Abstract
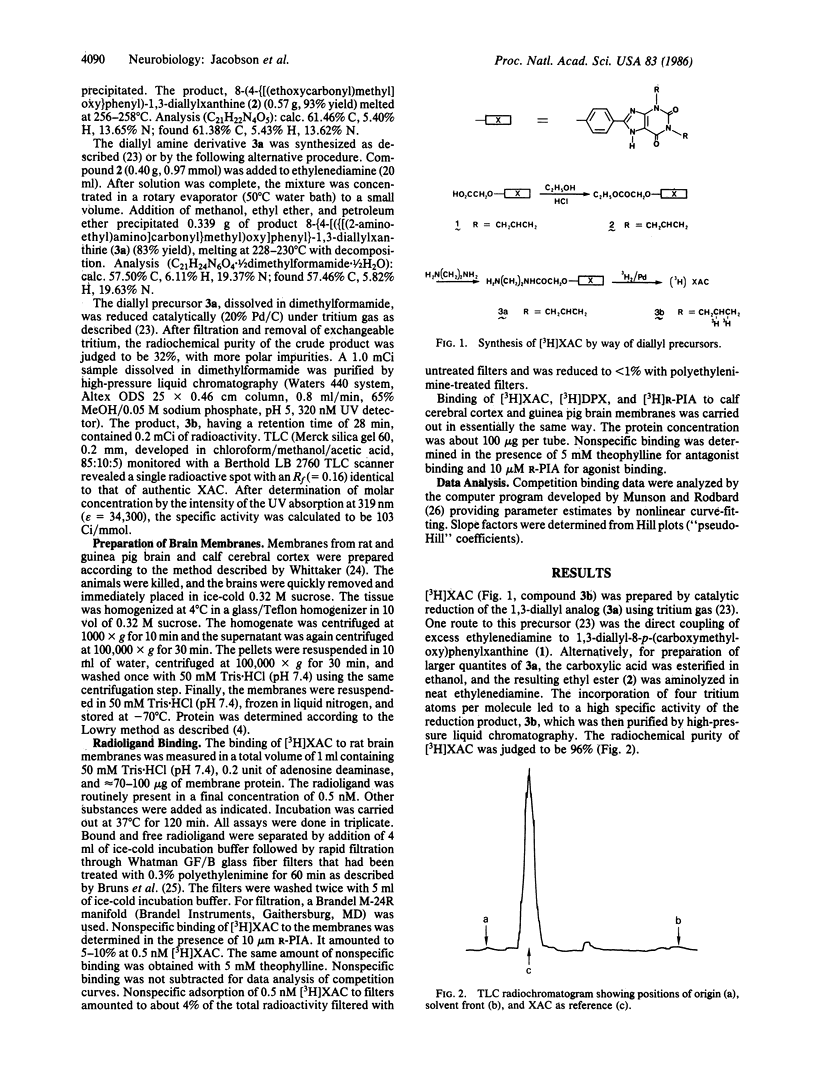
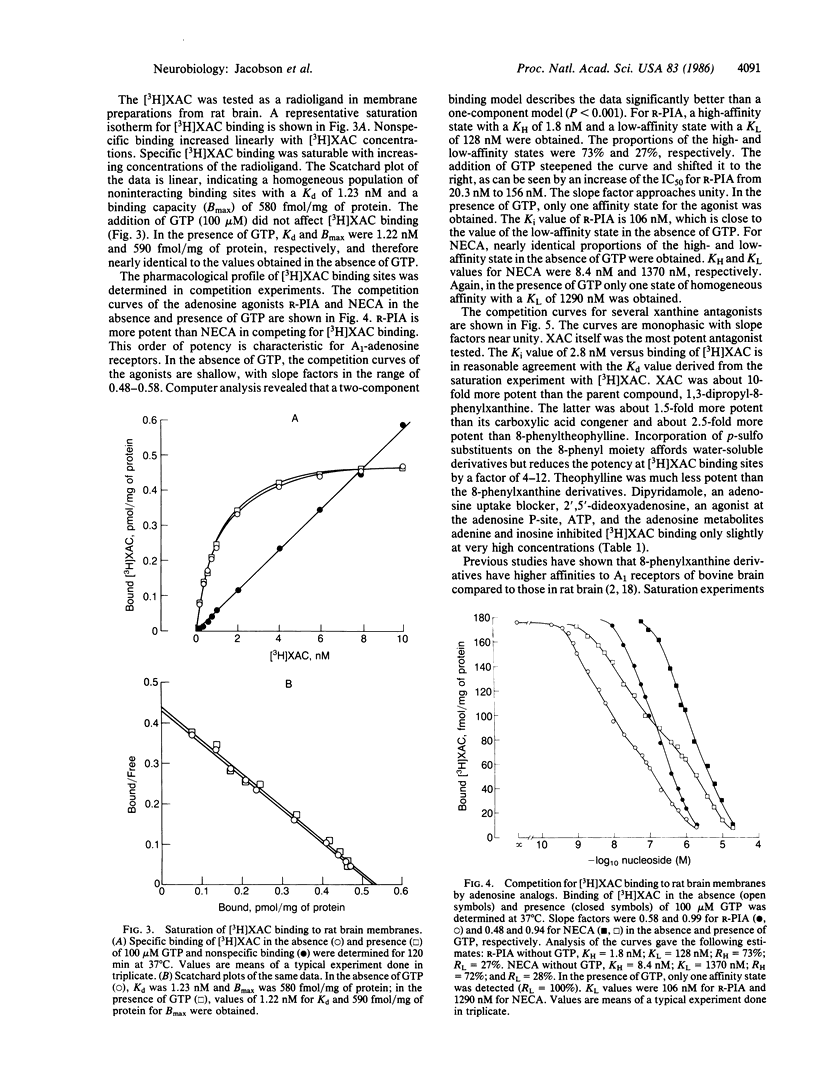
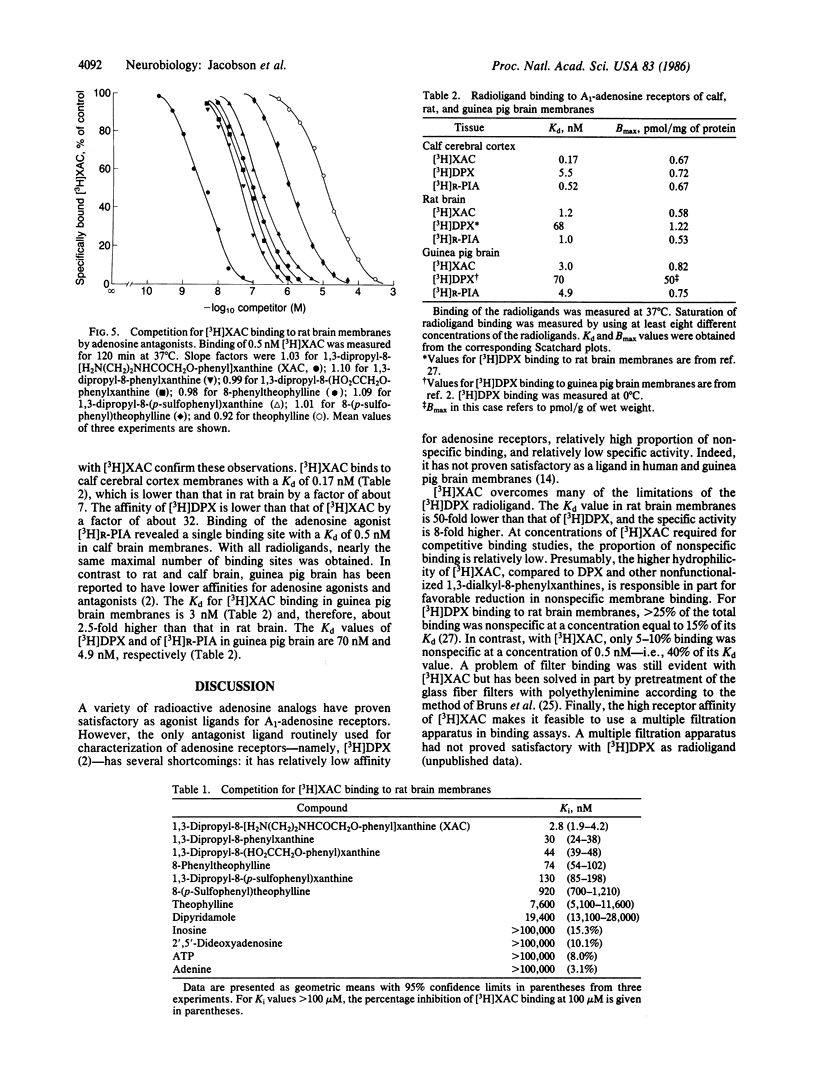
An amine-functionalized derivative of 1,3-dipropyl-8-phenylxanthine has been prepared in tritiated form as a xanthine amine congener ([3H]XAC) for use as an antagonist radioligand for adenosine receptors. [3H]XAC has higher receptor affinity, higher specific activity, lower nonspecific membrane binding, and more favorable hydrophilicity than 1,3-diethyl-8-[3H]phenylxanthine, the xanthine commonly used for adenosine receptor binding. In rat cerebral cortical membranes, [3H]XAC exhibits saturable, specific binding with a Kd of 1.23 nM and a Bmax of 580 fmol/mg of protein at 37 degrees C. N6-(R-Phenylisopropyl)adenosine is a more potent inhibitor of [3H]XAC binding than is 5'-N-ethylcarboxamidoadenosine, indicating that binding is to an A1-adenosine receptor. In the absence of GTP, the inhibition curves for adenosine agonists versus [3H]XAC binding are biphasic, indicating that [3H]XAC is binding to low- and high-affinity agonist states of the A1 receptor. In the presence of GTP, adenosine analogs exhibit monophasic, low-affinity inhibition of binding of [3H]XAC. Inhibition of [3H]XAC binding by theophylline or by various 8-phenylxanthines is monophasic, and the potencies are commensurate with the potencies of these xanthines as adenosine receptor antagonists. The receptor sites in calf brain membranes exhibit a higher affinity (Kd = 0.17 nM) for [3H]XAC, whereas sites in guinea pig exhibit a slightly lower affinity (Kd = 3.0 nM). Densities of [3H]XAC binding sites are similar in brain membranes from all species.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr, Thampy K. G. Subclasses of adenosine receptors in brain membranes from adult tissue and from primary cultures of chick embryo. J Neurochem. 1982 Sep;39(3):647–652. doi: 10.1111/j.1471-4159.1982.tb07941.x. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptor binding: structure-activity analysis generates extremely potent xanthine antagonists. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2077–2080. doi: 10.1073/pnas.80.7.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Lawson-Wendling K., Pugsley T. A. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal Biochem. 1983 Jul 1;132(1):74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Cooper M. J., Gavish M., Snyder S. H. Guanine nucleotide and cation regulation of the binding of [3H]cyclohexyladenosine and [3H]diethylphenylxanthine to adenosine A1 receptors in brain membranes. Mol Pharmacol. 1982 Mar;21(2):329–335. [PubMed] [Google Scholar]
- Green R. D. Reciprocal modulation of agonist and antagonist binding to inhibitory adenosine receptors by 5'-guanylylimidodiphosphate and monovalent cations. J Neurosci. 1984 Oct;4(10):2472–2476. doi: 10.1523/JNEUROSCI.04-10-02472.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton H. W., Ortwine D. F., Worth D. F., Badger E. W., Bristol J. A., Bruns R. F., Haleen S. J., Steffen R. P. Synthesis of xanthines as adenosine antagonists, a practical quantitative structure-activity relationship application. J Med Chem. 1985 Aug;28(8):1071–1079. doi: 10.1021/jm00146a016. [DOI] [PubMed] [Google Scholar]
- Jacobson K. A., Kirk K. L., Padgett W. L., Daly J. W. A functionalized congener approach to adenosine receptor antagonists: amino acid conjugates of 1,3-dipropylxanthine. Mol Pharmacol. 1986 Feb;29(2):126–133. [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., Kirk K. L., Padgett W. L., Daly J. W. Functionalized congeners of 1,3-dialkylxanthines: preparation of analogues with high affinity for adenosine receptors. J Med Chem. 1985 Sep;28(9):1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., Kirk K. L., Padgett W. L., Daly J. W. Functionalized congeners of adenosine: preparation of analogues with high affinity for A1-adenosine receptors. J Med Chem. 1985 Sep;28(9):1341–1346. doi: 10.1021/jm00147a039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson K. A., Kirk K. L., Daly J. W., Jonzon B., Li Y. O., Fredholm B. B. A novel 8-phenyl-substituted xanthine derivative is a selective antagonist at adenosine A1-receptors in vivo. Acta Physiol Scand. 1985 Oct;125(2):341–342. doi: 10.1111/j.1748-1716.1985.tb07725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M. J., Lenschow V., Schwabe U. Two affinity states of Ri adenosine receptors in brain membranes. Analysis of guanine nucleotide and temperature effects on radioligand binding. Mol Pharmacol. 1984 Jul;26(1):1–9. [PubMed] [Google Scholar]
- Lohse M. J., Ukena D., Schwabe U. Demonstration of Ri-type adenosine receptors in bovine myocardium by radioligand binding. Naunyn Schmiedebergs Arch Pharmacol. 1985 Jan;328(3):310–316. doi: 10.1007/BF00515559. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Trost T. Characterization of adenosine receptors in rat brain by (-)[3H]N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol. 1980 Sep;313(3):179–187. doi: 10.1007/BF00505731. [DOI] [PubMed] [Google Scholar]
- Schütz W., Steurer G., Tuisl E. Functional identification of adenylate cyclase-coupled adenosine receptors in rat brain microvessels. Eur J Pharmacol. 1982 Nov 19;85(2):177–184. doi: 10.1016/0014-2999(82)90463-0. [DOI] [PubMed] [Google Scholar]
- Schütz W., Tuisl E., Kraupp O. Adenosine receptor agonists: binding and adenylate cyclase stimulation in rat liver plasma membranes. Naunyn Schmiedebergs Arch Pharmacol. 1982 Apr;319(1):34–39. doi: 10.1007/BF00491475. [DOI] [PubMed] [Google Scholar]
- Ukena D., Daly J. W., Kirk K. L., Jacobson K. A. Functionalized congeners of 1,3-dipropyl-8-phenylxanthine: potent antagonists for adenosine receptors that modulate membrane adenylate cyclase in pheochromocytoma cells, platelets and fat cells. Life Sci. 1986 Mar 3;38(9):797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena D., Poeschla E., Schwabe U. Guanine nucleotide and cation regulation of radioligand binding to Ri adenosine receptors of rat fat cells. Naunyn Schmiedebergs Arch Pharmacol. 1984 Jun;326(3):241–247. doi: 10.1007/BF00505325. [DOI] [PubMed] [Google Scholar]
- Ukena D., Schirren C. G., Klotz K. N., Schwabe U. Evidence for an A2 adenosine receptor in guinea pig lung. Naunyn Schmiedebergs Arch Pharmacol. 1985 Oct;331(1):89–95. doi: 10.1007/BF00498856. [DOI] [PubMed] [Google Scholar]
- Williams M., Braunwalder A., Erickson T. J. Evaluation of the binding of the A-1 selective adenosine radioligand, cyclopentyladenosine (CPA), to rat brain tissue. Naunyn Schmiedebergs Arch Pharmacol. 1986 Feb;332(2):179–183. doi: 10.1007/BF00511410. [DOI] [PubMed] [Google Scholar]
- Williams M., Valentine H. L. Binding of [3H]cyclohexyladenosine to adenosine recognition sites in guinea pig ileal membranes: comparison with binding in brain membranes. Neurosci Lett. 1985 Jun 4;57(1):79–83. doi: 10.1016/0304-3940(85)90043-6. [DOI] [PubMed] [Google Scholar]
- Yeung S. M., Green R. D. Agonist and antagonist affinities for inhibitory adenosine receptors are reciprocally affected by 5'-guanylylimidodiphosphate or N-ethylmaleimide. J Biol Chem. 1983 Feb 25;258(4):2334–2339. [PubMed] [Google Scholar]
- Yeung S. M., Green R. D. [3H]5'-N-ethylcarboxamide adenosine binds to both Ra and Ri adenosine receptors in rat striatum. Naunyn Schmiedebergs Arch Pharmacol. 1984 Mar;325(3):218–225. doi: 10.1007/BF00495947. [DOI] [PubMed] [Google Scholar]
- Yeung S. M., Green R. D. [3H]5'-N-ethylcarboxamide adenosine binds to both Ra and Ri adenosine receptors in rat striatum. Naunyn Schmiedebergs Arch Pharmacol. 1984 Mar;325(3):218–225. doi: 10.1007/BF00495947. [DOI] [PubMed] [Google Scholar]


