Abstract
Breast cancer is a complex and heterogeneous disease. Gene expression profiling has contributed significantly to our understanding of this heterogeneity at a molecular level, refining taxonomy based on simple measures such as histological type, tumour grade, lymph node status and the presence of predictive markers like oestrogen receptor and human epidermal growth factor receptor 2 (HER2) to a more sophisticated classification comprising luminal A, luminal B, basal-like, HER2-positive and normal subgroups. In the laboratory, breast cancer is often modelled using established cell lines. In the present review we discuss some of the issues surrounding the use of breast cancer cell lines as experimental models, in light of these revised clinical classifications, and put forward suggestions for improving their use in translational breast cancer research.
Introduction
The first human cell line was established in a Baltimore laboratory over 50 years ago by George Gey [1]. This cell line was HeLa - named after Henrietta Lacks, the lady from whom the cell line was derived, who had cervical carcinoma. Gey's vision paved the way for cell culture as we know it today, allowing its widespread development into an important experimental tool in cancer research. One of the major benefits of using cultured cell lines in cancer research is that they offer an infinite supply of a relatively homogeneous cell population that is capable of self-replication in standard cell culture medium.
The first breast cancer cell line to be established was BT-20 in 1958 [2]. It was another 20 years, however, before establishing breast cancer cell lines became more widespread, including the MD Anderson series [3] and what still remains the most commonly used breast cancer cell line in the world, MCF-7 established in 1973 at the Michigan Cancer Foundation [4]. The popularity of MCF-7 is largely due to its exquisite hormone sensitivity through expression of oestrogen receptor (ER), making it an ideal model to study hormone response [5].
Despite these early accomplishments, relatively few breast cancer cell lines have been established in the more recent past, mainly because of difficulties in culturing homogeneous populations without significant stromal contamination and, at least in the United Kingdom, partly due to rigorous ethical regulations surrounding obtaining human tissue for research [6]. Successes include the SUM series of 10 cell lines derived from either breast primary tumours, pleural effusions or various metastatic sites in individual patients [7]. These cell lines are now widely available through commercial cell banks.
Breast cancer heterogeneity
Long before the advent of modern molecular profiling techniques, histopathologists recognised that breast cancer was heterogeneous through morphological observations. Classification was based on the following measures: histological type, tumour grade, lymph node status and the presence of predictive markers such as ER and, more recently, human epidermal growth factor receptor 2 (HER2). The development of molecular profiling using DNA microarrays proved this heterogeneity, demonstrating through gene expression profiling and the immunohistochemical expression of ERα, progesterone receptor (PR) and HER2 that breast cancer could be classified into at least five subtypes: luminal A, luminal B, HER2, basal and normal [8,9]. Molecular characteristics of these sub-types are summarised in Table 1.
Table 1.
Molecular classification of breast carcinoma
| Classification | Immunoprofile | Other characteristics | Example cell lines (adapted from [13,22]) |
|---|---|---|---|
| Luminal A | ER+, PR+/-, HER2- | Ki67 low, endocrine responsive, often chemotherapy responsive | MCF-7, T47D, SUM185 |
| Luminal B | ER+, PR+/-, HER2+ | Ki67 high, usually endocrine responsive, variable to chemotherapy. HER2+ are trastusumab responsive | BT474, ZR-75 |
| Basal | ER-, PR-, HER2- | EGFR+ and/or cytokeratin 5/6+, Ki67 high, endocrine nonresponsive, often chemotherapy responsive | MDA-MB-468, SUM190 |
| Claudin-low | ER-, PR-, HER2- | Ki67, E-cadherin, claudin-3, claudinin-4 and claudinin-7 low. Intermediate response to chemotherapy | BT549, MDA-MB-231, Hs578T, SUM1315 |
| HER2 | ER-, PR-, HER2+ | Ki67 high, trastusumab responsive, chemotherapy responsive | SKBR3, MDA-MB-453 |
EGFR, epidermal growth factor receptor; ER, oestrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Each subtype has different prognosis and treatment response [10]. Because ER is a therapeutic target, the luminal A and luminal B subtypes are amenable to hormone therapy. Similarly the HER2 group are potential candidates for trasuszumab therapy. In the current absence of expression of a recognised therapeutic target, basal tumours are difficult to treat, more biologically aggressive and often have a poor prognosis. Because the basal phenotype is characterised by the lack of expression of ERα, PR and HER2, it is sometimes referred to as triple-negative. Although there are similarities in the basal and triple-negative phenotypes, the terms are not strictly interchangeable; as outlined in a recent review, there is still no unifying definition for basal cancers and, while triple-negative enriches for basal breast cancer, the phenotypes are not identical [11].
More recently the claudin-low subtype was described by interrogating established human and murine datasets [12]. Initially clustered with the basal subtype as a result of a lack of ERα, PR and HER2 expression and associated poor prognosis, these tumours were shown to be unique by the additional downregulation of claudin-3 and claudinin-4, low expression of the proliferation marker Ki67, enrichment for markers associated with the epithelial-mesenchymal transition and expression of features associated with mammary cancer stem cells (CSCs) (for example, CD44+CD24-/low phenotype) [13].
Do current breast cancer cell line models reflect breast cancer heterogeneity?
Our group previously highlighted the pros and cons of using cell lines as in vitro models of breast cancer [14]. Although questions have been raised over how representative immortalised cell lines are of human breast cancer [15], when used in the right way these remain powerful experimental tools and in many instances the information derived from these has translated into clinical benefit. A good example was the recognition that anti-oestrogens regulated the growth of tamoxifen-stimulated MCF-7 cells [16,17], paving the way for the ultimate development and subsequent trials of fulvestrant (Faslodex®, AstraZeneca Pharmaceutical LP, Wilmington, DE, USA), a selective ER downregulator that is now recommended for the treatment of recurrent ER-positive metastatic breast cancer in the postmenopausal setting [18,19].
With the different molecular classifications of breast cancer now firmly established, researchers have turned their attention to breast cancer cell lines to determine whether the molecular profiles observed in breast carcinomas are reflected in cell line models of the disease. A comprehensive evaluation of breast cancer cell lines by Lacroix and Leclercq, conducted before molecular profiling of breast cancer was widespread, concluded that while breast cancer cell lines have advanced our understanding of breast cancer biology, gaps still remained in terms of how representative these are [20] - in particular, the extent to which a single cell line can mirror the heterogeneity associated with clinical samples, the limited coverage of specialised histopathological types and whether the phenotype of a breast tumour in vivo is maintained in cell culture. This conclusion was reinforced in a breast cancer gap analysis [21]. Application of sophisticated transcriptional profiling to breast cancer cell lines using various platforms has gone some way to address these issues. In general, these studies have shown that the luminal, basal, HER2 and claudin-low clusters identified in breast tumours can easily be distinguished in breast cancer cell lines (Table 1) [13,22-26]. Of note is the finding that the claudin-low subtype seems to be over-represented in breast cancer cell lines, possibly as a result of the ease of growth associated with cells that lack ERα, PR and HER2. These cell lines provide good opportunities for the further study of this phenotype, which will enhance our understanding of its biology.
In an estimate of therapeutic response, luminal breast cancer cell lines preferentially responded to the AKT inhibitor GSK690693 and the phosphoinositide 3-kinase inhibitor GSK1069615, while proliferation of basal cell lines was selectively inhibited by the MEK protein kinase inhibitor GSK1120212 [27]. The response to trastuzumab, an antibody that selectively binds HER2, was evaluated in a panel of nine breast cancer cell lines with known HER2 amplification, but only three out of nine cell lines showed an unequivocal response [22]. This is in line with clinical observations reporting an efficacy of 34% for trastuzumab [28] and serves to highlight that relying on a single cell line could generate incorrect or misleading data. These studies indicate the need for a more rational approach for screening potential new breast cancer therapies by taking into account the different subgroups and recognising that response may not always be identical even within subgroups.
Breast cancer cell lines as models of mammary cancer stem cells
Stem cells are characterised by their ability to yield new tumours when xenografted into immunodeficient mice. This was first demonstrated in breast cancer by Al-Hajj and colleagues, who showed that as few as 100 to 200 breast CSCs with the phenotype CD44+CD24-/lowLin- were capable of forming tumours when introduced into the mammary fat pad of NOD/SCID mice [29]. Nowadays, breast CSCs are identified by one or more of the following features: their ability to form tumours in vivo; mammosphere formation in vitro; expression of aldehyde dehydrogenase; or through expression of cell surface biomarkers, usually the CD44+/CD24-/low phenotype [30].
Increasingly demonstrated is that very small numbers of CSCs (often described as tumour-initiating cells) exist within human breast cancer cell lines [31,32]. There are clearly many advantages to working with CSCs derived from cell lines as they may be good models to further understand stem cell biology and develop CSC-specific therapeutic targets. Two major obstacles need to be overcome, however, before these can be developed for routine use: CSCs are very much in the minority within a given tumour population, and CSCs have extremely slow population-doubling times. Improved enrichment methods are required to provide sufficient numbers of CSCs to conduct these types of studies, and their slow proliferation rates are challenging when it comes to experimentally testing potential new therapeutics.
The cell culture environment
Complex inter-relationships that exist between cells in vivo are lost when cell lines are cultured on plastic in two dimensions, yet two-dimensional culture still remains the most favoured mechanism for in vitro studies in breast cancer research. In addition, cell lines are often sensitive to culture conditions - particularly the inclusion of growth factors that can sometimes alter the cell phenotype, resulting in inappropriate pathway activation or differentiation. For example, when epidermal growth factor - a common component in media designed to culture breast myoepithelial cells - is included in luminal epithelial cell culture, this can induce loss of expression of E-cadherin characteristic of epithelial to mesenchymal transition and the cells exhibit a more motile phenotype [33] Culture under inappropriate conditions can also dramatically influence cell morphology, cell-cell and cell-matrix interactions, cell polarity and differentiation [34,35], as well as altering signalling cascades and gene expression [36]. Identification of the most appropriate conditions to maintain the desired cell phenotype is thus a critical issue. As well as considering the molecular profiles of breast cancer cell lines, we also need to look beyond simple two-dimensional breast cancer models. There has thus been a shift in growing cells in more physiologically relevant three-dimensional systems with the increased complexity of including multiple cell types [34,37].
As highlighted by Kenny and colleagues, cell morphology in three dimensions is different from that observed in two dimensions on tissue culture plastic [38]. In two dimensions, luminal-like epithelial cells demonstrated the classic cobblestone morphology and expression of cell-cell adhesion molecules such as E-cadherin, whereas basal epithelial cells displayed a more elongated and spiky appearance and expressed markers of epithelial-mesenchymal transition such as vimentin. In contrast, cell lines grown in three-dimensional culture showed four different morphologies: round, mass, grape-like and stellate [38]. MCF12A normal mammary epithelial cells formed round polarised acini-like structures similar to those seen in normal human breast tissue. Luminal A T47D and MCF-7 cells and luminal B BT474 cells formed tightly cohesive structures displaying robust cell-cell adhesions. In contrast, basal MDA-MB-468, claudin-low MDA-MB-231, and HER2- positive MDA-MB-453 and SKBR3 all formed loosely cohesive grape-like or stellate structures consistent with the more invasive phenotype they demonstrate in vitro [22]. Examples of the type of cell morphology we routinely observe when luminal A and HER2-positive cells are grown in two-dimensional and three-dimensional cultures are shown in Figure 1 and present close parallels with the study by Kenny and colleagues [38].
Figure 1.
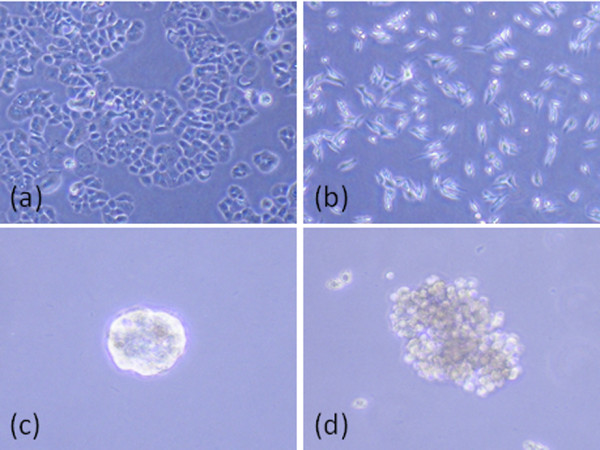
Cell morphology of cell lines grown in two-dimensional and three-dimensional cultures. Two-dimensional culture of (a) luminal A T47D and (b) HER2-positive MDA-MB-453 cell lines grown on tissue culture plastic. T47D cells demonstrate a tightly cohesive cobblestone appearance, whereas MDA-MB-453 cells have an elongated and spindly appearance. (c) T47D and (d) MDA-MB-453 cell lines cultured in three dimensions as previously described [46]. T47D cells form tightly cohesive mass structures displaying robust cell-cell adhesions, whereas MDA-MB-453 cells form loosely cohesive grape-like structures consistent with morphology observed by Kenny and colleagues [38].
Functional three-dimensional studies have led to a greater understanding of normal breast structure and development; for example, by defining a role for laminin V and desmogleins in epithelial cell polarity and maintenance of normal tissue architecture [35,39,40]. Three-dimensional models have also provided an insight into the biology of breast cancer by implicating a role for β1-integrin in breast cancer progression and by use of blocking antibodies to reverse the malignant phenotype of epithelial cells [41]. With the role of the stroma in regulating breast cancer behaviour receiving increased attention [42-44] and the recent recognition that basal and luminal breast cancers behave very differently when co-cultured with stromal fibroblasts [45], other three-dimensional breast cancer models have incorporated stromal cells such as fibroblasts [46], macrophages [47] and endothelial cells [48].
Increasing the complexity of these models is not without its problems, identification of individual cell populations within multicellular structures is particularly challenging and difficulties in quantifying structures formed remain an issue, although computer-based methods of morphological analysis show potential [49]. A recent study has successfully modelled preinvasive ductal carcinoma in situ by co-culturing tumour cells with myoepithelial cells, observing ductal carcinoma in situ structures similar to those seen in clinical specimens [46]. Further addition of tumour-associated fibroblasts resulted in tumour cell invasion and morphology reminiscent of invasive carcinoma [46]. Although this is a big advance towards modelling the stages of breast cancer progression, the gold standard that is yet to be achieved is to enable co-evolution of tumour and stromal components in vitro.
These complex multicellular three-dimensional cultures are not just a tool for understanding disease progression, but may have important implications in drug screening. This was highlighted recently by Pickl and Ries [50], who demonstrated a significantly higher response of SKBR3 cells to trastuzumab when the cells were cultured in three dimensions compared with cells cultured in two dimensions. Three-dimensional models may thus become a more widespread tool for research and drug screening, and while these models are technically challenging to establish, they are in the long term much more biologically relevant models for studying the disease in vitro.
While existing three-dimensional breast cancer models are moving towards the addition of some of the cellular components found within the complex breast tumour microenvironment [46,51], inclusion of CSCs has thus far been overlooked. Addition of stem cells derived from the various cell types within the breast tumour microenvironment may augment these in vitro three-dimensional studies. With the difficulties in enriching for CSCs and their slow proliferation rates, this is not a trivial task. Nevertheless, more complex heterotypic models are required to fully model the in vivo cellular environment - a systems biology approach is needed to tackle this.
The choice of cell culture medium becomes increasingly relevant, with complex cultures containing multiple cell types where media for one cell type may influence the phenotype of the co-cultured cell population. This in itself may present problems; having to rely on a single type of media to support cell types that may have quite different media requirements is challenging and emphasises the need for correct controls and robust standardisation of methodology.
Cell lines in xenograft studies
Whilst xenograft models provide a whole organism environment for tumour growth, these too have limitations. Experiments are usually performed in immunocompromised mice, which can impact on tumour formation and progression. The site of implantation is an important consideration, with injections into the mammary fat pad considered more physiologically relevant than subcutaneous injections even though the mouse and human mammary glands have quite different structures. Another confounding variable is the distinct difference between the stroma of human and mouse mammary tissue, which casts doubt on the relevance of xenograft models [52]. As discussed above, the stroma is now recognised to influence breast tumour cells. The differing biology of mouse and human stroma together with reports of spontaneous transformation of mouse stroma by human breast tumour xenografts, resulting in hybrid mouse-human nuclei within the xenograft [53], raise further concerns. Several groups have tried to overcome this by co-injecting human fibroblasts with cancer cell lines [54,55], but this does not allow for co-evolution of tumour and stroma that would happen during cancer development.
Of the cell lines commonly incorporated into xenograft models, ER-positive luminal A cell lines such as MCF-7 and T47D will only form tumours in the presence of oestrogen and, unsurprisingly, growth can be inhibited by anti-oestrogen therapy. Cell lines representing other subtypes (for example, BT474, MDA-MB-468 and MDA-MB-231) have also been shown to be tumourigenic; however, cells representing the HER2 subtype, including SKBR3 and MDA-MB-453 cells, have poor tumourigenic potential.
An unexpected finding with xenograft models is the limited ability of tumours to invade and metastasise, particularly given the often metastatic origin of cell lines (reviewed in [14]). If metastasis occurs it is usually to the lung, which is not the most common metastatic site in human breast cancer - thus breast cancer metastasis is often studied through intravenous injection, enabling colonisation of specific organs; for example, intracarotid artery injection for study of brain metastasis or left ventricle injection for metastasis to bone. Cell lines such as MDA-MB-231 that are regarded as invasive in vitro remain relatively poorly metastatic in vivo, although when introduced directly into the circulation the cell line has proved useful in models of experimental metastasis. Through rounds of in vivo selection, elegant experiments by Massague's group have developed highly metastatic derivatives of MDA-MB-231 cells that home to particular metastatic sites, enabling generation of gene expression signatures linked with a specific metastatic site [56]. Using the human breast cancer cell line SUM1315 derived from a clinical sample of a metastatic node, Kupperwasser and colleagues introduced this as an orthotopic model into immunodeficient (NOD/SCID) mice bearing grafts of human bone, and showed the cells preferentially and spontaneously metastasised to the human bone graft rather than mouse skeleton [57].
MDA-MB-435 cells
A review of this nature would be incomplete without reference to MDA-MB-435 cells, which are spontaneously metastatic. A catalogue of the genomic and molecular properties of a breast cell line panel classified MDA-MB-435 cells as basal B [22]. Hollestelle and colleagues also characterised MDA-MB-435 as a basal cell line through gene expression microarray profiling [26]. The provenance of this cell line, however, is hotly debated. Originally isolated as part of the MD Anderson series (hence the MDA prefix [58]), these cells were thought to be derived from a breast carcinoma, but subsequent microarray and immunohistochemistry data have indicated that MDA-MB-435 might originate from melanoma [59-61].
Despite clear controversies surrounding MDA-MB-435, many researchers continue to use this as a bona fide breast cancer cell line. We believe the persistent use of this cell line, including publications in high-impact journals - for example, where MDA-MB-435 was used as a model of triple-negative breast cancer [62] - and even in specialist breast cancer journals [63,64], is unacceptable as it is likely to generate potentially misleading data. Nevertheless researchers are now more aware of the provenance of MDA-MB-435 cells, with two recent papers using the cell line as a melanoma model [65,66] and its inclusion in a 2010 list of cell lines of questionable origin [67]. We urge researchers, members of grant review panels and journal reviewers and editors to be more aware of this. Indeed, many journals now have a policy of requesting some form of cell line authentication to accompany manuscript submission, which is something we support.
Breast cancer cell lines that still need to be developed
Although there are now a reasonable number of breast cancer cell lines available to reflect the molecular sub-groups, relevant models are lacking for some of the rarer histopathological types. There is a single report on the development of two cell lines from phyllodes tumours [68] but these do not seem have gained widespread use. Cell lines derived from inflammatory breast cancer are limited to SUM149 and SUM190 [7], but the prevalence of the basal phenotype in this group [69] suggests basal cell lines may be used as surrogates. To our knowledge there is no known cell line derived from male breast cancer and, given that the incidence of male breast cancer is rising [70], this poses a challenge for modelling in a functional sense.
Conclusions
Tremendous advances in our understanding of the biology of breast cancer have been made over the past several decades using breast cancer cell lines. We must now move beyond the 'one marker, one cell line' studies of the past and use knowledge gained through genetic and transcriptomic profiling to use cell lines or cell line panels more effectively as experimental models to study specific subgroups of breast cancer, because this is likely to have the greatest impact on improving outcome for breast cancer patients.
Abbreviations
CSC: cancer stem cell; ER: oestrogen receptor; HER2: human epidermal growth factor receptor 2; MEK: mitogen-activated protein/extracellular signal-regulated kinase kinase; PR: progesterone receptor.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Deborah L Holliday, Email: D.L.Holliday@leeds.ac.uk.
Valerie Speirs, Email: V.Speirs@leeds.ac.uk.
Acknowledgements
The authors are supported by the Breast Cancer Campaign, the Dr Hadwen Trust for Humane Research and the Lord Dowding Fund for Humane Research.
References
- Gey GO, Coffman WD, Kubicek MT. Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res. 1952;12:264–265. [Google Scholar]
- Lasfargues EY, Ozzello L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958;21:1131–1147. [PubMed] [Google Scholar]
- Cailleau R, Olivé M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- Soule HD, Vasquez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1413. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–3078. [PubMed] [Google Scholar]
- Human Tissue Authority. http://www.hta.gov.uk/
- Ethier SP, Mahacek ML, Gullick WJ, Frank Tja ND, Weber BL. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. Cancer Res. 1993;53:627–635. [PubMed] [Google Scholar]
- Perou CM, Jeffrey SS, van de Rijn M, Rees CA, Eisen MB, Ross DT, Pergamenschikov A, Williams CF, Zhu SX, Lee JC, Lashkari D, Shalon D, Brown PO, Botstein D. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc Natl Acad Sci USA. 1999;96:9212–9217. doi: 10.1073/pnas.96.16.9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lønning P, Børresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Rakha EA, Richardson AL, Schmitt FC, Tan PH, Tse GM, Weigelt B, Ellis IO, Reis-Filho JS. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24:157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdall SE, Hanby AM, Lansdown MRJ, Speirs V. Breast cancer cell lines: friend or foe? Breast Cancer Res. 2003;5:89–95. doi: 10.1186/bcr577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nat Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Hobbs K, Clark GM. Effects of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985;45:584–590. [PubMed] [Google Scholar]
- Gottardis MM, Robinson SP, Jordan VC. Estradiol-stimulated growth of MCF-7 tumors implanted in athymic mice: a model to study the tumoristatic action of tamoxifen. J Steroid Biochem. 1988;30:311–314. doi: 10.1016/0022-4731(88)90113-6. [DOI] [PubMed] [Google Scholar]
- Robertson JF, Osborne CK, Howell A, Jones SE, Mauriac L, Ellis M, Kleeberg UR, Come SE, Vergote I, Gertler S, Buzdar A, Webster A, Morris C. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women - a prospective combined analysis of two multicenter trials. Cancer. 2003;98:229–238. doi: 10.1002/cncr.11468. [DOI] [PubMed] [Google Scholar]
- Johnston SJ, Cheung KL. Fulvestrant - a novel endocrine therapy for breast cancer. Curr Med Chem. 2010;17:902–914. doi: 10.2174/092986710790820633. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- Thompson A, Brennan K, Cox A, Gee J, Harcourt D, Harris A, Harvie M, Holen I, Howell A, Nicholson R, Steel M, Streuli C. Evaluation of the current knowledge limitations in breast cancer research: a gap analysis. Breast Cancer Res. 2008;10:R26. doi: 10.1186/bcr1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay A, Tamber N, Fenwick K, Iravani M, Grigoriadis A, Dexter T, Lord CJ, Reis-Filho JS, Ashworth A. A high-resolution integrated analysis of genetic and expression profiles of breast cancer cell lines. Breast Cancer Res Treat. 2009;118:481–498. doi: 10.1007/s10549-008-0296-7. [DOI] [PubMed] [Google Scholar]
- Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, Kwei KA, Hernandez-Boussard T, Wang P, Gazdar AF, Minna JD, Pollack JR. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PJ, Lin AF, Arendt LM, Klebba I, Jones AD, Rudnick JA, Dimeo TA, Gilmore H, Jefferson DM, Graham RA, Naber SP, Schnitt S, Kuperwasser C. Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast Cancer Res. 2010;12:R87. doi: 10.1186/bcr2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M, Ng SS, French PJ, Peeters JK, Rozendaal MJ, Riaz M, Koopman DG, Ten Hagen TL, de Leeuw BH, Zwarthoff EC, Teunisse A, van der Spek PJ, Klijn JG, Dinjens WN, Ethier SP, Clevers H, Jochemsen AG, den Bakker MA, Foekens JA, Martens JW, Schutte M. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat. 2010;121:53–64. doi: 10.1007/s10549-009-0460-8. [DOI] [PubMed] [Google Scholar]
- Greshock J, Bachman KE, Degenhardt YY, Jing J, Wen YH, Eastman S, McNeil E, Moy C, Wegrzyn R, Auger K, Hardwicke MA, Wooster R. Molecular target class is predictive of in vitro response profile. Cancer Res. 2010;70:3677–3686. doi: 10.1158/0008-5472.CAN-09-3788. [DOI] [PubMed] [Google Scholar]
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.20.3.719. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J. Detection and analysis of mammary gland stem cells. J Pathol. 2009;217:229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno AM, Salcido CD, Gillet JP, Wu CP, Fostel JM, Mumau MD, Gottesman MM, Varticovski L, Ambudkar SV. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Natl Cancer Inst. 2010;102:1637–1652. doi: 10.1093/jnci/djq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Thiery JP, Lafont F, Stampfer F, Boyer B. Transient effect of epidermal growth factor on the motility of an immortalized mammary epithelial cell line. J Cell Sci. 1993;106:869–878. doi: 10.1242/jcs.106.3.869. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130:601–610. doi: 10.1016/j.cell.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Streuli CH Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro - a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15:405–412. doi: 10.1016/j.semcancer.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Debnath J, Brugge JS. Modelling glandular epithelial cancers in three-dimensional cultures. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, Lorenz K, Lee EH, Barcellos-Hoff MH, Petersen OW, Gray JW, Bissell MJ. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. doi: 10.1016/j.molonc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runswick SK, O'Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol. 2001;3:823–830. doi: 10.1038/ncb0901-823. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ. Modelling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Bissell MJ. The dominance of the microenvironment in breast and ovarian cancer. Semin Cancer Biol. 2002;12:97–104. doi: 10.1006/scbi.2001.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp JT, Elloumi F, Roman Perez E, Rein J, Stewart DA, Harrell JC, Perou CM, Troester MA. Interactions with fibroblasts are distinct in basal-like and luminal breast cancers. Mol Cancer Res. 2011;9:3–13. doi: 10.1158/1541-7786.MCR-10-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday DL, Brouilette KT, Markert A, Gordon LA, Jones JL. Novel multicellular organotypic models of normal and malignant breast: tools for dissecting the role of the microenvironment in breast cancer progression. Breast Cancer Res. 2009;11:R3. doi: 10.1186/bcr2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, Stanley ER, Segall JE, Condeelis JS. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- Shekhar MP, Werdell J, Tait L. Interaction with endothelial cells is a prerequisite for branching ductal-alveolar morphogenesis and hyperplasia of preneoplastic human breast epithelial cells: regulation by estrogen. Cancer Res. 2000;60:439–449. [PubMed] [Google Scholar]
- Han J, Chang H, Giricz O, Lee GY, Baehner FL, Gray JW, Bissell MJ, Kenny PA, Parvin B. Molecular predictors of 3D morphogenesis by breast cancer cell lines in 3D culture. PLoS Comput Biol. 2010;6:e1000684. doi: 10.1371/journal.pcbi.1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickl M, Ries CH. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene. 2009;28:461–468. doi: 10.1038/onc.2008.394. [DOI] [PubMed] [Google Scholar]
- Weigelt B, Bissell MJ. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin Cancer Biol. 2008;18:311–321. doi: 10.1016/j.semcancer.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovey RC, McFadden TB, Akers RM. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia. 1999;4:53–68. doi: 10.1023/A:1018704603426. [DOI] [PubMed] [Google Scholar]
- Jacobsen BM, Harrell JC, Jedlicka P, Borges VF, Varella-Garcia M, Horwitz KB. Spontaneous fusion with, and transformation of mouse stroma by, malignant human breast cancer epithelium. Cancer Res. 2006;66:8274–8279. doi: 10.1158/0008-5472.CAN-06-1456. [DOI] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuelten CH, Busch JI, Tang B, Flanders KC, Oshima A, Sutton E, Karpova TS, Roberts AB, Wakefi eld LM, Niederhuber JE. Transient tumor-fibroblast interactions increase tumor cell malignancy by a TGF-β mediated mechanism in a mouse xenograft model of breast cancer. PLoS One. 2010;5:e9832. doi: 10.1371/journal.pone.0009832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Kuperwasser C, Dessain S, Bierbaum BE, Garnet D, Sperandio K, Gauvin GP, Naber SP, Weinberg RA, Rosenblatt M. A mouse model of human breast cancer metastasis to human bone. Cancer Res. 2005;65:6130–6138. doi: 10.1158/0008-5472.CAN-04-1408. [DOI] [PubMed] [Google Scholar]
- Cailleau R, Olivé M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- Christgen M, Lehmann U. MDA-MB-435: the questionable use of a melanoma cell line as a model for human breast cancer is ongoing. Cancer Biol Ther. 2007;6:1355–1337. doi: 10.4161/cbt.6.9.4624. [DOI] [PubMed] [Google Scholar]
- Rae JM, Ramus SJ, Waltham M, Armes JE, Campbell IG, Clarke R, Barndt RJ, Johnson MD, Thompson EW. Common origins of MDA-MB-435 cells from various sources with those shown to have melanoma properties. Clin Exp Metastasis. 2004;21:543–552. doi: 10.1007/s10585-004-3759-1. [DOI] [PubMed] [Google Scholar]
- Ellison G, Klinowska T, Westwood RF, Docter E, French T, Fox JC. Further evidence to support the melanocytic origin of MDA-MB-435. Mol Pathol. 2002;55:294–299. doi: 10.1136/mp.55.5.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Osada T, Wang Y, Yu L, Sakakura K, Katayama A, McCarthy JB, Brufsky A, Chivukula M, Khoury T, Hsu DS, Barry WT, Lyerly HK, Clay TM, Ferrone S. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J Natl Cancer Inst. 2010;102:1496–1512. doi: 10.1093/jnci/djq343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Hussein F, Woeste A, Gründker C, Frontzek K, Emons G, Hawighorst T. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res Treat. 2011;128:337–46. doi: 10.1007/s10549-010-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning A, Friese K, Burges A, Mylonas I. Tamoxifen enhances the cytotoxic effects of nelfinavir in breast cancer cells. Breast Cancer Res. 2010;12:R45. doi: 10.1186/bcr2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh JH, Song JH, Eun JW, Kim JK, Jung KH, Bae HJ, Xie HJ, Ryu JC, Ahn YM, Wee SJ, Park WS, Lee JY, Nam SW. Systemic cell-cycle suppression by Apicidin, a histone deacetylase inhibitor, in MDA-MB-435 cells. Int J Mol Med. 2009;24:205–226. doi: 10.3892/ijmm_00000224. [DOI] [PubMed] [Google Scholar]
- Samadi N, Gaetano C, Goping IS, Brindley DN. Autotaxin protects MCF-7 breast cancer and MDA-MB-435 melanoma cells against Taxol-induced apoptosis. Oncogene. 2009;28:1028–1039. doi: 10.1038/onc.2008.442. [DOI] [PubMed] [Google Scholar]
- Capes-Davis A, Theodosopoulos G, Atkin I, Drexler HG, Kohara A, MacLeod RA, Masters JR, Nakamura Y, Reid YA, Reddel RR, Freshney RI. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int J Cancer. 2010;127:1–8. doi: 10.1002/ijc.25379. [DOI] [PubMed] [Google Scholar]
- Warso MA, Mehta RR, Hart GD, Graves JM, Green A. A cell line derived from a clinically benign phyllodes tumor: characterization and implications. Anticancer Res. 1995;15:399–404. [PubMed] [Google Scholar]
- Bertucci F, Finetti P, Birnbaum D, Viens P. Gene expression profiling of inflammatory breast cancer. Cancer. 2010;116(11 Suppl):2783–2793. doi: 10.1002/cncr.25165. [DOI] [PubMed] [Google Scholar]
- Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat. 2009;115:429–430. doi: 10.1007/s10549-008-0053-y. [DOI] [PubMed] [Google Scholar]


