitis in erythropoietic protoporphyria mouse model. Gastroenterology. 2011;141:1509–1519. doi: 10.1053/j.gastro.2011.06.078. [DOI] [PubMed] [Google Scholar]
- 111.Nonomura A., Kono N., Minato H. Diffuse biliary tract involvement mimicking primary sclerosing cholangitis in an experimental model of chronic graft-versus-host disease in mice. Pathol Int. 1998;48:421–427. doi: 10.1111/j.1440-1827.1998.tb03927.x. [DOI] [PubMed] [Google Scholar]
- 112.Worthington J., Chapman R. Primary sclerosing cholangitis. Orphanet J Rare Dis. 2006;1:41. doi: 10.1186/1750-1172-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wiencke K., Louka A.S., Spurkland A. Association of matrix metalloproteinase-1 and -3 promoter polymorphisms with clinical subsets of Norwegian primary sclerosing cholangitis patients. J Hepatol. 2004;41:209–214. doi: 10.1016/j.jhep.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 114.Satsangi J., Chapman R.W., Haldar N. A functional polymorphism of the stromelysin gene (MMP-3) influences susceptibility to primary sclerosing cholangitis. Gastroenterology. 2001;121:124–130. doi: 10.1053/gast.2001.25527. [DOI] [PubMed] [Google Scholar]
- 115.Mourelle M., Salas A., Vilaseca J. Induction of chronic cholangitis in the rat by trinitrobenzenesulfonic acid. J Hepatol. 1995;22:219–225. doi: 10.1016/0168-8278(95)80432-3. [DOI] [PubMed] [Google Scholar]
- 116.Orth T., Neurath M., Schirmacher P. A novel rat model of chronic fibrosing cholangitis induced by local administration of a hapten reagent into the dilated BD is associated with increased TNF-alpha production and autoantibodies. J Hepatol. 2000;33:862–872. doi: 10.1016/s0168-8278(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 117.Lichtman S.N., Wang J., Clark R.L. A microcholangiographic study of liver disease models in rats. Acad Radiol. 1995;2:515–521. doi: 10.1016/s1076-6332(05)80410-6. [DOI] [PubMed] [Google Scholar]
- 118.Stephens J., Cosyns M., Jones M. Liver and bile duct pathology following cryptosporidium parvum infection of immunodeficient mice. Hepatology. 1999;30:27–35. doi: 10.1002/hep.510300138. [DOI] [PubMed] [Google Scholar]
- 119.Ungar B.L., Burris J.A., Quinn C.A. New mouse models for chronic cryptosporidium infection in immunodeficient hosts. Infect Immun. 1990;58:961–969. doi: 10.1128/iai.58.4.961-969.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mead J.R., Arrowood M.J., Sidwell R.W. Chronic cryptosporidium parvum infections in congenitally immunodeficient SCID and nude mice. J Infect Dis. 1991;163:1297–1304. doi: 10.1093/infdis/163.6.1297. [DOI] [PubMed] [Google Scholar]
- 121.Ward J.M., Anver M.R., Haines D.C. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959–968. [PMC free article] [PubMed] [Google Scholar]
- 122.Georgiev P., Jochum W., Heinrich S. Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 2008;95:646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- 123.Numata Y., Tazuma S., Nishioka T. Immune response in mouse experimental cholangitis associated with colitis induced by dextran sulfate sodium. J Gastroenterol Hepatol. 2004;19:910–915. doi: 10.1111/j.1440-1746.2003.03333.x. [DOI] [PubMed] [Google Scholar]
- 124.Tjandra K., Le T., Swain M.G. Experimental colitis attenuates development of toxin-induced cholangitis in rats. Dig Dis Sci. 2002;47:1216–1223. doi: 10.1023/a:1015330809095. [DOI] [PubMed] [Google Scholar]
- 125.Orth T., Neurath M., Schirmacher P. Anti-neutrophil cytoplasmic antibodies in a rat model of trinitrobenzenesulphonic acid-induced liver injury. Eur J Clin Invest. 1999;29:929–939. doi: 10.1046/j.1365-2362.1999.00547.x. [DOI] [PubMed] [Google Scholar]
- 126.Beaussier M., Wendum D., Fouassier L. Adaptative bile duct proliferative response in experimental bile duct ischemia. J Hepatol. 2005;42:257–265. doi: 10.1016/j.jhep.2004.10.025. [DOI] [PubMed] [Google Scholar]