Abstract
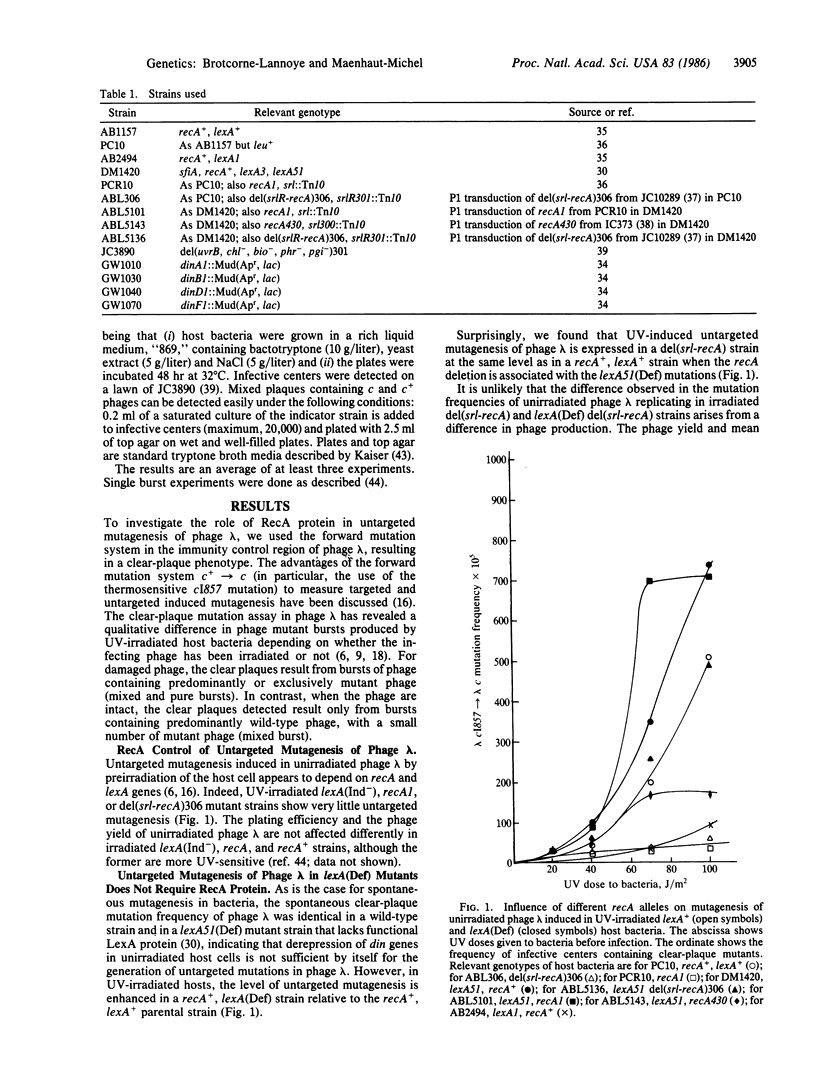
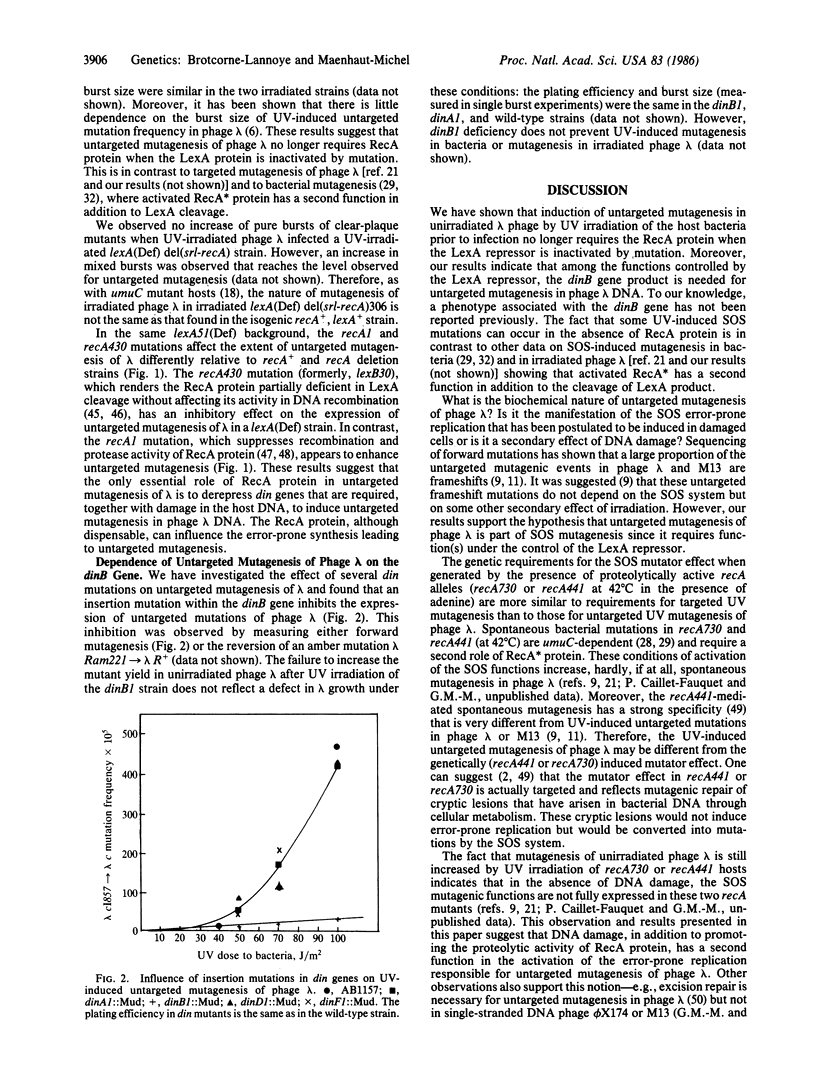
Untargeted UV mutagenesis of bacteriophage lambda--i.e., the increased recovery of lambda mutants when unirradiated lambda infects UV-irradiated Escherichia coli--is thought to be mediated by a transient decrease in DNA replication fidelity, generating mutations in the newly synthesized strands. Using the bacteriophage lambda cI857----lambda c mutation system, we provide evidence that the RecA protein, shown previously to be required for this mutagenic pathway, is no longer needed when the LexA protein is inactivated by mutation. We suggest that the error-prone DNA replication responsible for UV-induced untargeted mutagenesis is turned on by the presence of replication-blocking lesions in the host cell DNA and that the RecA protein is required only to derepress the relevant din gene(s). This is in contrast to mutagenesis of irradiated bacteria or irradiated phage lambda, in which activated RecA protein has a second role in mutagenesis in addition to the cleavage of the LexA protein. Among the tested din genes, the dinB gene product (in addition to the uvrA and uvrB gene products) was found to be required for untargeted mutagenesis of bacteriophage lambda. To our knowledge, a phenotype associated with the dinB gene has not been reported previously.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanco M., Herrera G., Collado P., Rebollo J. E., Botella L. M. Influence of RecA protein on induced mutagenesis. Biochimie. 1982 Aug-Sep;64(8-9):633–636. doi: 10.1016/s0300-9084(82)80102-8. [DOI] [PubMed] [Google Scholar]
- Brandenburger A., Godson G. N., Radman M., Glickman B. W., van Sluis C. A., Doubleday O. P. Radiation-induced base substitution mutagenesis in single-stranded DNA phage M13. Nature. 1981 Nov 12;294(5837):180–182. doi: 10.1038/294180a0. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P. Mutagenic DNA repair in Escherichia coli. III. Requirement for a function of DNA polymerase III in ultraviolet-light mutagenesis. Mol Gen Genet. 1976 Feb 27;144(1):53–58. doi: 10.1007/BF00277304. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Woodgate R. Mutagenic repair in Escherichia coli. X. The umuC gene product may be required for replication past pyrimidine dimers but not for the coding error in UV-mutagenesis. Mol Gen Genet. 1984;196(2):364–366. doi: 10.1007/BF00328073. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Woodgate R. Mutagenic repair in Escherichia coli: products of the recA gene and of the umuD and umuC genes act at different steps in UV-induced mutagenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4193–4197. doi: 10.1073/pnas.82.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M. Phage yield during W-reactivation of bacteriophage. Mutat Res. 1977 Nov;45(2):161–167. doi: 10.1016/0027-5107(77)90015-x. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Defais M., Radman M. Molecular mechanisms of induced mutagenesis. Replication in vivo of bacteriophage phiX174 single-stranded, ultraviolet light-irradiated DNA in intact and irradiated host cells. J Mol Biol. 1977 Nov 25;117(1):95–110. doi: 10.1016/0022-2836(77)90025-0. [DOI] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Maenhaut-Michel G., Radman M. SOS mutator effect in E. coli mutants deficient in mismatch correction. EMBO J. 1984 Apr;3(4):707–712. doi: 10.1002/j.1460-2075.1984.tb01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillet-Fauquet P., Maenhaut-Michel G., Radman M. SOS mutator effect in E. coli mutants deficient in mismatch correction. EMBO J. 1984 Apr;3(4):707–712. doi: 10.1002/j.1460-2075.1984.tb01873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Christensen R. B., Christensen J. R., Koenig I., Lawrence C. W. Untargeted mutagenesis induced by UV in the lacI gene of Escherichia coli. Mol Gen Genet. 1985;201(1):30–34. doi: 10.1007/BF00397982. [DOI] [PubMed] [Google Scholar]
- Cieśla Z. Plasmid pKM101-mediated mutagenesis in Escherichia coli is inducible. Mol Gen Genet. 1982;186(2):298–300. doi: 10.1007/BF00331866. [DOI] [PubMed] [Google Scholar]
- DEVORET R. INFLUENCE DU G'ENOTYPE DE LA BACT'ERIE H OTE SUR LA MUTATION DU PHAGE LAMBDA PRODUITE PAR LE RAYONNEMENT ULTRAVIOLET. C R Hebd Seances Acad Sci. 1965 Feb 1;260:1510–1513. [PubMed] [Google Scholar]
- Defais M. Role of the E. coli umuC gene product in the repair of single-stranded DNA phage. Mol Gen Genet. 1983;192(3):509–511. doi: 10.1007/BF00392198. [DOI] [PubMed] [Google Scholar]
- Ennis D. G., Fisher B., Edmiston S., Mount D. W. Dual role for Escherichia coli RecA protein in SOS mutagenesis. Proc Natl Acad Sci U S A. 1985 May;82(10):3325–3329. doi: 10.1073/pnas.82.10.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Ichikawa-Ryo H., Kondo S. Indirect mutagenesis in phage lambda by ultraviolet preirradiation of host bacteria. J Mol Biol. 1975 Sep 5;97(1):77–92. doi: 10.1016/s0022-2836(75)80023-4. [DOI] [PubMed] [Google Scholar]
- JACOB F. Mutation d'un bactériophage induite par l'irradiation des seules bactéries-hotes avant l'infection. C R Hebd Seances Acad Sci. 1954 Feb 8;238(6):732–734. [PubMed] [Google Scholar]
- KAISER A. D. A genetic study of the temperate coliphage. Virology. 1955 Nov;1(4):424–443. doi: 10.1016/0042-6822(55)90036-2. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. Expression of the E. coli uvrA gene is inducible. Nature. 1981 Feb 26;289(5800):808–810. doi: 10.1038/289808a0. [DOI] [PubMed] [Google Scholar]
- Krueger J. H., Elledge S. J., Walker G. C. Isolation and characterization of Tn5 insertion mutations in the lexA gene of Escherichia coli. J Bacteriol. 1983 Mar;153(3):1368–1378. doi: 10.1128/jb.153.3.1368-1378.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L., Saran B. R., Allen R., Jr Sequence analysis of ultraviolet-induced mutations in M13lacZ hybrid phage DNA. J Mol Biol. 1984 Dec 5;180(2):217–237. doi: 10.1016/s0022-2836(84)80001-7. [DOI] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L. Specificity of UV mutagenesis in the lac promoter of M13lac hybrid phage DNA. Nature. 1982 Jun 17;297(5867):596–598. doi: 10.1038/297596a0. [DOI] [PubMed] [Google Scholar]
- Maenhaut-Michel G., Caillet-Fauquet P. Effect of umuC mutations on targeted and untargeted ultraviolet mutagenesis in bacteriophage lambda. J Mol Biol. 1984 Jul 25;177(1):181–187. doi: 10.1016/0022-2836(84)90064-0. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Low K. B. Specificity of mutagenesis resulting from the induction of the SOS system in the absence of mutagenic treatment. Cell. 1984 Jun;37(2):675–682. doi: 10.1016/0092-8674(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutagenic specificity of ultraviolet light. J Mol Biol. 1985 Mar 5;182(1):45–65. doi: 10.1016/0022-2836(85)90026-9. [DOI] [PubMed] [Google Scholar]
- Miura A., Tomizawa J. I. Studies on radiation-sensitive mutants of E. coli. 3. Participation of the rec system in induction of mutation by ultraviolet irradiation. Mol Gen Genet. 1968;103(1):1–10. doi: 10.1007/BF00271151. [DOI] [PubMed] [Google Scholar]
- Morand P., Blanco M., Devoret R. Characterization of lexB mutations in Escherichia coli K-12. J Bacteriol. 1977 Aug;131(2):572–582. doi: 10.1128/jb.131.2.572-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P. L., Roberts J. W. RecA protein--promoted lambda repressor cleavage: complementation between RecA441 and RecA430 proteins in vitro. Mol Gen Genet. 1984;198(2):25–34. doi: 10.1007/BF00328696. [DOI] [PubMed] [Google Scholar]
- Mount D. W. A mutant of Escherichia coli showing constitutive expression of the lysogenic induction and error-prone DNA repair pathways. Proc Natl Acad Sci U S A. 1977 Jan;74(1):300–304. doi: 10.1073/pnas.74.1.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo J. E., Moreau P. L., Blanco M., Devoret R. Restoration of RecA protein activity by genetic complementation. Mol Gen Genet. 1984;195(1-2):83–89. doi: 10.1007/BF00332728. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L., Phizicky E. M. Activity of the Escherichia coli recA-gene product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):917–920. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Walker G. C., Dobson P. P. Mutagenesis and repair deficiencies of Escherichia coli umuC mutants are suppressed by the plasmid pKM101. Mol Gen Genet. 1979 Apr 17;172(1):17–24. doi: 10.1007/BF00276210. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis D. K., Uhlin B. E., Amini K. S., Clark A. J. Physical mapping of the srl recA region of Escherichia coli: analysis of Tn10 generated insertions and deletions. Mol Gen Genet. 1981;183(3):497–504. doi: 10.1007/BF00268771. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., Kogoma T. Involvement of the activated form of RecA protein in SOS mutagenesis and stable DNA replication in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7539–7543. doi: 10.1073/pnas.81.23.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M., McCall J. O., Volkert M. R., Wermundsen I. E. Constitutive expression of SOS functions and modulation of mutagenesis resulting from resolution of genetic instability at or near the recA locus of Escherichia coli. Mol Gen Genet. 1982;185(1):43–50. doi: 10.1007/BF00333788. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. The mutability toward ultraviolet light of recombination-deficient strains of Escherichia coli. Mutat Res. 1969 Jul-Aug;8(1):9–14. doi: 10.1016/0027-5107(69)90135-3. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Thermal enhancement of ultraviolet mutability in a tif-1 uvrA derivative of Escherichia coli B-r: evidence that ultraviolet mutagenesis depends upon an inducible function. Proc Natl Acad Sci U S A. 1974 May;71(5):1930–1934. doi: 10.1073/pnas.71.5.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M., Wermundsen I. E. Targeted and untargeted mutagenesis by various inducers of SOS functions in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):881–886. doi: 10.1101/sqb.1979.043.01.095. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Hutchinson F. Non-targeted mutagenesis of unirradiated lambda phage in Escherichia coli host cells irradiated with ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):293–305. doi: 10.1016/0022-2836(84)90122-0. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Skopek T. R., Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage by ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):273–291. doi: 10.1016/0022-2836(84)90121-9. [DOI] [PubMed] [Google Scholar]


