Abstract
Aim: Scientific evidence regarding exercise in migraine prophylaxis is required. Therefore this study aimed to evaluate the effects of exercise in migraine prevention.
Methods: In a randomized, controlled trial of adults with migraine, exercising for 40 minutes three times a week was compared to relaxation according to a recorded programme or daily topiramate use, which was slowly increased to the individual’s highest tolerable dose (maximum 200 mg/day). The treatment period lasted for 3 months, and migraine status, quality of life, level of physical activity, and oxygen uptake were evaluated. The primary efficacy variable was the mean reduction of the frequency of migraine attacks during the final month of treatment compared with the baseline.
Results: Ninety-one patients were randomized and included in the intention-to-treat analysis. The primary efficacy variable showed a mean reduction of 0.93 (95% confidence interval (CI) 0.31–1.54) attacks in the exercise group, 0.83 (95% CI 0.22–1.45) attacks in the relaxation group, and 0.97 (95% CI 0.36–1.58) attacks in the topiramate group. No significant difference was observed between the groups (p = 0.95).
Conclusion: Exercise may be an option for the prophylactic treatment of migraine in patients who do not benefit from or do not want to take daily medication.
Keywords: Headache, oxygen uptake, physical activity, prevention, quality of life
Introduction
Migraine is a serious health problem with huge consequences for individuals as well as for society (1). In the prophylactic treatment of migraine, topiramate is a drug of first choice, as it has been shown to be both effective and well tolerated (2,3). However, some patients prefer non-pharmacological treatments, which may be as effective as drugs (4). Behavioural therapies including relaxation, biofeedback, and stress management have been proven to be effective, and there is grade-A evidence of the effectiveness of relaxation therapy (5).
Regular exercise is often recommended in migraine treatment (6). Exercise is defined as physical activity that is planned, structured, repetitive, and purposeful in the sense that the improvement or maintenance of physical fitness is the objective (7). Several studies evaluating aerobic endurance training report beneficial effects on both frequency and intensity of migraine, as well as on the duration of the attacks and on patient well being (8–10). However, there is not enough scientific evidence available to draw conclusions about the effects of aerobic exercise in migraine treatment and more studies are imperative (6).
On the other hand, Kelman (11) found that 22% of participants reported exercise as a trigger factor for migraines, which might be a reason for some patients to avoid exercise. Individuals with migraine and other headaches have been shown to be less physically active than those without headaches (12). However, an exercise programme, based on indoor cycling three times a week, has been shown to be safe and useful in improving exercise capacity with no deterioration of the participant’s migraine status (13). The effectiveness of exercise in migraine prevention is still unclear, and we therefore undertook this randomized controlled trial in order to compare exercise with common pharmacological and non-pharmacological treatments with regard to migraine prevention.
Methods
Patients
This study was conducted at a specialist headache clinic in Sweden between January 2006 and March 2009. The participants were mostly recruited via newspaper advertisements, but also from the headache clinic. Those who were interested in participation were examined by a neurologist (ML), and a migraine diagnosis was given according to The International Classification of Headache Disorders criteria (ICHD-II) (14). During this meeting, they also received individual advice about acute medication and an information brochure about migraine. Participants who fulfilled the inclusion criteria and none of the exclusion criteria were invited to participate in the study. The inclusion criteria stated that the participants must: be aged between 18 and 65 years old; have migraine with or without aura with a frequency of 2–8 attacks per month, and have had migraine for at least 1 year before participating in the study and before the age of 50. The exclusion criteria were: interval headaches not distinguishable from migraine; medication-overuse headache; regular exercise (once or more per week during the 12 weeks prior to the study); earlier regular practice of relaxation; pregnancy; breastfeeding; use of daily migraine prophylaxis in the 12 weeks prior to the study; inability to understand Swedish; use of antipsychotic or antidepressive medication in the 12 weeks prior to the study; drug or alcohol abuse; and topiramate intolerance.
Randomization and masking
In order to confirm that they had a retrospective history of 2–8 migraine attacks/month, the participants underwent a prospective baseline period of at least 1 month, during which time they kept a migraine diary. The baseline period could be extended up to 3 months, depending on the date on which the treatment period could start. The participants who met the inclusion criteria after the baseline period were randomized into one of three groups: relaxation, exercise, or topiramate. The enrolling neurologist remained completely separate from the randomization procedure, which was conducted by an independent person according to a lottery method. Six pieces of paper, two for each group, were folded twice and put into an opaque envelope. One piece of paper was taken each time a patient entered the study. After six participants had been included, the procedure started again. After randomization, the participants were informed about the study and scheduled for treatment by the study secretary. As this study compared pharmacological and non-pharmacological treatments, blindness to treatment was not possible, but all of the completed assessment forms were encoded and returned to the study secretary in sealed envelopes. Therefore, the evaluator was effectively blinded.
Study design and procedures
The study was a single-centre, prospective, randomized controlled trial and was approved by the Regional Ethical Review Board in Gothenburg. Before enrolment, all of the study participants gave their written informed consent after receiving full oral and written information about the study. The study was designed according to established guidelines for randomized controlled studies and for clinical trials of prophylactic migraine treatments (15,16).
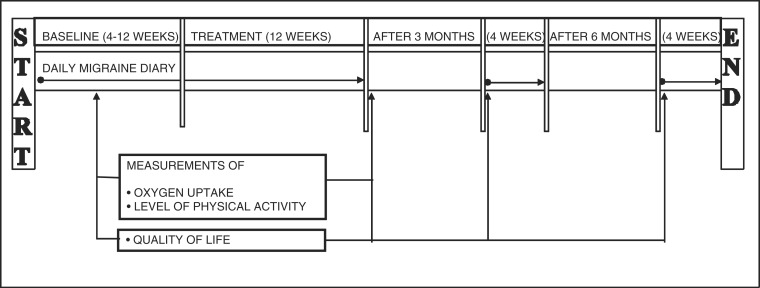
The study design, including the assessments, is described in Figure 1. After a 4–12 week baseline period, the 12-week treatment period started. Follow-up was carried out 3 and 6 months after treatment. No restrictions were placed on the use of concomitant acute medication. Over the course of the study, all of the participants were allowed to contact the physiotherapist or the neurologist with questions over the telephone or in the form of a scheduled visit.
Figure 1.
Study design.
Migraine status, including the primary efficacy variable, was assessed through the use of daily diaries regarding migraine, acute medication (doses of tablets, injections, nasal sprays, and suppositories) and the average pain that the migraines caused. These diaries were based on vertical visual-analogue scales, which have been shown to be valid for the registration of pain intensity in headache patients (17). The diaries were sent to the study secretary every 4 weeks. A migraine attack was defined as concomitant days with migraine headache and distinct attacks were counted if separated by ≥24 h free from headache (15). The participants were also asked to note any adverse events (AEs) (18) during the treatment period. All of the AEs reported in writing are presented, without any speculation as to their cause. Quality of life (QoL) was measured using the Swedish version of the Migraine-Specific Quality of Life Questionnaire (MSQoL), which consists of 20 items, each of which is rated using a response scale with four categories (1 = very much and 4 = not at all). The data were then standardized to a range of values from 0–100, where 0 represents the worst health status experienced by the participants and 100 represents the best health status (19). In order to evaluate aerobic capacity, the estimated VO2max was measured using Åstrand’s submaximal bicycle test (20,21). The tests were performed by an experienced physiotherapist, who was not involved in the treatment, on a 828E ergometer cycle (Monark, Varberg, Sweden), and pulse rate was assessed using a FS1 pulse watch (Polar, Guangzhou, China). Finally, the International Short Physical Activity Questionnaire (IPAQ) was used to assess the participants’ levels of physical activity (22). This questionnaire estimates the number of minutes per day or week during which an individual performs different activities. The level of activity is assessed by estimating the intensity of each type of activity according to its energy requirements, defined as metabolic equivalents (MET), in order to yield a score in MET-minutes. The numbers of sedentary hours/day were also estimated. The primary efficacy variable in the study was a reduction in the mean frequency of migraine attacks. All of the other variables were seen as secondary variables.
The participants who had been allocated to the relaxation group had to attend a scheduled individual appointment with a registered physiotherapist (EV) once a week. The relaxation programme described by Larsson and Andrasik (23) is based on common forms of relaxation, breathing, and stress-management techniques and includes a series of six exercises, each of which is based on the one before. Each relaxation exercise lasted for between 5 and 20 minutes, and verbal and written information was given out before the introduction of a new relaxation exercise. After each session, there was an opportunity for the participant to discuss their progress with the physiotherapist. If they were absent, they were contacted and informed about how to continue on their own. Between the scheduled sessions, the participants practised at home every day with a compact disc. For the purpose of this study, adherence to the relaxation treatment was defined as participating in six or more sessions at the clinic, as the programme included six different exercises. Verbal confirmation of practice at home was also required.
The participants who were allocated to the exercise group trained with a registered physiotherapist (EV) for 40 minutes, three times a week. The exercise programme was based on indoor cycling. The rate of perceived exertion (RPE) scale (intensity graded 6–20) was used to set the training intensity (24). Each training session included a 15-minute warm-up period (intensity on RPE scale 11–13), followed by a 20-minute exercise period (RPE scale 14–16) and a 5-minute cool-down period (RPE scale 11–13). The exercise programme has also been described earlier (13). The opportunity to discuss the exercise with the physiotherapist was given after every session. If the participant was absent, they exercised at home or at a local gym. All forms of continuous aerobic exercise were then accepted, and participants were instructed to reproduce the same intensity and duration used in the exercise programme. Based on recommendations for increasing VO2max (25), participants who exercised once per week on average at the clinic and a total of two or more times a week were seen as adhering to the treatment.
The participants in the medication group visited a neurologist (ML) before starting a course of topiramate. Topiramate is an antiepileptic drug in use for over 10 years and has been registered in Sweden (Topimax; Janssen-Cilag, Sollentuna, Sweden) for the treatment of migraine since 2005. These participants also received written information about the drug. Their dosage was slowly increased by 25 mg every week until the dosage reached the highest dose that the individual could tolerate, with a maximum of 200 mg/day (18). They were allowed to call the neurologist at any time of day during the treatment period and to book a scheduled visit if needed. At least one follow-up visit was scheduled. Adherence was defined as using the medicine >2 months in accordance with the prescription and was measured using self-reports (26).
Statistical analysis
A power analysis was conducted in order to detect a clinically relevant difference between the groups in terms of a change in the mean monthly migraine frequency from the baseline, defined as 1.0 number of attacks. Assuming a standard deviation of 1.2, and a power level of 80%, a two sided-test with an alpha level of 0.05 showed that it was necessary to have 30 subjects in each group.
The null hypothesis was defined as follows: there is no difference between the three treatment regimes. This hypothesis was used for all of the efficacy variables.
Changes from the baseline at all points in time in terms of migraine days/month, mean pain intensity, acute medication used, QoL, MET-minutes/week, sedentary hours/day and VO2max were evaluated as secondary efficacy variables. The response rate (≥50% improvement in the primary efficacy variable) was also evaluated. All of the analyses were carried out and reported as decided beforehand in the statistical protocol.
The primary analysis was performed using the intention-to-treat (ITT) population, with randomized participants who met the inclusion criteria. For missing data, the last observation was carried forward (assuming no change for non-completers). All of the analyses were also carried out per protocol (PP). The PP population incorporated all of the ITT subjects who did not deviate in any major way from the protocol, including at least one post-baseline measure of the primary efficacy variable and adherence to the actual treatment. All of the data are presented using descriptive statistics, i.e. the number of observations, means and standard deviations for continuous variables, and frequencies and percentages for categorical variables. Continuous data were analysed using ANCOVA, in which treatment was used as a fixed factor and the baseline levels as a covariate in the model. The data are presented with the least square means and the corresponding 95% confidence intervals (CI). Categorical data were analysed using the chi-square test. All of the tests were two-sided and p<0.05 was regarded as statistically significant. All of the analyses were performed using STATISTICA 9.0 (Stat Soft, Tulsa OK, USA).
Results
Participants
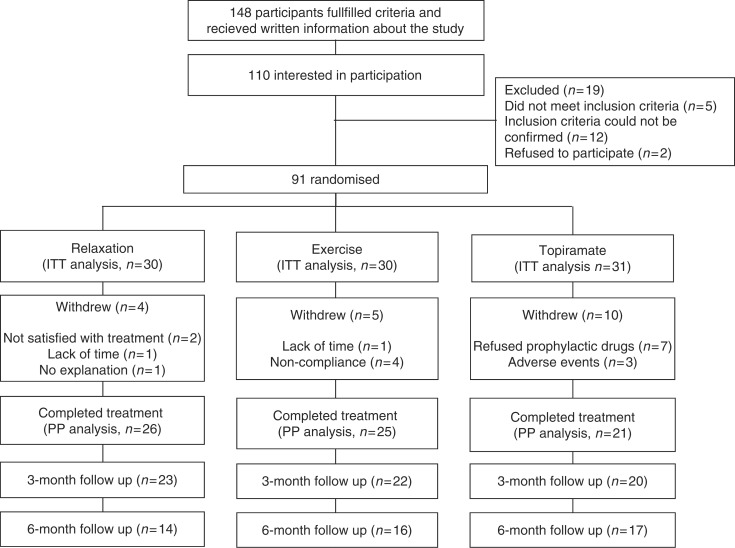
Of the 148 participants who received written information about the study, 91 could be randomized and included in the ITT analysis. The flow of participants through each stage of the trial is presented in Figure 2. Among the participants, 44 had migraine without aura, seven had migraine with aura and 40 had both diagnoses. One of the patients had chronic migraine. The demographic data and baseline outcome measures are presented in Table 1.
Figure 2.
Flow of participants throughout all stages of the study.
Table 1.
Baseline demographic characteristics of the intention-to-treat (ITT) and the per-protocol (PP) populations
| Characteristic | Relaxation (n = 30) | Exercise (n = 30) | Topiramate (n = 31) | Total ITT (n = 91) | Total PP (n = 72) |
|---|---|---|---|---|---|
| Age (years) | 41.5 ± 11. 4 | 47.0 ± 10.8 | 44.4 ± 9.2 | 44.3 ± 10.6 | 44.4 ± 11.3 |
| Sex | |||||
| Male | 2 (7) | 5 (17) | 2 (6) | 9 (10) | 6 (8) |
| Female | 28 (93) | 25 (83) | 29 (94) | 82 (90) | 66 (92) |
| Weight (kg) | 69.8 ± 9.8 | 70.1 ± 13.3 | 75.6 ± 15.0 | 72.1 ± 13.0 | 72.1 ± 12.7 |
| Height (cm) | 170 ± 6.5 | 170 ± 8.4 | 170 ± 6.4 | 170 ± 7.1 | 170 ± 6.4 |
| Body mass index (kg/m2) | 24.1 ± 2.3 | 24.9 ± 4.1 | 26.4 ± 4.8 | 25.1 ± 3.9 | 25.1 ± 3.9 |
| Smoking | 1 (3) | 2 (7) | 3 (10) | 6 (7) | 5 (7) |
| Type of migraine | |||||
| Migraine with aura | 1 (3) | 3 (10) | 3 (10) | 7 (8) | 5 (7) |
| Migraine without aura | 16 (53) | 11 (37) | 17 (35) | 44 (48) | 34 (47) |
| Migraine with and without aura | 13 (43) | 16 (53) | 11 (55) | 40 (44) | 33 (46) |
| Duration of disease (years) | 22.2 ± 11.8 | 28.8 ± 11.0 | 25.1 ± 11.4 | 25.4 ± 11.6 | 25.7 ± 11.9 |
| Baseline frequency of migraine (days) | 7.6 ± 3.8 | 7.0 ± 3.8 | 7.5 ± 3.9 | 7.3 ± 3.8 | 7.2 ± 3.6 |
| Baseline frequency of migraine attacks (numbers of attacks) | 4.2 ± 1.6 | 4.3 ± 2.0 | 3.6 ± 1.6 | 4.0 ± 1.8 | 4.1 ± 1.7 |
| Baseline frequency of headache medication used (doses) | 6.5 ± 4.6 | 6.9 ± 4.1 | 7.1 ± 5.3 | 6.8 ± 4.6 | 7.4 ± 4.8 |
| Baseline intensity of pain (VAS) | 39 (26–55) | 50 (26–64) | 40 (29–58) | 40 (29–61) | 45 (30–63) |
| Baseline MSQoL | 58 (51–67) | 60 (43–77) | 60 (48–73) | 60 (47–72) | 59 (50–71) |
| Baseline oxygen uptake (ml x kg−1 x min−1) | 34.9 ± 2.4 | 33.8 ± 1.7 | 30.9 ± 2.1 | 33.2 ± 1.2 | 32.7 ± 1.1 |
Data are expressed as number (%), mean±standard deviation or median (interquartile range). MSQoL = Migraine-specific quality of life; VAS, visual-analogue scale.
A significant difference in the change in body weight from the baseline values was seen between the groups after treatment (PP population, p = 0.007). The mean change was 1.0 (0.1–1.9) kg within the relaxation group, –0.5 (−1.5 to 0.4) kg within the exercise group and −1.3 (−2.4 to −0.2) kg within the topiramate group.
Primary efficacy variable
The change in the number of migraine attacks during the last month of treatment compared with the baseline showed a mean reduction of 0.93 (95% CI 0.31–1.54) attacks in the exercise group, 0.83 (95% CI 0.22–1.45) attacks in the relaxation group, and 0.97 (95% CI 0.36–1.58) attacks in the topiramate group. No significant difference was observed between the groups (p = 0.95). This result was confirmed in the PP analysis (p = 0.77). An overall test for within individual changes over time for the entire group showed a significant reduction (p<0.05).
Secondary efficacy variables
Among the secondary efficacy variables, the reduction in pain intensity from the baseline to the mean of the 3-month treatment period significantly favoured the topiramate group (p = 0.044). However, no differences were seen when comparing the proportions of subjects with a change in pain intensity defined numerically as improved or non-improved (p = 0.29). In the exercise group, maximal oxygen uptake increased significantly (p = 0.008). No statistically significant differences between the groups were found for any of the other secondary efficacy variables at any point in time. The PP analysis confirmed all of these results, accept for acute medication use 6 month after treatment. The results are presented in Table 2 and 3.
Table 2.
Differences from baseline in efficacy variables at different points in time for the intention-to-treat (ITT) and the per-protocol (PP) populations
| Variable | ITT |
PP |
||||||
|---|---|---|---|---|---|---|---|---|
| Relaxation | Exercise | Topiramate | p-value | Relaxation | Exercise | Topiramate | p-value | |
| Attack frequency (n/month) | ||||||||
| Treatment period | −0.97 ± 0.23 | −0.65 ± 0.23 | −0.70 ± 0.23 | 0.57 | −1.17 ± 0.25 | −0.66 ± 0.26 | −0.95 ± 0.28 | 0.37 |
| Last month of treatment | −0.83 ± 0.31 | −0.93 ± 0.31 | −0.97 ± 0.31 | 0.95 | −1.03 ± 0.35 | −0.90 ± 0.36 | −1.27 ± 0.39 | 0.77 |
| 3 months after treatment | −0.94 ± 0.28 | −0.98 ± 0.28 | −0.68 ± 0.28 | 0.71 | −1.15 ± 0.31 | −0.87 ± 0.32 | −0.87 ± 0.34 | 0.78 |
| 6 months after treatment | −0.95 ± 0.27 | −0.86 ± 0.27 | −0.73 ± 0.27 | 0.85 | −1.15 ± 0.30 | −0.80 ± 0.31 | −0.96 ± 0.34 | 0.72 |
| Migraine days (n/month) | ||||||||
| Treatment period | −1.40 ± 0.43 | −1.15 ± 0.43 | −1.49 ± 0.43 | 0.85 | −1.57 ± 0.60 | −1.89 ± 0.58 | −3.15 ± 0.63 | 0.16 |
| Last month of treatment | −1.32 ± 0.55 | −1.98 ± 0.55 | −2.13 ± 0.54 | 0.54 | −1.65 ± 0.44 | −1.06 ± 0.45 | −2.18 ± 0.49 | 0.25 |
| 3 months after treatment | −1.47 ± 0.55 | −2.23 ± 0.55 | −2.08 ± 0.54 | 0.59 | −1.75 ± 0.57 | −2.14 ± 0.58 | −3.05 ± 0.63 | 0.31 |
| 6 months after treatment | −1.83 ± 0.52 | −1.71 ± 0.52 | −1.98 ± 0.51 | 0.93 | −2.17 ± 0.53 | −2.03 ± 0.54 | −2.86 ± 0.59 | 0.55 |
| Mean pain intensity (VAS 0–100) | ||||||||
| Treatment period | −3.1 ± 2.3 | −4.7 ± 2.3 | −11.1 ± 2.3 | 0.04a | −3.6 ± 2.6 | −5.1 ± 2.7 | −14.9 ± 2.9 | 0.01a |
| Last month of treatment | −6.2 ± 3.2 | ⊟8.8 ± 3.2 | −14.5 ± 3.2 | 0.18 | −7.1 ± 3.7 | −10.2 ± 3.7 | −20.0 ± 4.1 | 0.06 |
| 3 months after treatment | −5.1 ± 3.5 | −7.1 ± 3.5 | −13.7 ± 3.4 | 0.19 | −5.9 ± 4.0 | −8.0 ± 4.1 | −18.7 ± 4.5 | 0.09 |
| 6 months after treatment | −4.6 ± 3.6 | −5.9 ± 3.6 | −11.3 ± 3.5 | 0.37 | −5.3 ± 4.2 | −7.3 ± 4.3 | −15.2 ± 4.7 | 0.27 |
| Acute medication use (doses/month) | ||||||||
| Treatment period | −1.33 ± 0.54 | −1.08 ± 0.56 | −1.89 ± 0.54 | 0.57 | −1.84 ± 0.77 | −2.43 ± 0.82 | −2.94 ± 0.88 | 0.64 |
| Last month of treatment | −1.56 ± 0.65 | −1.98 ± 0.68 | −2.15 ± 0.65 | 0.81 | −1.59 ± 0.64 | −1.49 ± 0.68 | −2.54 ± 0.73 | 0.52 |
| 3 months after treatment | −2.84 ± 0.54 | −2.72 ± 0.55 | −2.71 ± 0.54 | 0.98 | −3.34 ± 0.59 | −3.10 ± 0.63 | −3.63 ± 0.68 | 0.85 |
| 6 months after treatment | −2.91 ± 0.52 | −2.30 ± 0.53 | −3.64 ± 0.52 | 0.20 | −3.42 ± 0.54 | −2.97 ± 0.57 | −5.02 ± 0.62 | 0.05 a |
| Quality of life (points 1–100) | ||||||||
| After treatment | 3.4 ± 1.9 | 5.7 ± 1.9 | 1.9 ± 1.9 | 0.37 | 4.3 ± 2.2 | 5.8 ± 2.2 | 2.9 ± 2.4 | 0.68 |
| 3 months after treatment | 3.1 ± 2.4 | 5.0 ± 2.3 | 2.4 ± 2.3 | 0.73 | 4.0 ± 2.7 | 4.9 ± 2.7 | 3.7 ± 3.0 | 0.95 |
| 6 months after treatment | 4.0 ± 2.2 | 5.5 ± 2.2 | 2.5 ± 2.2 | 0.62 | 4.9 ± 2.6 | 5.1 ± 2.6 | 3.7 ± 2.8 | 0.93 |
| Level of physical activity (MET-minutes/week) | ||||||||
| After treatment | 0 (−453 to 480) | 277 (0–1107) | 0 (0–403) | 0.23 | 20 (−480 to 480) | 318 (0–1080) | 132 (0–633) | 0.34 |
| Sedentary (hours/day) | ||||||||
| After treatment | 0 (−2 to 0) | 0 (−2 to 0) | 0 (0–0) | 0.20 | 0 (−2 to 0) | 0 (−2 to 0) | 0 (0–1) | 0.32 |
| Oxygen uptake (ml/kg/min) | ||||||||
| After treatment | 1.1 ± 1.0 | 3.9 ± 0.9 | −0.4 ± 1.0 | 0.008a | 0.9 ± 1.0 | 4.8 ± 1.2 | −0.8 ± 1.2 | 0.002a |
Values are least square means ± standard error or median (interquartile range). aSignificant result.
Table 3.
Participants who responded to the different treatments
| Response | ITT |
PP |
||||
|---|---|---|---|---|---|---|
| Relaxation (n = 30) | Exercise (n = 30) | Topiramate (n = 31) | Relaxation (n = 26) | Exercise (n = 25) | Topiramate (n = 21) | |
| Responders (≥50% improvement) | 7 (23) | 9 (30) | 8 (26) | 7 (27) | 7 (28) | 8 (38) |
| Somewhat improved (25⊟49% improvement) | 5 (17) | 5 (17) | 3 (10) | 5 (19) | 5 (20) | 3 (14) |
| Not clinically improved (≤25% improvement) | 18 (60) | 16 (53) | 20 (65) | 14 (54) | 13 (52) | 10 (48) |
Values are n (%). ITT: p = 0.86; PP: p = 0.93.
Adverse events
No AEs were documented for participants in the relaxation or exercise groups. AEs were documented by eight participants in the topiramate group (33%). The safety population (i.e. the patients who took at least one dose of medicine) consisted of 24 individuals. Three of them (12.5%) reported AEs as the cause of their withdrawal from the study. The most common AEs reported were paresthesia (n = 5), fatigue (n = 3), a depressed mood (n = 3), vertigo (n = 2), and infrequent bowel movements (n = 2). Headaches, tremors, muscle twitching, mood swings, dysgeusia, nausea, dry eyes, epistaxis, a dry mouth, urinary incontinence, amnesia, cognitive disorders, diarrhoea, and musculoskeletal chest pain were each reported by one patient. The same patients often reported several symptoms. No serious AEs were reported.
Discussion
Scientific evidence regarding exercise in migraine prophylaxis is required. In this randomized controlled study, participants doing regular exercise experienced an improvement in the frequency of their migraine attacks that was not significantly different from the change achieved with well-evaluated treatment alternatives. This finding was also true for most secondary efficacy variables and confirmed through PP analysis.
Exercise treatment resulted in a significant increase in maximal oxygen uptake in comparison to the other treatments, which was expected. It did not, however, significantly affect the participants’ levels of physical activity (MET-minutes/week) or sedentary time. Topiramate yielded a greater improvement in terms of mean pain intensity, which was one of the secondary efficacy variables. However, no difference was seen in the proportions of subjects with a change in pain intensity defined numerically as improved or non-improved. The absolute change was relatively small, and it can therefore be discussed whether it is clinically relevant. Furthermore, we cannot exclude type I errors. It may be important from a clinical perspective that AEs were only reported in the topiramate group. No serious AEs were reported. Although in a low proportion of patients, topiramate has the potential to cause such AEs (18). AEs are a well-known reason for patients to reject prophylactic drugs, which makes it important to further evaluate non-pharmacological options. However, this finding must be interpreted with caution, as written information about potential AEs was provided in the medication group only. Furthermore, the standard method used for capturing AEs stems from drug-related research and is not necessarily sensitive to AEs resulting from non-pharmacological interventions, which may seem more natural to participants. As we know from previous studies that topiramate and relaxation are effective as means of migraine prophylaxis, we assume from our study results that exercise is also effective. Our findings are also supported by earlier studies and reports on migraine and exercise (8–10,27). This has a direct implication for patients who are unwilling to take medication or cannot tolerate it.
Although the study has acceptable power, we cannot exclude the notion that differences between these treatments exist. However, it is our opinion that if these differences are too small to discover in a sample of this size, other factors than effect might be just as important to consider when choosing prophylactic treatment. Such may include, for example, patients’ preferences, beliefs with regard to medicines (28) and how much time they want to devote to prophylactic actions.
We chose to have active treatments as controls, instead of placebos or ‘waiting lists’. The issue of placebo-controlled trials has been debated (29), and our decision was based on the conclusion of the Declaration of Helsinki (2008), that a placebo may be used only where no current proven intervention exists or where there are compelling and scientifically sound methodological reasons (30). A further reason for this design was the need to compare exercise with both standard medical therapy and the most common non-pharmacological option. The study compared supervised group treatment (exercise) with individual treatment (relaxation) and medication with only a few scheduled appointments. This was primarily in order to reflect our normal clinical practice. Regarding the risk that the patients received unequal amounts of attention, which could affect the results, we believe that individual relaxation therapy once a week may have resulted in the individual patients receiving as much attention as those performing exercises in small groups three times a week. The participants in the topiramate group had the opportunity to call the neurologist at any time during the day with questions and for support, which many of them did. The exercise group met other participants, but possible group effects in migraine treatment are poorly evaluated. We doubt that meeting others can prevent migraine as well as well-evaluated prophylactic medication.
It is methodologically challenging to compare pharmacological and non-pharmacological treatments. Participants often have a preference for drug or behavioural therapy, which may undermine their adherence for therapy, influence dropout rate, and even affect treatment response (31). This was true for some participants in our study, who were unsatisfied with the randomization. Dropout may have affected our results accordingly, but as we performed our analyses based on both the ITT and PP samples without finding any differences, we believe that this problem was controlled for. For ethical reasons, the participants were assured of their right to drop out at any time without giving a reason.
There are some limitations to be considered in the interpretation of our results. This study was based on a self-selected sample of patients and the external validity of our findings is not obvious. In an attempt to increase the generalizability of our findings, we predominately recruited participants via newspaper advertisements. The decision not to include patients who already undertook regular exercise could be criticized, as individuals who are unaccustomed to exercise do not necessarily represent a typical migraine population,(6) although migraine sufferers in general have been found to exercise less with the increasing severity of the disease (12). Regarding QoL, the patients in our study were comparable to patients with migraine in a large study of 1383 patients (19).
When interpreting our results, we cannot exclude that regression to the mean and the natural course of the disorder play a role in the improvements during and after treatment. Since the study was randomized, the three groups should, however, have been equally affected. Nor can we, with this study design, determine the relative role of the placebo effect in the different treatment groups. The treatment effects in our study were somewhat smaller than expected and the effects in the medicine group are smaller compared to other studies of topiramate There are though some possible explanations for this. First, in comparison to studies of migraine prevention, our participants were slightly older and had a longer duration of disease (32,33). Furthermore, we chose not to exclude participants who had severe migraine and those who had failed adequate courses of treatment with ≥3 migraine prophylactic agents. In other studies these are seen as exclusion criteria (32,34). Owing to these factors, our patients may have been treatment-refractory to a greater extent, which may have affected our results negatively. A further reason that the effects seen in our study is inferior to what is seen in other topiramate studies can be that we defined ITT more generously (3,35,36). Our PP-population is therefore more suitable to use in a comparison of treatment effects with the studies referred to. Some studies only report the PP-population (37,38). The efficacy variables and the definition of a migraine attack also sometimes differ. Using the PP-population to compare our topiramate group to earlier important topiramate studies regarding 50% improvement, days with migraine and, if similarly defined, also migraine attack frequency show that our results are superior to the placebo groups in all studies and in some cases similar to the topiramate groups (3,35–38). We therefore find it unlikely, that the favorable results in our study are entirely caused by the placebo effect in any of the groups. Since the results in the non-pharmacological treatment groups were not significantly inferior to those in the topiramate group in this adequately powered study, we argue that those treatments are indeed effective, and assume that this is not exclusively caused by the placebo effect. The major strengths of our study include the randomized controlled trial design, and that established guidelines for randomized controlled studies and for clinical trials of prophylactic migraine treatments were adhered to (15,16). Other strong points include the participation of an enrolling neurologist with a great deal of experience in diagnosing migraine patients, the use of two well-documented treatment methods as controls, and the use of valid and reliable outcome measures. The overall results from our study indicate that exercise is a non-pharmacological treatment option for migraine. The results are of great value, as non-pharmacological options for migraine treatment are often sought after by patients. From a wider health-based perspective, it should be stressed that patients with migraine are less physically active than the general population (12), and that exercise has positive effects in terms of general well-being and the prevention of disease (39). We welcome additional studies to verify our results.
In conclusion, exercise was found to be equal to the well-documented methods of relaxation and topiramate with regard to the reduction of migraine frequency. This non-pharmacological approach may therefore be an option for the prophylactic treatment of migraine in patients who do not benefit from or do not want daily medication.
Acknowledgements
The authors would like to thank biostatistician Jan Kowalski, JK Biostatistics, Stockholm, Sweden, for conducting the statistical analyses, Maarit Bengtsson for assistance with the exercise group, and Björn Erkholm for testing aerobic capacity. Thanks to Fysiken Fitness Centre, Spinnaren Health Centre, and Läkarhuset Göteborg for the use of their facilities.
Funding
This work was supported by the Swedish Research Council (Vetenskapsrådet) (DNR: 2009-376), the Gothenburg Research and Development Council (VGFOUGSB-6147), Praktikertjänst, Stockholm, Sweden, the Minnesfonden at the Swedish Association of Registered Physiotherapists, the Renée Eander fund (Renée Eanders hjälpfond), The Neurological Research Foundation (Insamlingsstiftelsen för neurologisk forskning), the Olle Engkvists Byggmästare Foundation (Stiftelsen Olle Engkvist Byggmästare), GlaxoSmithKlein and AstraZeneca.
Conflicts of interests
No conflicts of interest were reported.
References
- 1. Menken M, Munsat TL, Toole JF. The global burden of disease study: implications for neurology. Arch Neurol 2000; 57(3): 418–420. [DOI] [PubMed] [Google Scholar]
- 2. Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, et al. EFNS guideline on the drug treatment of migraine ⊟ revised report of an EFNS task force. Eur J Neurol 2009; 16(9): 968–981. [DOI] [PubMed] [Google Scholar]
- 3. Diener HC, Tfelt-Hansen P, Dahlof C, Lainez MJ, Sandrini G, Wang SJ, et al. Topiramate in migraine prophylaxis ⊟ results from a placebo-controlled trial with propranolol as an active control. J Neurol 2004; 251(8): 943–950. [DOI] [PubMed] [Google Scholar]
- 4. Holroyd KA, Penzien DB. Pharmacological versus non-pharmacological prophylaxis of recurrent migraine headache: a meta-analytic review of clinical trials. Pain 1990; 42(1): 1–13. [DOI] [PubMed] [Google Scholar]
- 5. Campbell JK, Penzien DB and Wall EM. Evidence-based guidelines for migraine headache: behavioral and physical treatments. http://www.aan.com/professionals/practice/pdfs/gl0089.pdf (2010, consulted March 2011).
- 6. Busch V, Gaul C. Exercise in migraine therapy − is there any evidence for efficacy? A critical review. Headache 2008; 48(6): 890–899. [DOI] [PubMed] [Google Scholar]
- 7. Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003; 107(24): 3109–3116. [DOI] [PubMed] [Google Scholar]
- 8. Narin SO, Pinar L, Erbas D, Ozturk V, Idiman F. The effects of exercise and exercise-related changes in blood nitric oxide level on migraine headache. Clin Rehabil 2003; 17(6): 624–630. [DOI] [PubMed] [Google Scholar]
- 9. Lockett DM, Campbell JF. The effects of aerobic exercise on migraine. Headache 1992; 32(1): 50–54. [DOI] [PubMed] [Google Scholar]
- 10. Koseoglu E, Akboyraz A, Soyuer A, Ersoy AO. Aerobic exercise and plasma beta endorphin levels in patients with migrainous headache without aura. Cephalalgia 2003; 23(10): 972–976. [DOI] [PubMed] [Google Scholar]
- 11. Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007; 27(5): 394–402. [DOI] [PubMed] [Google Scholar]
- 12. Varkey E, Hagen K, Zwart JA, Linde M. Physical activity and headache: results from the Nord-Trondelag Health Study (HUNT). Cephalalgia 2008; 28(12): 1292–1297. [DOI] [PubMed] [Google Scholar]
- 13. Varkey E, Cider A, Carlsson J, Linde M. A study to evaluate the feasibility of an aerobic exercise program in patients with migraine. Headache 2009; 49(4): 563–570. [DOI] [PubMed] [Google Scholar]
- 14. Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. 2nd ed. Cephalalgia 2004; 24 (Suppl 1): 1–160. [PubMed] [Google Scholar]
- 15. Tfelt-Hansen P, Block G, Dahlof C, Diener HC, Ferrari MD, Goadsby PJ, et al. Guidelines for controlled trials of drugs in migraine. 2nd ed. Cephalalgia 2000; 20(9): 765–786. [DOI] [PubMed] [Google Scholar]
- 16. Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Int Med 2001; 134(8): 663–694. [DOI] [PubMed] [Google Scholar]
- 17. Lundqvist C, Benth JS, Grande RB, Aaseth K, Russell MB. A vertical VAS is a valid instrument for monitoring headache pain intensity. Cephalalgia 2009; 29(10): 1034–1041. [DOI] [PubMed] [Google Scholar]
- 18. Edvinsson L, Linde M. New drugs in migraine treatment and prophylaxis: telcagepant and topiramate. Lancet 2010; 376(9741): 645–655. [DOI] [PubMed] [Google Scholar]
- 19. Patrick DL, Hurst BC, Hughes J. Further development and testing of the migraine-specific quality of life (MSQOL) measure. Headache 2000; 40(7): 550–560. [DOI] [PubMed] [Google Scholar]
- 20. Macsween A. The reliability and validity of the Astrand nomogram and linear extrapolation for deriving VO2max from submaximal exercise data. J Sports Med Phys Fitness 2001; 41(3): 312–317. [PubMed] [Google Scholar]
- 21. Astrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol 1954; 7(2): 218–221. [DOI] [PubMed] [Google Scholar]
- 22. Ekelund U, Sepp H, Brage S, Becker W, Jakes R, Hennings M, et al. Criterion-related validity of the last 7-day, short form of the International Physical Activity Questionnaire in Swedish adults. Public Health Nutr 2006; 9(2): 258–265. [DOI] [PubMed] [Google Scholar]
- 23. Larsson B, Andrasik F. Guidetti V, Russell G, Sillanpää M, Winner P. Relaxation treatment of recurrent headaches in children and adolescents. Headache and migraine in childhood and adolescence. London: Martin Dunitz, 2002. 307–316. [Google Scholar]
- 24. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 1970; 2(2): 92–98. [PubMed] [Google Scholar]
- 25. American College of Sports and Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 1998; 30(6): 975–991. [DOI] [PubMed] [Google Scholar]
- 26. Evans RW, Linde M. Expert opinion: adherence to prophylactic migraine medication. Headache 2009; 49(7): 1054–1058. [DOI] [PubMed] [Google Scholar]
- 27. Kumar KK. Exercise for prophylaxis of migraine. Headache 1988; 28(3): 228–228. [DOI] [PubMed] [Google Scholar]
- 28. Hedenrud T, Jonsson P, Linde M. Beliefs about medicines and adherence among Swedish migraineurs. Ann Pharmacother 2008; 42(1): 39–45. [DOI] [PubMed] [Google Scholar]
- 29. Franklin G, Howard B. What makes placebo-controlled trials unethical? Am J Bioeth 2002; 2(2): 3–9. [DOI] [PubMed] [Google Scholar]
- 30. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. http://www.wma.net/en/30publications/10policies/b3/index.html (2011, consulted March 2011).
- 31. Schwartz CE, Chesney MA, Irvine MJ, Keefe FJ. The control group dilemma in clinical research: applications for psychosocial and behavioral medicine trials. Psychosom Med 1997; 59(4): 362–371. [DOI] [PubMed] [Google Scholar]
- 32. Streng A, Linde K, Hoppe A, Pfaffenrath V, Hammes M, Wagenpfeil S, et al. Effectiveness and tolerability of acupuncture compared with metoprolol in migraine prophylaxis. Headache 2006; 46(10): 1492–1502. [DOI] [PubMed] [Google Scholar]
- 33. Diener HC, Kronfeld K, Boewing G, Lungenhausen M, Maier C, Molsberger A, et al. Efficacy of acupuncture for the prophylaxis of migraine: a multicentre randomised controlled clinical trial. Lancet Neurol 2006; 5(4): 310–316. [DOI] [PubMed] [Google Scholar]
- 34. Cady RK, Mathew N, Diener HC, Hu P, Haas M, Novak GP. Evaluation of carisbamate for the treatment of migraine in a randomized, double-blind trial. Headache 2009; 49(2): 216–226. [DOI] [PubMed] [Google Scholar]
- 35. Silberstein SD, Neto W, Schmitt J, Jacobs D. Topiramate in migraine prevention: results of a large controlled trial. Arch Neurol 2004; 61(4): 490–495. [DOI] [PubMed] [Google Scholar]
- 36. Brandes JL, Saper JR, Diamond M, Couch JR, Lewis DW, Schmitt J, et al. Topiramate for migraine prevention: a randomized controlled trial. JAMA 2004; 291(8): 965–973. [DOI] [PubMed] [Google Scholar]
- 37. Storey JR, Calder CS, Hart DE, Potter DL. Topiramate in migraine prevention: a double-blind, placebo-controlled study. Headache 2001; 41: 968–975. [DOI] [PubMed] [Google Scholar]
- 38. Mei D, Capuano A, Vollono C, Evangelista M, Ferraro D, Tonali P, et al. Topiramate in migraine prophylaxis: a randomised double-blind versus placebo study. Neurol Sci 2004; 25(5): 245–250. [DOI] [PubMed] [Google Scholar]
- 39. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116(9): 1081–1093. [DOI] [PubMed] [Google Scholar]