Abstract
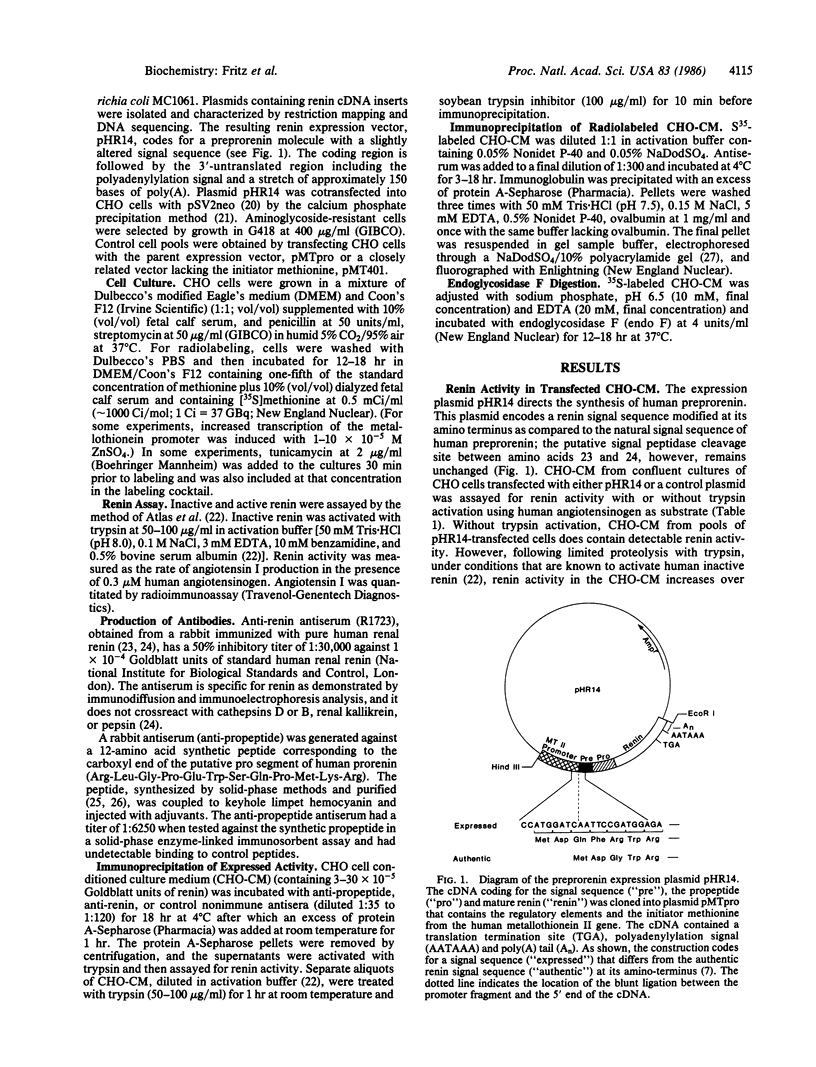
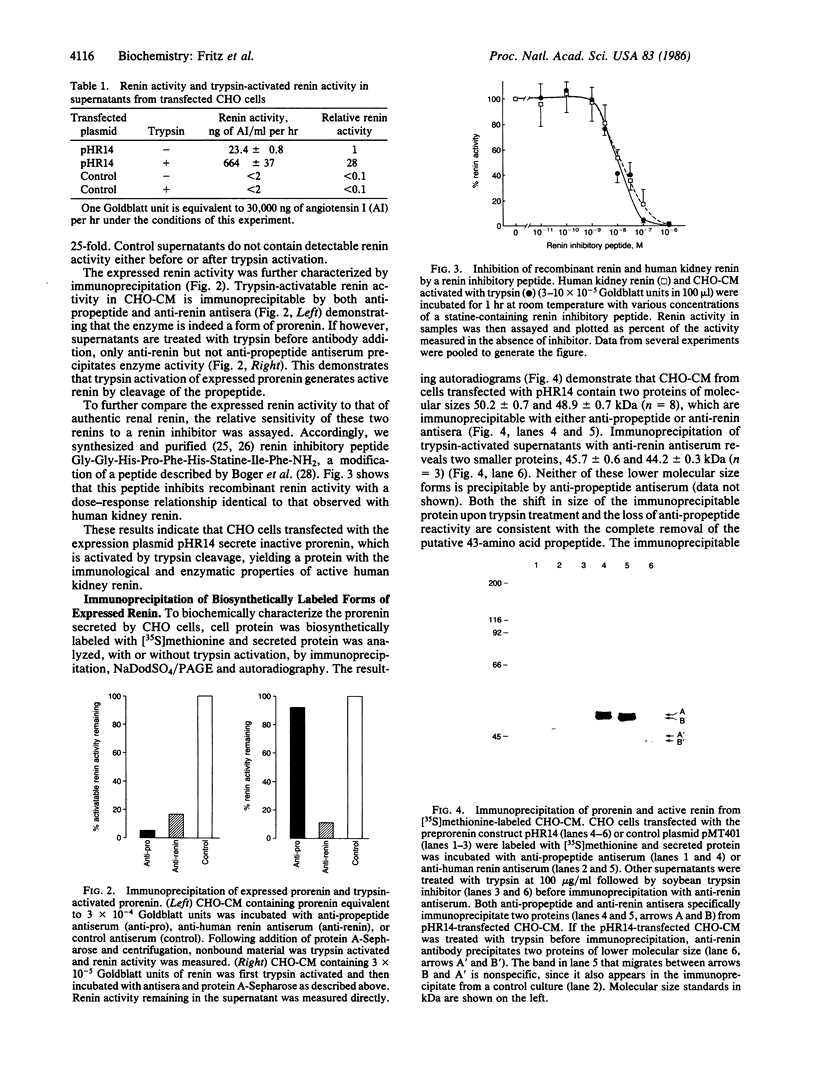
Human preprorenin was synthesized in Chinese hamster ovary (CHO) cells transfected with an expression vector containing renin cDNA sequences. These cells secrete an inactive form of renin (EC 3.4.23.15) that can be activated by trypsin. This inactive renin is precipitable by antibody generated against purified human renal renin and also by antisera generated to a synthetic peptide derived from the amino acid sequence of the pro segment of preprorenin (anti-propeptide), indicating that the secreted inactive enzyme is a form of prorenin. Analysis of [35S]methionine-labeled proteins immunoprecipitated from CHO cell conditioned culture medium indicates that prorenin is expressed in CHO cells as two distinct forms that differ in their degree of glycosylation. In vitro trypsin activation of prorenin cleaves approximately 4.5 kDa from the protein, rendering it unreactive with the antipropeptide antiserum but still recognizable by anti-renal renin antibody. These results show directly that the prorenin expressed by CHO cells is an inactive enzyme that is activated by trypsin cleavage of the pro segment. The ability to express human renin in this form will allow for the purification of both active and inactive forms of the enzyme in quantities sufficient for detailed physiological and structural studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker G. M., Galen F. X., Devaux C., Foote S., Papernik E., Pesty A., Menard J., Corvol P. Human chorionic cells in primary culture: a model for renin biosynthesis. J Clin Endocrinol Metab. 1982 Nov;55(5):902–909. doi: 10.1210/jcem-55-5-902. [DOI] [PubMed] [Google Scholar]
- Akahane K., Umeyama H., Nakagawa S., Moriguchi I., Hirose S., Iizuka K., Murakami K. Three-dimensional structure of human renin. Hypertension. 1985 Jan-Feb;7(1):3–12. doi: 10.1161/01.hyp.7.1.3. [DOI] [PubMed] [Google Scholar]
- Atlas S. A., Christofalo P., Hesson T., Sealey J. E., Fritz L. C. Immunological evidence that inactive renin is prorenin. Biochem Biophys Res Commun. 1985 Nov 15;132(3):1038–1045. doi: 10.1016/0006-291x(85)91911-4. [DOI] [PubMed] [Google Scholar]
- Atlas S. A., Hesson T. E., Sealey J. E., Dharmgrongartama B., Laragh J. H., Ruddy M. C., Aurell M. Characterization of inactive renin ("prorenin") from renin-secreting tumors of nonrenal origin. Similarity to inactive renin from kidney and normal plasma. J Clin Invest. 1984 Feb;73(2):437–447. doi: 10.1172/JCI111230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger J., Lohr N. S., Ulm E. H., Poe M., Blaine E. H., Fanelli G. M., Lin T. Y., Payne L. S., Schorn T. W., LaMont B. I. Novel renin inhibitors containing the amino acid statine. Nature. 1983 May 5;303(5912):81–84. doi: 10.1038/303081a0. [DOI] [PubMed] [Google Scholar]
- Bouhnik J., Fehrentz J. A., Galen F. X., Seyer R., Evin G., Castro B., Menard J., Corvol P. Immunologic identification of both plasma and human renal inactive renin as prorenin. J Clin Endocrinol Metab. 1985 Feb;60(2):399–401. doi: 10.1210/jcem-60-2-399. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Craik C. S., Kelly R. B. The exocrine protein trypsinogen is targeted into the secretory granules of an endocrine cell line: studies by gene transfer. J Cell Biol. 1985 Aug;101(2):639–645. doi: 10.1083/jcb.101.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson W., Karplus M., Haber E. Construction of a model for the three-dimensional structure of human renal renin. Hypertension. 1985 Jan-Feb;7(1):13–26. doi: 10.1161/01.hyp.7.1.13. [DOI] [PubMed] [Google Scholar]
- Duksin D., Mahoney W. C. Relationship of the structure and biological activity of the natural homologues of tunicamycin. J Biol Chem. 1982 Mar 25;257(6):3105–3109. [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. Isolation, cloning and sequence analysis of the cDNA for the alpha-subunit of human chorionic gonadotropin. Nature. 1979 Oct 4;281(5730):351–356. doi: 10.1038/281351a0. [DOI] [PubMed] [Google Scholar]
- Hardman J. A., Hort Y. J., Catanzaro D. F., Tellam J. T., Baxter J. D., Morris B. J., Shine J. Primary structure of the human renin gene. DNA. 1984 Dec;3(6):457–468. doi: 10.1089/dna.1.1984.3.457. [DOI] [PubMed] [Google Scholar]
- Hirose S., Kim S., Miyazaki H., Park Y. S., Murakami K. In vitro biosynthesis of human renin and identification of plasma inactive renin as an activation intermediate. J Biol Chem. 1985 Dec 25;260(30):16400–16405. [PubMed] [Google Scholar]
- Hobart P. M., Fogliano M., O'Connor B. A., Schaefer I. M., Chirgwin J. M. Human renin gene: structure and sequence analysis. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5026–5030. doi: 10.1073/pnas.81.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh W. A., Carlson E. J., Dzau V. J. Characterization of inactive renin from human kidney and plasma. Evidence of a renal source of circulating inactive renin. J Clin Invest. 1983 Mar;71(3):506–517. doi: 10.1172/JCI110795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh W. A., Luetscher J. A., Carlson E. J., Grislis G. Inactive renin of high molecular weight (big renin) in normal human plasma. Activation by pepsin, trypsin, or dialysis to pH 3.3 and 7.5. Hypertension. 1980 Nov-Dec;2(6):750–756. doi: 10.1161/01.hyp.2.6.750. [DOI] [PubMed] [Google Scholar]
- Imai T., Miyazaki H., Hirose S., Hori H., Hayashi T., Kageyama R., Ohkubo H., Nakanishi S., Murakami K. Cloning and sequence analysis of cDNA for human renin precursor. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7405–7409. doi: 10.1073/pnas.80.24.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagami T., Okamoto H., Ohtsuki K., Shimamoto K., Chao J., Margolius H. S. Human plasma inactive renin: purification and activation by proteases. J Clin Endocrinol Metab. 1982 Oct;55(4):619–627. doi: 10.1210/jcem-55-4-619. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Hirose S., Miyazaki H., Ueno N., Higashimori K., Morinaga S., Kimura T., Sakakibara S., Murakami K. Identification of plasma inactive renin as prorenin with a site-directed antibody. Biochem Biophys Res Commun. 1985 Jan 31;126(2):641–645. doi: 10.1016/0006-291x(85)90232-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laub O., Rutter W. J. Expression of the human insulin gene and cDNA in a heterologous mammalian system. J Biol Chem. 1983 May 25;258(10):6043–6050. [PubMed] [Google Scholar]
- Leckie B. J. Inactive renin: an attempt at a perspective. Clin Sci (Lond) 1981 Feb;60(2):119–130. doi: 10.1042/cs0600119. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T. Use of recombinant DNA technology to program eukaryotic cells to synthesize rat proinsulin: a rapid expression assay for cloned genes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5798–5802. doi: 10.1073/pnas.79.19.5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRIFIELD R. B. SOLID-PHASE PEPTIDE SYNTHESIS. 3. AN IMPROVED SYNTHESIS OF BRADYKININ. Biochemistry. 1964 Sep;3:1385–1390. doi: 10.1021/bi00897a032. [DOI] [PubMed] [Google Scholar]
- McIntyre G. D., Pau B., Hallett A., Leckie B. J., Szelke M. The purification of a high-molecular-weight, enzymatically inactive renin precursor from human kidney. J Hypertens. 1984 Jun;2(3):305–310. [PubMed] [Google Scholar]
- Misono K. S., Chang J. J., Inagami T. Amino acid sequence of mouse submaxillary gland renin. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4858–4862. doi: 10.1073/pnas.79.16.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H., Fukamizu A., Hirose S., Hayashi T., Hori H., Ohkubo H., Nakanishi S., Murakami K. Structure of the human renin gene. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5999–6003. doi: 10.1073/pnas.81.19.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. P., Walker M. D., Lee F., Kelly R. B. Expressing a human proinsulin cDNA in a mouse ACTH-secreting cell. Intracellular storage, proteolytic processing, and secretion on stimulation. Cell. 1983 Dec;35(2 Pt 1):531–538. doi: 10.1016/0092-8674(83)90187-3. [DOI] [PubMed] [Google Scholar]
- Panthier J. J., Foote S., Chambraud B., Strosberg A. D., Corvol P., Rougeon F. Complete amino acid sequence and maturation of the mouse submaxillary gland renin precursor. Nature. 1982 Jul 1;298(5869):90–92. doi: 10.1038/298090a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D. B., Johnson L. K., Schwartz K., Sista H., Scarborough R. M., Lewicki J. A. Distinct atrial natriuretic factor receptor sites on cultured bovine aortic smooth muscle and endothelial cells. Biochem Biophys Res Commun. 1985 Mar 15;127(2):433–442. doi: 10.1016/s0006-291x(85)80179-0. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H. Prorenin and other large molecular weight forms of renin. Endocr Rev. 1980 Fall;1(4):365–391. doi: 10.1210/edrv-1-4-365. [DOI] [PubMed] [Google Scholar]
- Sibanda B. L., Blundell T., Hobart P. M., Fogliano M., Bindra J. S., Dominy B. W., Chirgwin J. M. Computer graphics modelling of human renin. Specificity, catalytic activity and intron-exon junctions. FEBS Lett. 1984 Aug 20;174(1):102–111. doi: 10.1016/0014-5793(84)81086-8. [DOI] [PubMed] [Google Scholar]
- Skeggs L. T., Dorer F. E., Levine M., Lentz K. E., Kahn J. R. The biochemistry of the renin-angiotensin system. Adv Exp Med Biol. 1980;130:1–27. doi: 10.1007/978-1-4615-9173-3_1. [DOI] [PubMed] [Google Scholar]
- Slater E. E., Strout H. V., Jr Pure human renin. Identification and characterization and of two major molecular weight forms. J Biol Chem. 1981 Aug 10;256(15):8164–8171. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Warren T. G., Shields D. Expression of preprosomatostatin in heterologous cells: biosynthesis, posttranslational processing, and secretion of mature somatostatin. Cell. 1984 Dec;39(3 Pt 2):547–555. doi: 10.1016/0092-8674(84)90461-6. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]