Abstract
Witnessing domestic violence (WDV) is a traumatic childhood experience associated with increased risk for depression, posttraumatic stress disorder and reduced IQ scores. Specific affects of WDV on brain development have not been assessed. We sought to ascertain whether WDV was associated with abnormalities in white matter (WM) tract integrity using diffusion tensor imaging (DTI). Twenty subjects who witnessed domestic violence (16F/ 4M, mean age 22.4±2.48 yrs) but were not physically or sexually abused were compared to 27 healthy controls (19F/ 8M, 21.9±1.97 yrs) without exposure to trauma or Axis I and II disorders. DTI images were acquired with a 3T Siemens Trio scanner. Group differences in fractional anisotropy (FA), covaried by age, gender, parental education, perceived financial sufficiency, IQ and degree of exposure to parental verbal aggression were assessed using tract-based spatial statistics (TBSS), which projects FA values onto an alignment-invariant fiber tract representation. FA values in the inferior longitudinal fasciculus of left lateral occipital lobe were significantly lower (p<0.05 corrected for multiple comparison) in the WDV group. FA values correlated inversely with ratings of depression, anxiety, somatization, ‘limbic irritability’ and neuropsychological measures of processing speed. Measures of radial but not axial diffusivity were affected suggesting alterations in myelination. Degree of FA reduction was associated with duration of witnessing interparental verbal aggression and with exposure between ages 7 – 13 years. The inferior longitudinal fasciculus connects occipital and temporal cortex and is the main component of the visual–limbic pathway that subserves emotional, learning and memory functions that are modality specific to vision. This finding is consistent with the hypothesis that exposure to childhood maltreatment is associated with alterations in fiber pathways that convey the adverse experience to frontal, temporal or limbic regions.
1. Introduction
Each year millions of children are exposed to acts of physical violence between their parents. A nationally representative sample of dual-parent households in the USA (McDonald et al., 2006) and a National Longitudinal Survey of Children and Youth in Canada (Moss, 2003) both reported that 8% of children had in a given year seen violence at home. Retrospective reports from convenience samples paint a similar picture with about 14% of US undergraduates (Henning et al., 1997) and adults in a major Health Maintenance Organization (Edwards et al., 2003) witnessing at least one act of interparental violence (IPV). Although many parents try to shelter their children from witnessing domestic violence (WDV), they commonly see, hear, and intervene in these episodes (Fantuzzo et al., 1997).
WDV is a serious form of emotional abuse. We found in a sample of 18-22 year olds that it was associated with moderately large effects on ratings of depression, anger-hostility, dissociation and limbic ‘irritability’, and with very large effect sizes when combined with exposure to other forms of maltreatment (Teicher et al., 2006a). WDV increases risk for depression (Nicodimos et al., 2009) and aggression (Abrahams and Jewkes, 2005; Casiano et al., 2009) by 2-4 fold, and is one of the most common causes of childhood PTSD (Luthra et al., 2009; Silva et al., 2000).
Several studies have shown an association between exposure to childhood abuse and alterations in gray and white matter (see (Teicher et al., 2002; Teicher et al., 2006b) for reviews). While half or more of maltreated children with PTSD in previous neuroimaging studies witnessed domestic violence (e.g., (Carrion et al., 2001; De Bellis et al., 2002), it is unclear the degree to which reported effects could be attributed to WDV versus exposure to sexual or physical abuse.
To ascertain if there are specific effects associated with WDV we conducted a diffusion tensor imaging (DTI) analysis of white matter (WM) tract integrity in young adults with childhood exposure. DTI, which analyzes the restricted diffusion of water molecules, provides a more detailed assessment of fiber tracts than conventional MRI (Catani, 2006). It has emerged as a powerful technique for studying the role of neural connectivity in health and disease (Catani, 2006). Stress can affect fiber tract development as corticosteroids can suppress the final mitosis of glial cells necessary for myelination. Although long axonal connections are established in early development, the diameter and microtubular structure of axons continues to develop into adulthood (Keshavan et al., 2002) and postnatal experience can affect the proportion of myelinated and unmyelinated fibers of different diameters within a tract (Juraska and Kopcik, 1988). Corpus callosum abnormalities have been observed in several studies of childhood abuse (Andersen et al., 2008; De Bellis et al., 1999; De Bellis et al., 2002; Jackowski et al., 2008; Teicher et al., 2004; Teicher et al., 1997).
We recently reported alterations in fractional anisotropy (FA) in portions of the arcuate fasciculus, cingulum bundle and fornix of young adults exposed to parental verbal aggression – another form of emotional abuse (Choi et al., 2009). The arcuate fasciculus interconnects Wernicke’s and Broca’s area via a relay station in premotor/ motor areas (Bernal and Ardila, 2009) and it plays a significant role in speech comprehension (Breier et al., 2008; Choi et al., 2009). The cingulum bundle interconnects cortical and limbic regions and the portion affected was located in the parahippocampal gyrus and associated with symptoms of depression, ‘limbic irritability’ and dissociation (Choi et al., 2009). The fornix interconnects hippocampus with septal area and mammillary bodies and alterations in FA in this tract correlated with ratings of anxiety and somatization (Choi et al., 2009). These findings fit with our hypothesis that exposure to early abuse targets corticolimbic regions engaged in emotional regulation as well as sensory regions and pathways involved in the perception and emotional reaction to adverse events (Teicher et al., 2006b). A priori, we hypothesized that eye witnessing IPV would specifically target fiber tracts involved in processing and conveying visual information.
2. Methods
2.1 Participants
Our goal was to recruit for neuroimaging right-handed subjects 18-25 years of age from the community who were exposed to IPV (and an equivalent number of unexposed controls) but were not exposed to other forms of abuse or other factors that could adversely affect brain development. Subjects were excluded who had a history of sexual abuse, parental loss, neglect, or significant physical abuse or exposure to harsh corporal punishment, as well as exposure to war, gang violence, motor vehicle accidents, near drowning, fires, natural disasters or animal attacks. Subjects were also required to be free from any neurological disease or insult, including any degree of head trauma resulting in loss of consciousness > 5 minutes or migraine headaches. Subjects were excluded with a history of premature birth or birth complications; a history of being shaken in infancy or childhood, maternal substance abuse during pregnancy; or medical disorders that could affect brain development. Subjects were selected to have no more than minimal use of drugs and alcohol.
Maximal cutoff for degree of substance use was ≤ 2 times per month (85% never used drugs). Maximal degree of alcohol use was limited to drinking only on weekends, with no history of binge drinking or drug or alcohol abuse. Subjects tested negative for drugs and alcohol on each visit.
Potential subjects responded to advertisements with the title “Memories of Childhood”, were briefly screened by phone, and then completed an online assessment instrument with 2342 entry fields that provide a vast array of information regarding childhood history, development, and symptomatology. They were unaware that WDV was the focus of the study. Data were collected on 1,271 subjects. History of exposure to physical abuse was obtained by self-report to the question: “Have you ever been physically hurt or attacked by someone such as a parent, another family member or friend (for example have you ever been struck, kicked, bitten, pushed or otherwise physically hurt)?” If so, they were asked to provide information on their relationship to this individual, the number of times they were hurt, age of initiation and termination of these episodes, whether the abuse received, or should have received medical attention, and whether the abuse resulted in permanent injuries or scars (Teicher et al., 2006a). An individual was defined as having experienced physical abuse if they reported any episode of inflicted physical injury that received or should have received medical treatment or resulted in permanent injury, or if they reported at least 4 episodes in which they felt that they had been attacked to a less serious degree.
Individuals were defined as having experienced sexual abuse if they responded affirmatively to the question: “Have you ever been forced into doing more sexually than you wanted to do or were too young to understand? (By “sexually” we mean being forced against your will into contact with the sexual parts of your body or his/her body)” (Teicher et al., 2006a). They were also asked to provide information on their relationship to this individual, number of times they were forced, age of first and last abuse, and whether or not they felt terrified or had their life or another person’s life threatened.
Twenty-one percent (n=272) of subjects who provided online data responded affirmatively to the question “have you ever witnessed serious domestic violence”, but only 5% of the sample (n=68) endorsed exposure to WDV in the absence of physical or sexual abuse. Thirty of these were excluded for exposure to other forms of trauma or medical factors.
All potentially eligible subjects (n=38) were invited to the laboratory and n=32 underwent detailed evaluation, including Structural Clinical Interviews for DSM-IV Axis I and II psychiatric disorders (SCID) (First et al., 1997), supplemented by the ADHD section of the K-SADS-PL (Kaufman et al., 1996). Exposure to interfamilial violence and other forms of maltreatment were assessed using the 100-item semi-structured Traumatic Antecedents Interview, developed by Herman et al., (1989). This interview was designed to evaluate reports of physical or sexual abuse, witnessing violence, physical or emotional neglect, significant separations or losses, verbal abuse, or parental discord (Roy and Perry, 2004). The reliability of TAI variables ranges from acceptable to excellent (median intraclass R = 0.73) (Roy and Perry, 2004). Subjects were also evaluated using the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 1997; Bernstein et al., 1994), and both self-report and interview versions of the Conflict-Tactic Scales (Straus et al., 1998). Certified mental health clinicians (Ph.D. psychologists, clinical nurse specialists) conducted the assessment and evaluation interviews. A panel of three doctoral-level psychiatric clinicians with extensive experience treating trauma-exposed children reviewed information on potential subjects and assignments were made by full consensus. WDV subjects were selected without regard to psychiatric history, except for alcohol or drug abuse. Selecting subjects meeting criteria for a specific disorder could bias results by only including the most severely affected subjects. Conversely, selecting subjects without any psychiatric history could bias results in the opposite direction. The intent was to recruit a balanced sample that would provide a rigorous test of our proposed hypotheses. The Institutional Review Board of McLean Hospital approved the study. All subjects provided written informed consent.
Twenty subjects (16F/ 4M, 22.4±2.5 years old) with a history of WDV and 27 healthy age-equivalent controls (19F/ 8M, 21.9±2.0 years old) who met full criteria were imaged. Subjects in the WDV group reported seeing and hearing years of intense verbal aggression between their parents, which culminated in some years in acts of physical violence. Overall, they reported that they witnessed (between ages 3-16) 4.4 ± 2.7 (mean ± S.D) years of exposure to IP physical plus verbal aggression along with 4.8 ± 4.0 years of exposure to IP verbal aggression without physical violence, for a total exposure duration of 9.2 ± 2.8 years. Most subjects indicated that the perpetrator of the violence was the father (55%) or mother’s boyfriend (10%). Fifteen percent indicated that mother was the perpetrator of the violence and 10% indicated both mother and father. (Two subjects did not divulge the perpetrators identity). Seventy-five percent endorsed that WDV incidents were ‘very’ or ‘extremely’ upsetting and 80% stated that WDV had a ‘moderate’ or ‘great’ effect on their lives.
2.2 Assessment Measures
IQ was measured by neuropsychologists using the Wechsler Adult Intelligence Scale – III (WAIS-III) (Wechsler, 1997). The Memory Assessment Scale (MAS) (Williams, 1991) was used to assess short-term (STM), verbal and visuospatial memory. Ratings of dissociation, ‘limbic irritability’, depression and anxiety were obtained using the Dissociative Experience Scale, (Bernstein and Putnam, 1986) limbic system checklist–33 (Teicher et al., 1993), and Kellner’s Symptom Questionnaire (Kellner, 1987), respectively. These measures were selected, a priori, as we had previously reported that they were markedly elevated in a separate sample of young adults with a history of WDV (Teicher et al., 2006a). Sociodemographic variables are risk factors for psychopathology. The two primary components of socioeconomic status (SES) are parental education and income. We used these as separate factors as opposed to combining them into a single SES score, as research suggest that they may have more value as independent covariates (Duncan and Magnuson, 2003). Subjects were often uncertain about parental income, but were well aware of the degree of perceived financial sufficiency, or stress, experienced while growing up. This was rated on a scale ranging from 1 (much less than enough money for our needs) to 5 (much more than enough money for our needs). This measure correlated better with symptom ratings than their estimates of parental income and was used instead as a covariate.
2.3 Image Acquisition
MRI examinations were conducted at the Brain Imaging Center. The head was stabilized with cushions and tape to help minimize movement. Multiple diffusion-weighted images (DWIs), with 12 encoding directions and an additional T2-weighted scan, were acquired using a 3T Siemens Trio scanner with standard single shot, spin echo, echo planar acquisition sequence with eddy current balanced diffusion weighting gradient pulses to reduce distortion (Reese et al., 2003). Scan parameters were: b=1000 sec/ mm2, TE/ TR=81msec/ 5sec; matrix=128×128 on 220mm×220mm FOV; slices 5mm without gap resulting in voxels of 1.71875×1.71875×5mm. Four magnitude averages provided sufficient signal-to-noise ratios. Volumetric T1-weighted anatomic reference images were acquired using an MPRAGE sequence (TE/ TR/ TI=2.74ms/ 2.1s/ 1/ 1s; 256×256×128 martix for 1×1×1.3 mm voxels).
2.4 Image Processing and Analysis
DTI preprocessing, including skull stripping and Eddy current correction, were performed using the FMRIB Software Library (FSL, Oxford, U.K.). A diffusion tensor model was fit to each voxel to create fractional anisotropy (FA), radial diffusivity (RD) and axial diffusivity (AD) images. The tract-based spatial statistics (TBSS) tool in FSL was used to calculate tract-based differences in FA values between the WDV group and controls. TBSS is a new approach for group difference determination that uses an anatomically-based carefully tuned nonlinear registration procedure to project results onto an alignment-invariant tract representation (the “mean FA skeleton”) for voxelwise analysis of multi-subject diffusion data (Smith et al., 2006). TBSS computes a group mean FA skeleton, which represents the centers of all fiber bundles that are common to the subjects involved in the study (Smith et al., 2006). Each subject’s aligned FA image was projected onto the skeleton, by filling the skeleton with FA values from the nearest relevant tract center. Group-based skeletonized FA maps were used for statistical analysis, applying a General Linear Model covariate analysis. Requisite significance was set, a priori, to detect voxel clusters in which group differences corrected for multiple comparisons were significant at P<0.05. Analyses were also conducted assessing group differences in axial and radial diffusivity focusing on regions of interest identified by the FA maps. Studies suggest that alterations in radial diffusivity but not axial diffusivity result from effects on myelin rather than axon numbers (Brubaker et al., 2009; Song et al., 2002).
2.5 Statistical Analyses
Data analyses were conducted using R (R Development Core Team, 2010). Differences between groups were evaluated using analysis of covariance. Exploratory correlation analyses assessed whether regional differences in FA could potentially account for a significant portion of the variance in pre-specified symptom ratings. Similarly, exploratory correlation analyses were performed for possible associations between FA in identified regions and neurocognitive measures on the WAIS-III (Wechsler, 1997). We hypothesized that since WDV has been associated with reduced IQ scores in childhood (Koenen et al., 2003) and infancy (Delaney-Black et al., 2002), that there may be correlations between WAIS-III index scores and white matter tract integrity in subjects exposed to WDV. Both cases and controls were included in the primary analyses, as we were interested in assessing potential functional correlates of these delineated WM segments in the general population, not just in subjects with WDV. Examining correlations in only one group, as sometimes advocated, restricts the range of the independent variable and can significantly bias results. Range restrictions deflate correlation coefficients if they reduce the standard deviation of the distribution of scores on one or both variables, and inflate r values if they increase the standard deviation, and should be avoided when possible (Russo, 2003). Further, the size of the full sample provided sufficient power (0.8) to detect medium size effects (r~0.4). Analysis of the individuals groups only provided sufficient power (0.8) to reliably detect large effects (r~0.6).
Spearman rank order correlations, partialling out effects of gender, were used to assess the degree of relationship between clinical variables and FA, as rating scale scores were not normally distributed (Kolmogrov-Smirnov and Shapiro-Wilk normality tests). Within group analyses were calculated for variables that showed a significant relationship with FA in the entire subject pool to determine if the regressive relationship applied more to one group than the other.
We assessed the effects of WDV at different ages using random forest regression (R package random Forest 4.5-34). This is a new analytic procedure, developed by Breiman (2001) as an extension of the decision tree approach. It is a form of “ensemble learning” in which a large number of unpruned decision trees are generated and their results aggregated. The random part comes in as each tree is constructed using a different bootstrap sample of the data, and each node is split using the best among a subset of predictors randomly chosen at that node. As Liaw and Wiener indicate (2002) this somewhat counterintuitive strategy performs very well compared to many other classifiers, including discriminant analysis, logistic regression, support vector machines and neural networks, and is robust against overfitting (Breiman, 2001). Advantages of random forest regression include: (1) very high classification accuracy; (2) a novel method of determining variable importance; and (3) no restrictions regarding the distribution and scaling properties of the data (Cutler et al., 2007). It is primarily use is in data mining and in genomic analysis, such as microarray studies. We coded each subjects exposure to IPV from ages 3 – 16, with 0 for no exposure in a given year, 1 for witnessing intense verbal aggression between their parental figures that year, and 2 for witnessing both intense verbal aggression and physical violence between their parental figures. Based on this information random forest regression analysis calculated the percent of variance in FA that could be explained by exposure across these ages, and determined the importance of exposure at each age. This was assessed by calculating the percent increase in mean square error following permutation of a variable. In other words, how much was the overall fit degraded by eliminating the contribution of a given age to the regression.
2.6 Tract-tracing
Detailed Tractography was performed in representative WDV and control subjects using MedINRIA (Medical Image Navigation and Research Tool by INRIA; http://www.sop.inria.fr/asclepios/software/–MedINRIA/) to better localize regions of statistically significant differences (determined by TBSS) along the likely pathways identified by probabilistic tract tracing. Regions of interest were selected on the directionally encoded tensor maps similar to the methods described by Vernooij et al. (2007) and Concha et al. (2005). Minimum fiber length was set to 5 mm and the smoothness of reconstructed fiber to 20. Other parameters for fiber tracking used MedINRIA default values. Tracking of fibers terminated when FA fell below a threshold of 0.18.
3. Results
3.1 Demographics and clinical measures
As indicated in Table I, the two groups did not significantly differ in age, gender ratio, years of education, and degree of drug or alcohol use. Subjects in these two groups also had nearly identical mean performance scores on memory tests. There was about a four-point difference between groups in IQ measures, which were not significant. However, there were significant differences between groups in parental education, perceived financial sufficiency and degree of exposure to parental verbal aggression. Parents of subjects in the WDV group had, on average, 1.7 fewer years of education. None of the subjects in the control group indicated that they came from families with less than enough money to meet their needs, while this was reported by 40% of the subjects in the WDV group. Similarly, none of the subjects in the control group were exposed to levels of parental verbal aggression previously identified as abusive (Teicher et al., 2006a), but 55% of subjects in the WDV group reported this degree of exposure. Hence, neuroimaging analyses were adjusted to control for differences in parental education, financial sufficiency and degree of exposure to parental verbal aggression. Parameters with standardized difference scores > 0.1 can also serve as potential confounding factors in cohort studies (Mamdani et al., 2005), so gender, age and full-scale IQ (which encompasses all of the WAIS-III measures) were included as additional covariates.
Table I.
Demographic characteristics, neurocognitive and psychiatric ratings of control and witnessing dor violence groups.
| Characteristics | Controls mean (S.D.) | Witness Domestic Violence mean (S.D). | Statistics | Standardized Difference | |
|---|---|---|---|---|---|
| (Anova, other) | p-value | ||||
| Gender (Males/Females) | 8M/19F | 4M/16F | Fisher, φ | 0.52 | 0.11 |
| Age (years) | 21.4 (1.9) | 22.1 (2.5) | 0.97 | 0.3 | 0.29 |
| Parental Education (years) | 16.2 (2.4) | 14. 5 (2.8) | 5.44 | 0.03 | 0.66 |
| Preceived Financial Sufficiency | 3.8 (0.7) | 2.7 (0.7) | 28.78 | 10-5 | 1.25 |
| Parental Verbal Abuse Score | 14.1 (7.7) | 39.0 (21.6) | 30.74 | 10-5 | 1.27 |
| Subject Education (years) | 14.3 (1.5) | 14.3 (1.8) | 0.02 | 0.8 | 0.04 |
| Drug use (days/month)a,b | 0.12 (0.41) | 0.14 (0.47) | 0.23 | 0.6 | 0.07 |
| Alcohol use (drinks/month)a,b | 14.6 (33.7) | 14.0 (20.6) | 0.00003 | 1.0 | 0.02 |
| Memory Assessment Scale | |||||
| Short-term memory | 105.8 (14.2) | 105.3 (16.0) | 0.02 | 0.9 | 0.04 |
| Verbal memory | 115.5 (13.9) | 113.9 (12.4) | 0.14 | 0.7 | 0.10 |
| Visual memory | 110.0 (9.9) | 110.9 (10.6) | 0.08 | 0.8 | 0.09 |
| Global memory | 114.5 (8.8) | 114.4 (10.3) | 0.0004 | 1.0 | 0.01 |
| Wechsler Adult Intelligence Scale | |||||
| Verbal IQ | 126.0 (14.1) | 121.1 (11.0) | 1.52 | 0.2 | 0.38 |
| Performance IQ | 117.0 (9.8) | 114.4 (13.8) | 0.53 | 0.5 | 0.23 |
| Full Scale IQ | 123.5 (11.8) | 119.8 (11.7) | 1.00 | 0.3 | 0.31 |
| Verbal Comprehension Index | 126.1 (12.8) | 123.4 (9.18) | 0.63 | 0.4 | 0.24 |
| Perceptual Organization Index | 118.8 (9.69) | 117.3 (16.7) | 0.13 | 0.7 | 0.11 |
| Working Memory Index | 114.1 (16.1) | 109.5 (15.2) | 0.92 | 0.3 | 0.29 |
| Processing Speed Index | 113.9 (16.6) | 108.5 (12.1) | 1.41 | 0.2 | 0.36 |
| Kellner Symptom Questionnaire | |||||
| Anxietya,b | 4.5 (3.5) | 8.4 (4.6) | 10.18 | 0.003 | 0.88 |
| Depressiona,b | 3.5 (3.7) | 7.7 (4.7) | 10.62 | 0.002 | 0.91 |
| Somatizationa,b | 3.8 (3.6) | 7.5 (3.6) | 11.89 | 0.001 | 0.92 |
| Anger-hostilitya,b | 3.3 (2.3) | 5.6 (3.2) | 6.69 | 0.01 | 0.78 |
| Dissociationa,b | 4.2 (4.0) | 11.9 (7.2) | 22.47 | 10-4 | 1.18 |
| Limbic Irritabilitya,b | 9.9 (7.3) | 24.5 (15.3) | 17.02 | 0.0002 | 1.12 |
Adjusted for
age,
gender
Subjects in the WDV group had increased ratings of anxiety, depression, somatization, anger-hostility, dissociation and ‘limbic irritability’. These differences were associated with large effect sizes. Twelve subjects in the WDV group meet DSM-IV criteria for one or more disorders. There were 8 subjects who met criteria for past history of major depression. Four were in remission. The remainder had recurrent episodes of depression and/ or dysthymia. Two subjects met current criteria for PTSD, 2 met for generalized anxiety disorder, and 2 for social phobia. One subject had ADHD, and two had a past history of eating disorders.
3.2 Neuroimaging measures
TBSS revealed one region of significantly reduced FA in the WDV group. This region was located in the left lateral occipital lobe and was characterized by a 13.2% reduction in FA (p < 0.05 corrected for multiple comparison, cluster size= 58, centered at MNI x=-2, y=-79, z=11) (Figure 1). Tract tracing identified this region as a portion of the inferior longitudinal fasciculus (ILF). The course of this fiber tract, extending from the left occipital cortex to the left temporal lobe, and passing through the identified region of reduced FA is illustrated in Figure 2.
Figure 1.
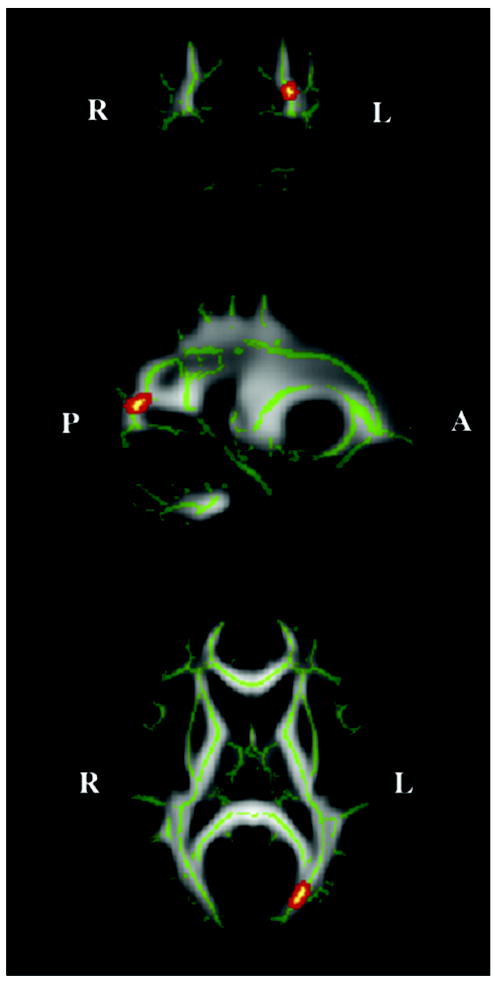
Coronal, sagittal and axial location of white matter tract region (shown in red, centered at x=-2, y=-79, z=11) that differed most significantly in fractional anisotropy (FA) between subjects with history of witnessing domestic violence and healthy controls. Green shows the mean FA skeleton (which represents the centers of white matter fiber bundles that are common to the subjects involved in this study). The background images are MNI152 templates.
Figure 2.
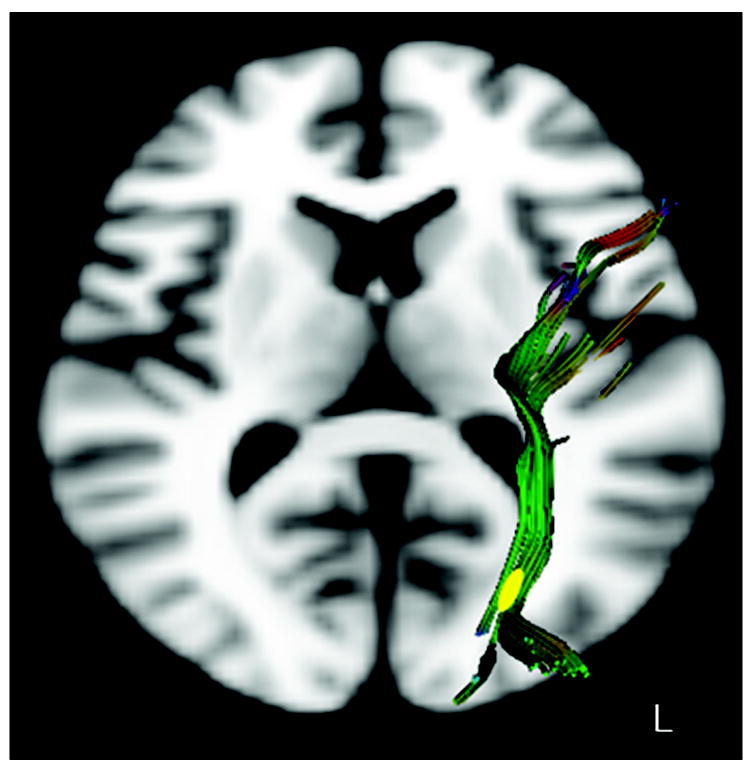
Detailed tractography of left inferior longitudinal fasciculus fibers in a representative subject color coded by fiber direction. Yellow region marks segment of the pathway delineated by tract-based spatial statistics as having significantly lower FA in subjects who witnessed domestic violence versus controls.
ANCOVA analyses indicated that FA in this region was markedly affected by exposure to IPV (F = 13.42, df = 1,39, P < 0.001) with gender (F = 6.6, df = 1,39, P < 0.02) as the only significant covariate (all other P values > 0.3). Within this region of reduced FA there was an 18.7% increase in radial diffusivity (F = 4.56, df = 1,38, P < 0.04), but only a non-significant 8.3% increase in axial diffusivity (F = 2.97, df = 1, 38, P < 0.10).
FA in this portion of the ILF appeared to be associated with both the duration and timing of exposure. The strongest association was between FA and duration of exposure to IP verbal aggression unaccompanied by physical violence (Figure 3). Across all subjects there was a Spearman rank order correlation (rs) of −0.557 (P < 0.0001), and a correlation rs = −0.670 (P < 0.002) for subjects in the WDV group. FA values in subjects with lengthy exposure to IP verbal aggression and only brief exposure to IP physical plus verbal aggression were more significantly affected than FA values in subjects with lengthy exposure to IP physical plus verbal aggression and correspondingly brief exposure to IP verbal aggression alone. This is illustrated in Figure 4 in which subjects in the WDV group were categorized into those with longer durations of exposure to IP verbal aggression alone than IP physical plus verbal aggression (IP verbal > physical, n=10) and the opposite (IP physical > verbal, n=9). (One subject could not be assigned because the history they provided was insufficiently detailed). There was a significant main effect of subgroup (F = 10.42, df = 2, 39, P < 0.0002). Average FA values were not significantly lower in subjects with IP physical > verbal than controls (TukeyHSD = -266.4, P > 0.3). In contrast, subjects with IP verbal > physical had much lower FA values than controls (TukeyHSD = -850.1, P = 0.0001) and lower FA values than subjects with IP physical > verbal (TukeyHSD = -583.6, P < 0.05). There was no difference between IP verbal > physical and IP physical > verbal subgroups in their total duration of exposure to IP aggression (F = 0.84, df = 1, 17, P > 0.3). On average subjects in the IP verbal > physical subgroup experience 7.6±3.3 years of exposure to IP verbal aggression alone and 2.2±1.9 years exposure to IP physical plus verbal aggression. In contrast, subjects in the IP physical > verbal subgroup experienced 1.8±1.8 years of exposure to IP verbal aggression alone and 6.8±3.3 years of IP physical plus verbal aggression.
Figure 3.
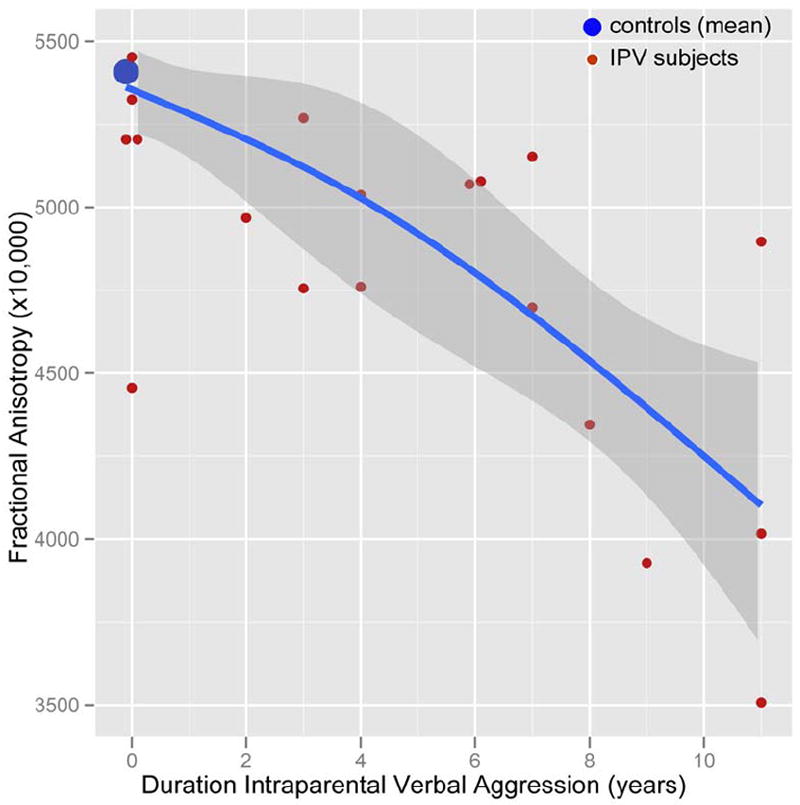
Scatter plot showing the relationship between years of exposure to interparental verbal aggression (unaccompanied by physical violence) and fractional anisotropy in the inferior longitudinal fasciculus. Blue line and confidence limits fit by natural spline with 2 degrees of freedom. IPV = interparental violence.
Figure 4.
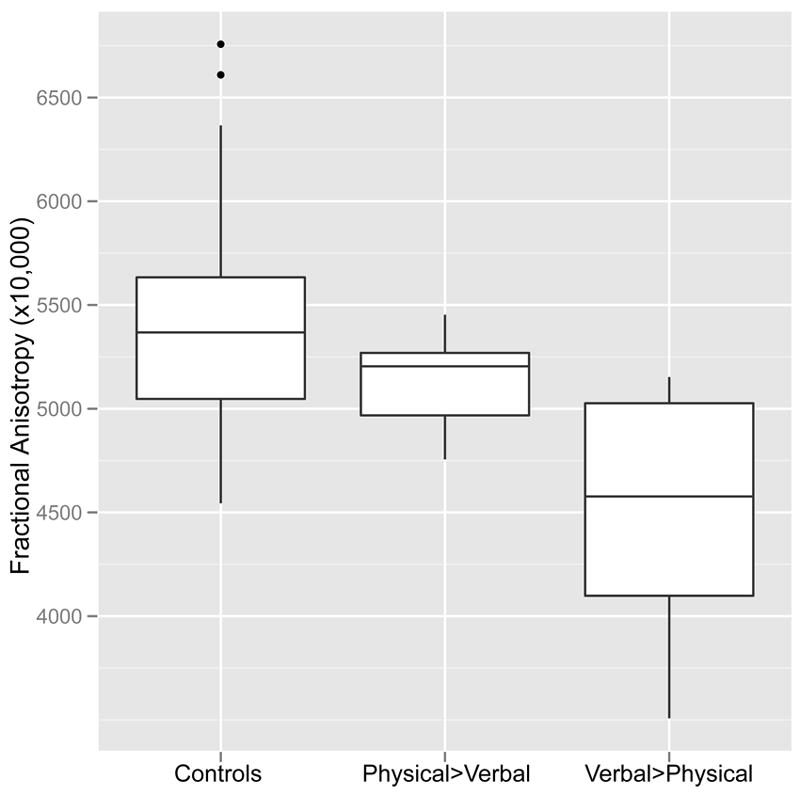
Boxplot showing differences in fractional anisotropy in the inferior longitudinal fasciculus between healthy controls, subjects exposed to more years of interparental physical plus verbal aggression than interparental verbal aggression alone (Physical>Verbal), and subjects exposed to more years of interparental verbal aggression than interparental physical plus verbal aggression (Verbal>Physical).
Random forest regression (20,000 trees, 4 variables tried per split) indicated that age of exposure accounted for 18.7% of the variance in FA measures in this portion of the ILF. As illustrated in Figure 5 there were marked apparent differences between ages in their relative importance. Ages 13, 12, 9 and 11 contributed most strongly to the fit. Overall, it appeared that the ILF was most susceptible to exposure to IP aggression from ages 7-13.
Figure 5.
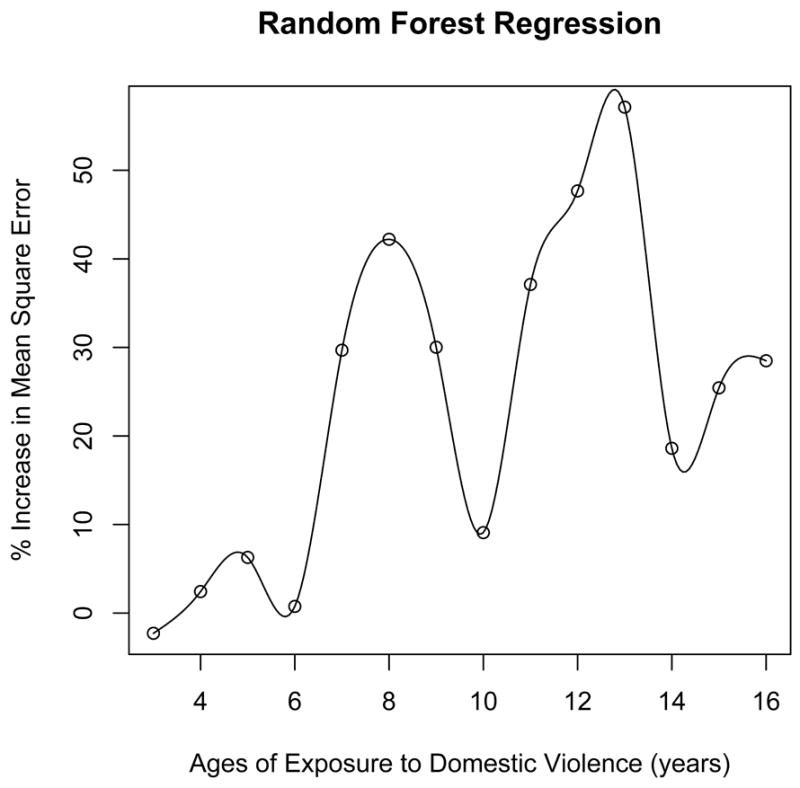
Results from random forest regression showing the importance of witnessing domestic violence at different ages on fractional anisotropy in the left inferior longitudinal fasciculus. The importance of exposure at a given age is indicated by the extent to which the mean square error of the overall fit is increased by eliminating the contribution of exposure at that age to the overall regression.
As indicated in Table II there were significant rank order correlations between FA in ILF and ratings of somatization, depression, anger-hostility, limbic irritability, anxiety and dissociation. Correlations between FA and psychiatric symptom rating were stronger in the WDV group than controls (e.g., depression: WDV group rs = -0.445, p < 0.05, controls rs = -0.113; anger-hostility: WDV group rs = -0.418, p < 0.06, controls rs = -0.098). There was also a significant correlation between FA in this ROI and the processing speed index, which was stronger in the controls (rs = 0.513, p < 0.01) than in WDV group (rs = 0.007). No other index or quotient on the WAIS-III correlated significantly with FA in this region.
Table II.
Relationship between FA in portion of the inferior longitudinal fasciculus and psychiatric ratings and nerocognitive measures.
| Measure | rs* | p value |
|---|---|---|
| Dissociation | -0.305 | 0.03 |
| Limbic Irritability’ | -0.411 | 0.003 |
| Anxiety | -0.343 | 0.02 |
| Depression | -0.424 | 0.002 |
| Somatization | -0.451 | 0.001 |
| Anger-hostility | -0.383 | 0.006 |
| Short Term Memory | 0.037 | 0.8 |
| Verbal Memory | 0.131 | 0.4 |
| Visual Memory | -0.114 | 0.5 |
| Global Memory | 0.004 | 0.98 |
| Verbal IQ | 0.218 | 0.2 |
| Performance IQ | 0.230 | 0.1 |
| Full Scale IQ | 0.227 | 0.1 |
| Verbal Comprehension Index | 0.224 | 0.1 |
| Perceptual Organization Index | 0.127 | 0.4 |
| Working Memory Index | 0.137 | 0.4 |
| Processing Speed Index | 0.356 | 0.02 |
Spearman rank order correlation with effect of gender partialled out
One concern in examining the potential effects of exposure to WDV is that WDV may be due to parental mental illness, and differences observed may be more a consequence of heredity than exposure. To test this possibility we reexamined the association covarying for history of maternal and paternal mental illness. Scores of zero were given for no history, 1 for a definite history and 0.5 for a possible history. Two-thirds of controls indicated that neither parent had a definite or possible history of mental illness versus only 20% of WDV subjects (χ2 = 11.61, df = 4, p = 0.02). Group differences remained significant after this adjustment (F = 14.97 df = 1,37, P = 0.0004), suggesting that the association between exposure to WDV and reduced regional FA, was not simply an artifact of parental mental illness.
Another possible concern is whether alterations in FA in the ILF were not actually related to WDV but to the subject’s symptoms. To address this issue an ANCOVA analysis was conducted controlling for all reported symptoms. The only symptom that emerged as a significant covariate was depression ratings (all other p values > 0.5). Group differences remained significant (F = 5.84, df = 1,43, p < 0.02) even after controlling for depression scores and gender.
4. DISCUSSION
We provide the first evidence demonstrating an association between WDV and regional development of white matter tracts. Specifically, WDV was associated with reduced FA and increased radial diffusivity in a portion of the left ILF. The ILF arises in extrastriate visual ‘association’ areas and projects to lateral and medial anterior temporal regions (Catani et al., 2003). This pathway appears to mediate the fast transfer of visual signals to anterior temporal regions and neuromodulatory back - projections from the amygdala to early visual areas (Catani et al., 2003). The ILF is a key component of the visual limbic pathway that subserves emotional, learning and memory functions that a re modality specific to vision. Clinical syndromes associated with ILF lesions include visual agnosia (inability to recognize objects or persons), prosopagnosia (inability to recognize faces), and impaired recent visual memory (Tusa and Ungerleider, 1985).
DTI studies have reported correlations between reduced FA in portions of the ILF and impairments of higher visuoperceptual functions (Rudrauf et al., 2008) including object and picture naming (Mandonnet et al., 2009; Shinoura et al.), dyslexia (Rollins et al., 2009; Steinbrink et al., 2008) and alexia (Epelbaum et al., 2008). Turken et al (2008) reported that FA in the left ILF specifically correlated with processing speed on the WISC-III. Our current finding that FA in the identified portion of the left ILF correlated with processing speed on the WAIS-III helps to confirm this observation. Hence, it is likely that the left ILF plays an important role in focusing attention and quickly scanning, discriminating between, and sequentially ordering visual information.
We found it surprising that WDV was specifically associated with reduced FA in left but not right ILF. We had hypothesized that WDV might target right hemisphere pathways based on the notion that WDV was primarily a visual event. One thing however that we learned in interviewing these subjects was that WDV has a strong auditory component, and that the worst episodes were perceived in a multimodal manner. Hence, the left ILF may have been affected as it also plays a role in language processing (Mandonnet et al., 2007).
Perhaps even more germane is the observation by Pourtois et al (2005) that while fearful facial expressions alone activate the right amygdala the combined presentation of fearful facial expressions and fearful voices specifically activates the left medial temporal gyrus (Pourtois et al., 2005). Similarly, Ethofer et al (2006) reported that subjects rated fearful and neutral facial expressions as more fearful when accompanied by a fearful voice, and that this shift in ratings of facial expression by a fearful voice correlated with hemodynamic response in the left amygdala and periamygdaloid cortex (Ethofer et al., 2006). Together, these findings suggest that crossmodal effects on the cognitive assessment of emotional valence are mediated via a convergence region situated in the left temporal cortex. Hence, in hindsight it seems reasonable that combined exposure to fearful facial expressions plus fearful voices may have overactivated portions of the left ILF and led, either through damage or compensatory down regulation, to suppressed development of this pathway. It is possible that reduced FA in this region may have had the beneficial effect of decreasing their stress or fear from seeing and hearing IPV. The price of this ‘adaptation’ however may be a reduction in visual processing speed (Turken et al., 2008), and an increased risk for dyslexia (Delaney-Black et al., 2002 Rollins et al., 2009; Steinbrink et al., 2008). There may also be significant psychiatric consequences, as seen in this sample.
FA in the identified portion of the left ILF correlated with ratings of anger-hostility, ‘limbic irritability’, depression, anxiety, dissociation and somatization, particularly in the WDV group. It is conceivable that affecting FA in the left ILF leads to an unbalanced right greater than left-sided limbic response to visual stimuli. In many individuals the right hemisphere appears specialized to have more emotional, particularly negative emotional responses to stimuli (Schiffer et al., 2007). We suspect that an imbalance between the integrity of the left and right ILF may predispose individuals to interpret relatively neutral stimuli in a more negative way, which may increase their vulnerability to psychopathology. Most of the subjects with WDV in this study were also exposed to parental verbal abuse. Although we controlled for this variable statistically, it is possible that alterations in arcuate fasciculus, fornix and cingulum bundle, seen in subjects exposed to parental verbal abuse (Choi et al., 2009), contributed to the neurocognitive and emotional difficulties observed in WDV subjects.
Results from random forest regression suggest that this portion of the left ILF was most susceptible to WDV between 7-13 years of age. Random forest regression is a relatively new technique with strengths and weaknesses that are not fully known. It may inflate prediction errors with certain data sets and provide good but not necessarily precise performance parameters in others. Nevertheless, the ages indicated as most susceptible by random forest regression coincided with the development of this pathway. FA in the left ILF was reported to increase with age between 6 – 17 years (Eluvathingal et al., 2007). However, no differences were reported in FA in the ILF between adolescence (14-21) and adulthood (22-64) (Schneiderman et al., 2007), suggesting that FA plateaus in early adolescence. Indeed, it appears from a graph presented in Hasan et al. (2010) that development of FA in left ILF plateaus at about 15 years of age. Hence, ages 7-13 may be a particularly vulnerable time period when FA values in this pathway are rapidly changing due to myelination. We have previously reported that there are sensitive periods when gray and white matter structures are most susceptible to exposure to childhood sexual abuse, and these sensitive periods tend to correspond to times of rapid developmental change (Andersen et al., 2008).
The finding that FA in the identified portion of the left ILF correlated with the duration of seeing and hearing intense interparental verbal arguments but not actual bouts of physical violence was interesting and unexpected. Blumenthal and colleagues (1998) had previously reported in college-age individuals that degree of exposure to interparental verbal aggression was a stronger predictor of psychiatric symptomatology than exposure to interparental violence. It may be the case that parents tried harder to protect children from seeing episodes likely to turn violent but had less concern about their witnessing non-physical confrontations. It may also be he case that the most powerful visual features were hurt and enraged facial expressions rather than actual blows. Acts of violence also tended to bring about a quick change in the confrontation. Sometimes the perpetrator would stop and apologize. In other cases one of the parties might leave, ending the immediate confrontation, and in some instances the police were called. Purely verbal fights could persist producing unabated feeling of stress and uncertainty. Subjects in the physical > verbal subgroup indicated that they were more upset by these episodes than subjects in the verbal > physical subgroup (0-5 scale, 4.7±0.5 vs. 4.1±0.5, F = 7.25, df = 1,17, p = 0.015), but both subgroups indicated that the impact of these events on their lives were equally profound (F = 0.20, df = 1,17, p > 0.6). We wonder if the ILF is also affected in subjects who only experienced intense verbal confrontations between parents in the absence of any interparental physical violence. Although the definition of domestic or intimate partner violence includes emotional, economic and sexual abuse, society tends to focus on the physical battery.
Herting et al., (2010) recently reported a reduction in FA in the left ILF and right optic radiation in children 11-15 years of age with family histories of alcoholism. Alcohol abuse is one of if not the leading risk factor for domestic violence (Kyriacou et al., 1999), and may have contributed to their finding, though they provided no data on WDV.
TBSS did not show evidence for FA reduction in the arcuate fasiculus, cingulum bundle, or fornix – tracts we identified as affected in a separate group of subjects who were exposed to parental verbal abuse but not IPV (Choi et al., 2009). This was expected as we considered degree of exposure to parental verbal aggression to be a nuisance factor in the present study and controlled for it in the analyses. There was also no evidence for FA differences in corpus callosum in this sample though we, and others, have consistently identified corpus callosum abnormalities in maltreated children using conventional MRI (Andersen et al., 2008; De Bellis et al., 1999; De Bellis et al., 2002; Teicher et al., 2004; Teicher et al., 1997) or DTI (Jackowski et al., 2008; Teicher et al., 2010). It is our impression that corpus callosum abnormalities are predominant in samples that contain subjects exposed to multiple different types of maltreatment, but may be overshadowed by alterations in more modality specific pathways in subjects exposed to only a single type of maltreatment.
This paper focused exclusively on fiber-tract differences associated with WDV. Differences in gray matter volume (GMV) and cortical thickness will be presented in a separate paper. Consistent with the present findings there were GMV differences in primary and secondary visual cortex, inferior temporal gyrus, parahippocampal gyrus, posterior cingulate and insula. Alterations in these regions may also contribute to observed clinical differences between WDV subjects and healthy controls. However, not all individuals exposed to WDV experience psychiatric difficulties – some appear to be remarkably resilient. Three factors may be of particular importance. First, polymorphisms such as the Val66Met substitution in brain-derived neurotrophic factor may influence susceptibility to both the clinical and neurobiological consequences of early-life stress (Gatt et al., 2009). Second, there may be, as this study suggests, sensitive periods when brain regions or fiber-tracts are maximally susceptible to early stress (Andersen and Teicher, 2008; Andersen et al., 2008). Exposure outside these ‘windows of vulnerability’ may be far less consequential. Third, there is probably an array of protective factors that may either occur prior to exposure to inoculate individuals against subsequent stressors, or occur following exposure to help rescue individuals (Kaufman et al., 2004).
The study is limited by the modest sample size, and by group differences in sociodemographic factors that were adjusted for. However, more definitive conclusions will require samples that are matched for sociodemographic factors (De Bellis et al., 2002). It is also limited by reliance on retrospective self-report. Some critics have raised concern about recall bias, suggesting that subjects who are currently in emotional distress will describe their childhood as more stressful or abusive (Pope and Hudson, 1995). Others have raised concerns about false or ‘recovered ’ memories (Allen, 1995). Based on these criticisms one might expect a high false positive rate for adult reports of childhood abuse. The opposite is actually the case (Williams, 1994). For example, Shaffer et al (2008) reported in a group of subjects assessed both prospectively and retrospectively that subjects often minimize their degree of exposure on retrospective report. Individuals reporting abuse retrospectively were those who typically endured the most severe abuse on prospective assessment. This fits with other studies showing that adult reports of abuse are verifiable (Chu et al., 1999). As an added layer of protection we solicited information regarding exposure to myriad forms of adversity along with a host of positive experiences, without informing candidates what specific experiences we were studying and what our enrollment criteria were. Our sample consisted of individuals reporting WDV but who denied experiencing a host of other stressful childhood experiences. These were not individuals who simply rated their childhoods as more stressful. The self-reported incidence of exposure to WDV in our screened sample was similar to previously published exposure rates (Edwards et al., 2003; Henning et al., 1997; McDonald et al., 2006; Moss, 2003).
Results from this study should be applicable to subjects exposed to IPV with or without exposure to other forms of emotional abuse such as parental verbal aggression. Subjects who witnessed IPV but also endured physical or sexual abuse might be expected to have additional neurobiological abnormalities (Carrion et al., 2001; De Bellis et al., 1999; De Bellis et al., 2002; Hanson et al., 2010). This study provides support for our hypothesis that exposure to early abuse targets corticolimbic regions or pathways engaged in emotional regulation as well as sensory regions and pathways mediating the perception of adverse events (Teicher et al., 2006b). The present findings also provide a potential mechanism to help explain some of the psychiatric and neurocognitive symptoms reported in children, adolescents and adults who have witnessed IPV.
Research Highlights.
Reduced fractional anisotropy in the left inferior longitudinal fasciculus – visual limbic pathways in young adults who witnessed domestic violence during childhood.
Reduction in fractional anisotropy in inferior longitudinal fasciculus associated with duration of exposure to intraparental verbal aggression rather than physical aggression.
Degree of reduction in fractional anisotropy in inferior longitudinal fasciculus correlated with ratings of depression, somatization and anger-hostility.
Degree of reduction in fractional anisotropy in inferior longitudinal fasciculus correlated with reduced processing speed on Wechsler Adult Intelligence Scale.
Axial diffusivity was affected in inferior longitudinal fasciculus suggesting affects on myelination.
Acknowledgments
This work was supported, in part, by RO1 awards to MHT from the National Institute of Mental Health under grant number MH-66222 and from the National Institute on Drug Abuse under grant numbers DA-016934 and DA-017846. This research was also supported, in part, by the Original Technology Research Program for Brain Science through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2010-0029266: B.S.J.). The authors thank Ms. Cynthia McGreenery for subject recruitment; and Carryl Navalta, Ph.D., Danielle Webster R.N., M.S., C.S., Katherine Flagg Ph.D., Keren Rabi M.A. and Hanako Suzuki M.A. for conducting or assisting in the clinical and neuropsychological assessments.
Footnotes
Disclosure of biomedical financial interests and potential conflicts of interest None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams N, Jewkes R. Effects of South African men’s having witnessed abuse of their mothers during childhood on their levels of violence in adulthood. Am J Public Health. 2005;95:1811–1816. doi: 10.2105/AJPH.2003.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JG. The spectrum of accuracy in memories of childhood trauma. Harv Rev Psychiatry. 1995;3:84–95. doi: 10.3109/10673229509017171. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomoda A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal B, Ardila A. The role of the arcuate fasciculus in conduction aphasia. Brain. 2009;132:2309–2316. doi: 10.1093/brain/awp206. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. see comments. [DOI] [PubMed] [Google Scholar]
- Bernstein EM, Putnam FW. Development, reliability and validity of a dissociation scale. J Nerv Ment Dis. 1986;174:727–735. doi: 10.1097/00005053-198612000-00004. [DOI] [PubMed] [Google Scholar]
- Blumenthal DR, Neemann J, Murphy CM. Lifetime exposure to interparental physical and verbal aggression and symptom expression in college students. Violence Vict. 1998;13:175–196. [PubMed] [Google Scholar]
- Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. Random Forests. Machine Learning. 2001;45:5–32. [Google Scholar]
- Brubaker CJ, Schmithorst VJ, Haynes EN, Dietrich KN, Egelhoff JC, Lindquist DM, Lanphear BP, Cecil KM. Altered myelination and axonal integrity in adults with childhood lead exposure: a diffusion tensor imaging study. Neurotoxicology. 2009;30:867–875. doi: 10.1016/j.neuro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Casiano H, Mota N, Afifi TO, Enns MW, Sareen J. Childhood maltreatment and threats with weapons. J Nerv Ment Dis. 2009;197:856–861. doi: 10.1097/NMD.0b013e3181be9c55. [DOI] [PubMed] [Google Scholar]
- Catani M. Diffusion tensor magnetic resonance imaging tractography in cognitive disorders. Curr Opin Neurol. 2006;19:599–606. doi: 10.1097/01.wco.0000247610.44106.3f. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu JA, Frey LM, Ganzel BL, Matthews JA. Memories of childhood abuse: dissociation, amnesia, and corroboration. Am J Psychiatry. 1999;156:749–755. doi: 10.1176/ajp.156.5.749. [DOI] [PubMed] [Google Scholar]
- Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26:2267–2274. [PMC free article] [PubMed] [Google Scholar]
- Cutler DR, Edwards TC, Jr, Beard KH, Cutler A, Hess KT, Gibson J, Lawler JJ. Random forests for classification in ecology. Ecology. 2007;88:2783–2792. doi: 10.1890/07-0539.1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. Developmental traumatology. Part II: Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Ondersma SJ, Nordstrom-Klee B, Templin T, Ager J, Janisse J, Sokol RJ. Violence exposure, trauma, and IQ and/ or reading deficits among urban children. Arch Pediatr Adolesc Med. 2002;156:280–285. doi: 10.1001/archpedi.156.3.280. [DOI] [PubMed] [Google Scholar]
- Duncan GL, Magnuson KA. Off With Hollingshead: Socioeconomic Resources, Parenting, and Child Development. In: Bornstein MH, Bradley RH, editors. Socioeconomic Status, Parenting, and Child Development. Lawrence Erlbaum; Mahwah, New Jersey: 2003. pp. 83–106. [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex. 2007;17:2760–2768. doi: 10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L. Pure alexia as a disconnection syndrome: new diffusion imaging evidence for an old concept. Cortex. 2008;44:962–974. doi: 10.1016/j.cortex.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Anders S, Erb M, Droll C, Royen L, Saur R, Reiterer S, Grodd W, Wildgruber D. Impact of voice on emotional judgment of faces: an event-related fMRI study. Hum Brain Mapp. 2006;27:707–714. doi: 10.1002/hbm.20212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzo J, Boruch R, Beriama A, Atkins M, Marcus S. Domestic violence and children: prevalence and risk in five major U.S. cities. J Am Acad Child Adolesc Psychiatry. 1997;36:116–122. doi: 10.1097/00004583-199701000-00025. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders - clinician version (SCID-CV) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009 doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. J Neurosci. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Abid H, Kramer LA, Fletcher JM, Ewing-Cobbs L. Quantification of the spatiotemporal microstructural organization of the human brain association, projection and commissural pathways across the lifespan using diffusion tensor tractography. Brain Struct Funct. 2010;214:361–373. doi: 10.1007/s00429-009-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning K, Leitenberg H, Coffey P, Bennett T, Jankowski MK. Long-term psychological adjustment to witnessing interparental physical conflict during childhood. Child Abuse Negl. 1997;21:501–515. doi: 10.1016/s0145-2134(97)00009-4. [DOI] [PubMed] [Google Scholar]
- Herman JL, Perry JC, van der Kolk BA. Traumatic Antecedents Interview. The Trauma Center; Boston: 1989. [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay discounting behavior and white matter microstructure abnormalities in youth with a family history of alcoholism. Alcohol Clin Exp Res. 2010;34:1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski AP, Douglas-Palumberi H, Jackowski M, Win L, Schultz RT, Staib LW, Krystal JH, Kaufman J. Corpus callosum in maltreated children with posttraumatic stress disorder: a diffusion tensor imaging study. Psychiatry Res. 2008;162:256–261. doi: 10.1016/j.pscychresns.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Kopcik JR. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan ND. Kiddie-SADS PL. Western Psychiatric Institute and Clinic. University of Pittsburgh Medical Centre; 1996. [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner R. A symptom questionnaire. Journal of Clinical Psychiatry. 1987;48:268–273. [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, DeBellis M, Dick E, Kotwal R, Rosenberg DR, Sweeney JA, Minshew N, Pettegrew JW. Development of the corpus callosum in childhood, adolescence and early adulthood. Life Sci. 2002;70:1909–1922. doi: 10.1016/s0024-3205(02)01492-3. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Caspi A, Taylor A, Purcell S. Domestic violence is associated with environmental suppression of IQ in young children. Dev Psychopathol. 2003;15:297–311. doi: 10.1017/s0954579403000166. [DOI] [PubMed] [Google Scholar]
- Kyriacou DN, Anglin D, Taliaferro E, Stone S, Tubb T, Linden JA, Muelleman R, Barton E, Kraus JF. Risk factors for injury to women from domestic violence against women. N Engl J Med. 1999;341:1892–1898. doi: 10.1056/NEJM199912163412505. [DOI] [PubMed] [Google Scholar]
- Liaw A, Wiener M. Classification and Regression by random Forest. R News. 2002;2/3:18–22. [Google Scholar]
- Luthra R, Abramovitz R, Greenberg R, Schoor A, Newcorn J, Schmeidler J, Levine P, Nomura Y, Chemtob CM. Relationship between type of trauma exposure and posttraumatic stress disorder among urban children and adolescents. J Interpers Violence. 2009;24:1919–1927. doi: 10.1177/0886260508325494. [DOI] [PubMed] [Google Scholar]
- Mamdani M, Sykora K, Li P, Normand SL, Streiner DL, Austin PC, Rochon PA, Anderson GM. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. Bmj. 2005;330:960–962. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandonnet E, Gatignol P, Duffau H. Evidence for an occipito-temporal tract underlying visual recognition in picture naming. Clin Neurol Neurosurg. 2009;111:601–605. doi: 10.1016/j.clineuro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain. 2007;130:623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- McDonald R, Jouriles EN, Ramisetty-Mikler S, Caetano R, Green CE. Estimating the number of American children living in partner-violent families. J Fam Psychol. 2006;20:137–142. doi: 10.1037/0893-3200.20.1.137. [DOI] [PubMed] [Google Scholar]
- Moss K. Witnessing violence--aggression and anxiety in young children. Health Rep. 2003;14(Suppl):53–66. [PubMed] [Google Scholar]
- Nicodimos S, Gelaye BS, Williams MA, Berhane Y. Associations between witnessing parental violence and experiencing symptoms of depression among college students. East Afr J Public Health. 2009;6:184–190. doi: 10.4314/eajph.v6i2.51764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Hudson JI. Does childhood sexual abuse cause adult psychiatric disorders? Essential of methodology. J Psychiatry Law. 1995;1995:363–381. [Google Scholar]
- Pourtois G, de Gelder B, Bol A, Crommelinck M. Perception of facial expressions and voices and of their combination in the human brain. Cortex. 2005;41:49–59. doi: 10.1016/s0010-9452(08)70177-1. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Rollins NK, Vachha B, Srinivasan P, Chia J, Pickering J, Hughes CW, Gimi B. Simple developmental dyslexia in children: alterations in diffusion-tensor metrics of white matter tracts at 3 T. Radiology. 2009;251:882–891. doi: 10.1148/radiol.2513080884. [DOI] [PubMed] [Google Scholar]
- Roy CA, Perry JC. Instruments for the assessment of childhood trauma in adults. J Nerv Ment Dis. 2004;192:343–351. doi: 10.1097/01.nmd.0000126701.23121.fa. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Grabowski TJ. Disconnection’s renaissance takes shape: Formal incorporation in group-level lesion studies. Cortex. 2008;44:1084–1096. doi: 10.1016/j.cortex.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Russo R. Statistics for the Behavioral Sciences: An Introduction. Psychology Press; 2003. [Google Scholar]
- Schiffer F, Teicher MH, Anderson C, Tomoda A, Polcari A, Navalta CP, Andersen SL. Determination of hemispheric emotional valence in individual subjects: A new approach with research and therapeutic implications. Behav Brain Funct. 2007;3:1–22. doi: 10.1186/1744-9081-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman JS, Buchsbaum MS, Haznedar MM, Hazlett EA, Brickman AM, Shihabuddin L, Brand JG, Torosjan Y, Newmark RE, Tang C, Aronowitz J, Paul-Odouard R, Byne W, Hof PR. Diffusion tensor anisotropy in adolescents and adults. Neuropsychobiology. 2007;55:96–111. doi: 10.1159/000104277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer A, Huston L, Egeland B. Identification of child maltreatment using prospective and self-report methodologies: a comparison of maltreatment incidence and relation to later psychopathology. Child Abuse Negl. 2008;32:682–692. doi: 10.1016/j.chiabu.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Tsukada M, Yoshida M, Yamada R, Tabei Y, Saito K, Koizumi T, Yagi K. Deficits in the left inferior longitudinal fasciculus results in impairments in object naming. Neurocase. 16:135–139. doi: 10.1080/13554790903329174. [DOI] [PubMed] [Google Scholar]
- Silva RR, Alpert M, Munoz DM, Singh S, Matzner F, Dummit S. Stress and vulnerability to posttraumatic stress disorder in children and adolescents. Am J Psychiatry. 2000;157:1229–1235. doi: 10.1176/appi.ajp.157.8.1229. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the Parent-Child Conflict Tactics Scales: development and psychometric data for a national sample of American parents. Child Abuse Negl. 1998;22:249–270. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Dumont NL, Ito Y, Vaituzis C, Giedd JN, Andersen SL. Childhood neglect is associated with reduced corpus callosum area. Biol Psychiatry. 2004;56:80–85. doi: 10.1016/j.biopsych.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Glod CA, Surrey J, Swett C., Jr Early childhood abuse and limbic system ratings in adult psychiatric outpatients. Journal of Neuropsychiatry & Clinical Neurosciences. 1993;5:301–306. doi: 10.1176/jnp.5.3.301. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Sciences. 1997;821:160–175. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, McGreenery CE. Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. Am J Psychiatry. 2006a;163:993–1000. doi: 10.1176/ajp.2006.163.6.993. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Sheu YS, Polcari A, McGreenery CE. Hurtful words: association of exposure to peer verbal abuse with elevated psychiatric symptom scores and corpus callosum abnormalities. Am J Psychiatry. 2010;167:1464–1471. doi: 10.1176/appi.ajp.2010.10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006b;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusa RJ, Ungerleider LG. The inferior longitudinal fasciculus: a reexamination in humans and monkeys. Ann Neurol. 1985;18:583–591. doi: 10.1002/ana.410180512. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: a combined fMRI and DTI study. Neuroimage. 2007;35:1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. The Psychological Corporation; New York: 1997. [Google Scholar]
- Williams JM. Memory Assessment Scales: Professional Manual. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Williams LM. Recall of childhood trauma: a prospective study of women’s memories of child sexual abuse. J Consult Clin Psychol. 1994;62:1167–1176. doi: 10.1037//0022-006x.62.6.1167. [DOI] [PubMed] [Google Scholar]


