Abstract
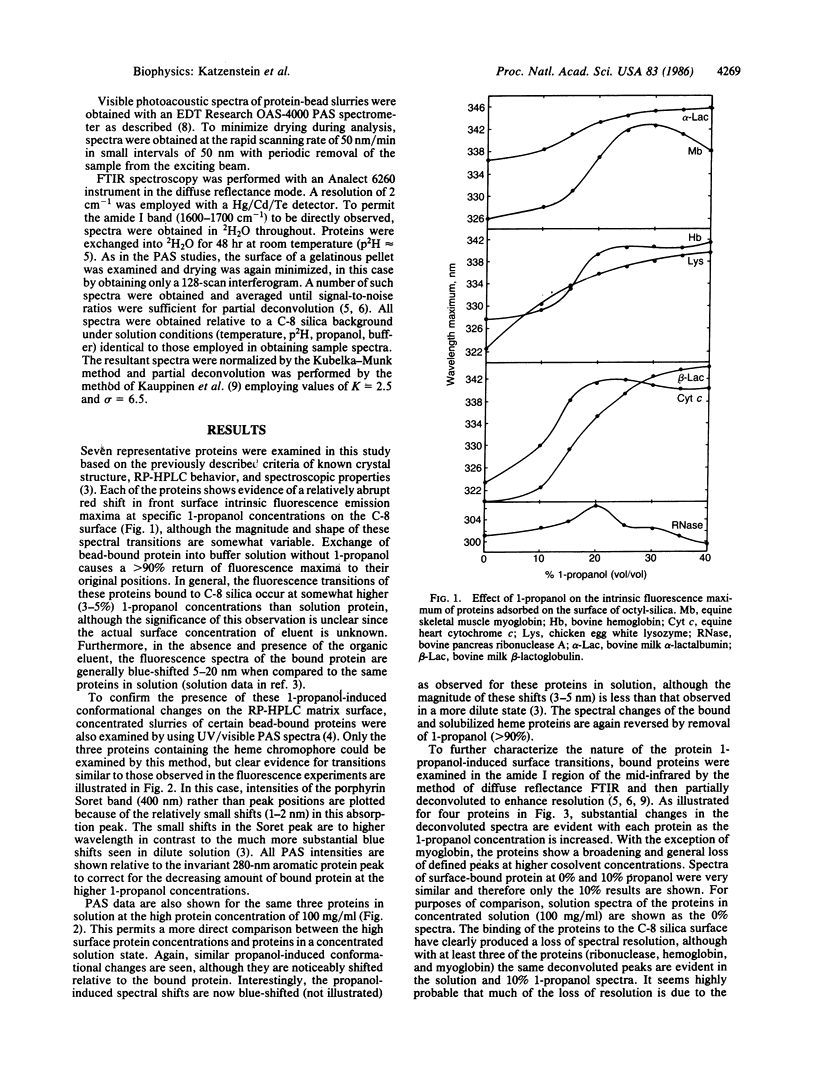
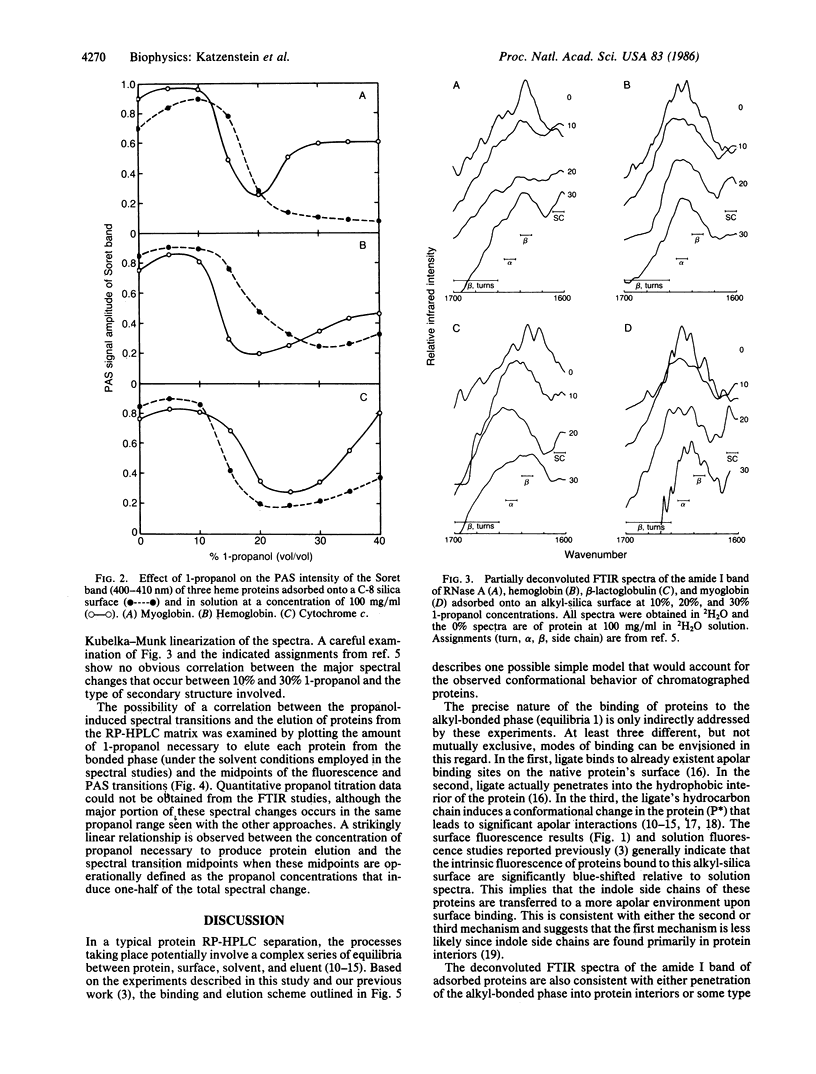
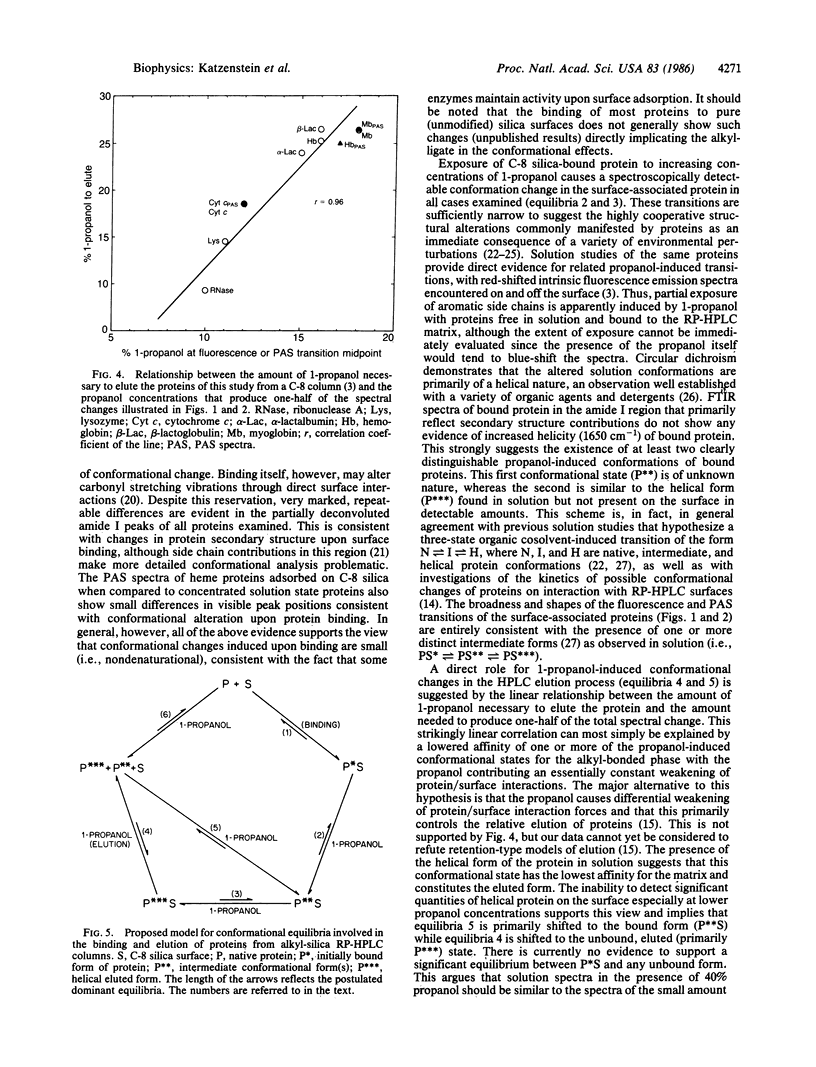
To test the hypothesis that conformational alterations might be involved in the elution of proteins from reversed-phase HPLC columns, the conformations of proteins bound onto a C-8 alkyl-bonded silica surface have been examined in the presence of increasing concentrations of the commonly employed eluent, 1-propanol. Using a combination of photoacoustic, diffuse reflectance deconvolution Fourier transform infrared and front face fluorescence spectroscopic techniques (to minimize interference from light scattering), the existence of surface-associated protein conformational changes induced by propanol is unequivocally demonstrated. The linear relationship found between the amount of propanol needed to elute proteins from C-8 columns and the midpoint of spectrally observed structural transitions is consistent with a role for conformational changes in the elution process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Goddette D. The mechanism of helical transition of proteins by organic solvents. Arch Biochem Biophys. 1985 Jul;240(1):21–32. doi: 10.1016/0003-9861(85)90004-9. [DOI] [PubMed] [Google Scholar]
- Benedek K., Dong S., Karger B. L. Kinetics of unfolding of proteins on hydrophobic surfaces in reversed-phase liquid chromatography. J Chromatogr. 1984 Dec 28;317:227–243. doi: 10.1016/s0021-9673(01)91662-0. [DOI] [PubMed] [Google Scholar]
- Burstein E. A., Vedenkina N. S., Ivkova M. N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol. 1973 Oct;18(4):263–279. doi: 10.1111/j.1751-1097.1973.tb06422.x. [DOI] [PubMed] [Google Scholar]
- Chirgadze Y. N., Fedorov O. V., Trushina N. P. Estimation of amino acid residue side-chain absorption in the infrared spectra of protein solutions in heavy water. Biopolymers. 1975 Apr;14(4):679–694. doi: 10.1002/bip.1975.360140402. [DOI] [PubMed] [Google Scholar]
- Cohen S. A., Benedek K., Tapuhi Y., Ford J. C., Karger B. L. Conformational effects in the reversed-phase liquid chromatography of ribonuclease A. Anal Biochem. 1985 Jan;144(1):275–284. doi: 10.1016/0003-2697(85)90117-4. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Flores J. Front-face fluorometry of liquid samples. Anal Biochem. 1979 Apr 1;94(1):15–21. doi: 10.1016/0003-2697(79)90783-8. [DOI] [PubMed] [Google Scholar]
- Finney J. L., Gellatly B. J., Golton I. C., Goodfellow J. Solvent effects and polar interactions in the structural stability and dynamics of globular proteins. Biophys J. 1980 Oct;32(1):17–33. doi: 10.1016/S0006-3495(80)84913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X., Regnier F. E. Retention model for proteins in reversed-phase liquid chromatography. J Chromatogr. 1984 Jul 27;296:15–30. doi: 10.1016/s0021-9673(01)96399-x. [DOI] [PubMed] [Google Scholar]
- Hearn M. T. Reversed-phase high-performance liquid chromatography. Methods Enzymol. 1984;104:190–212. doi: 10.1016/s0076-6879(84)04090-8. [DOI] [PubMed] [Google Scholar]
- Herskovits T. T., Gadegbeku B., Jaillet H. On the structural stability and solvent denaturation of proteins. I. Denaturation by the alcohols and glycols. J Biol Chem. 1970 May 25;245(10):2588–2598. [PubMed] [Google Scholar]
- Macritchie F. Proteins at interfaces. Adv Protein Chem. 1978;32:283–326. doi: 10.1016/s0065-3233(08)60577-x. [DOI] [PubMed] [Google Scholar]
- Meek J. L. Prediction of peptide retention times in high-pressure liquid chromatography on the basis of amino acid composition. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1632–1636. doi: 10.1073/pnas.77.3.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins. Proteins which do not present a single cooperative system. Adv Protein Chem. 1982;35:1–104. [PubMed] [Google Scholar]
- Privalov P. L. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- Purcell J. M., Susi H. Solvent denaturation of proteins as observed by resolution-enhanced Fourier transform infrared spectroscopy. J Biochem Biophys Methods. 1984 Jul;9(3):193–199. doi: 10.1016/0165-022x(84)90024-1. [DOI] [PubMed] [Google Scholar]
- Rosencwaig A. Photoacoustic spectroscopy. Annu Rev Biophys Bioeng. 1980;9:31–54. doi: 10.1146/annurev.bb.09.060180.000335. [DOI] [PubMed] [Google Scholar]
- Sadler A. J., Horsch J. G., Lawson E. Q., Harmatz D., Brandau D. T., Middaugh C. R. Near-infrared photoacoustic spectroscopy of proteins. Anal Biochem. 1984 Apr;138(1):44–51. doi: 10.1016/0003-2697(84)90766-8. [DOI] [PubMed] [Google Scholar]
- Sadler A. J., Micanovic R., Katzenstein G. E., Lewis R. V., Middaugh C. R. Protein conformation and reversed-phase high-performance liquid chromatography. J Chromatogr. 1984 Dec 28;317:93–101. doi: 10.1016/s0021-9673(01)91650-4. [DOI] [PubMed] [Google Scholar]
- Susi H., Byler D. M. Protein structure by Fourier transform infrared spectroscopy: second derivative spectra. Biochem Biophys Res Commun. 1983 Aug 30;115(1):391–397. doi: 10.1016/0006-291x(83)91016-1. [DOI] [PubMed] [Google Scholar]
- Wu C. S., Yang J. T. Sequence-dependent conformations of short polypeptides in a hydrophobic environment. Mol Cell Biochem. 1981 Oct 30;40(2):109–122. doi: 10.1007/BF00224754. [DOI] [PubMed] [Google Scholar]


