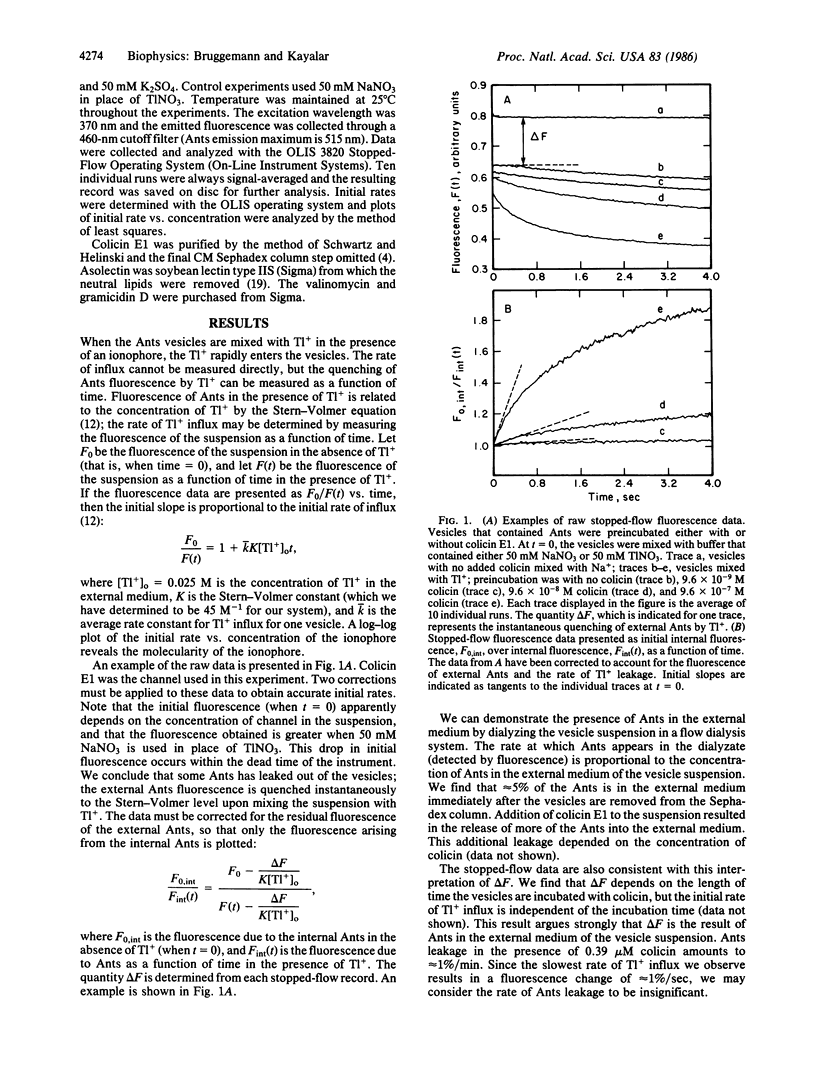
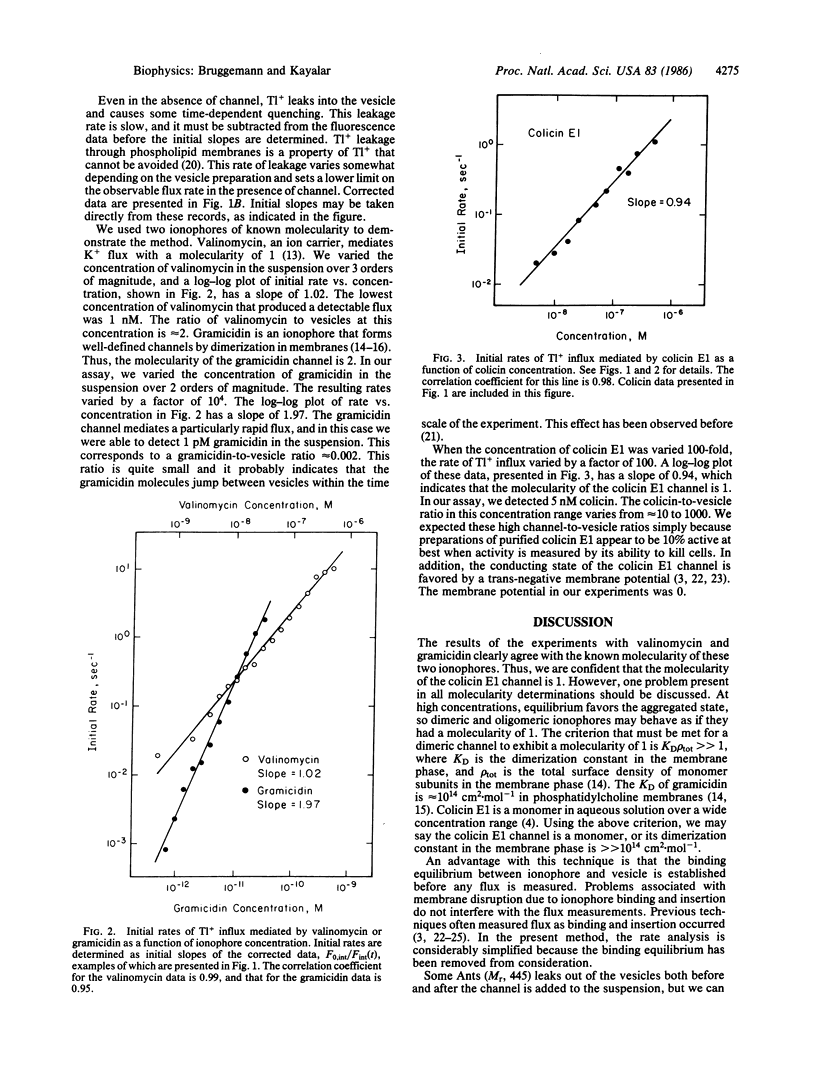
Abstract
A fluorescence technique that measures fast ion fluxes across liposome membranes was used to determine the molecularity of the colicin E1 channel. The rate of flux of Tl+ (used as a K+ analogue) into large unilamellar vesicles was measured by its ability to quench the fluorescence of a membrane-impermeable fluorophore entrapped in the vesicles. The dependence of Tl+ flux rate on the concentration of ionophore in the vesicle suspension reveals the molecularity of the ionophore. The method is demonstrated with two ionophores, valinomycin and gramicidin, whose molecularities are known to be one and two, respectively. The molecularity of the colicin E1 channel was determined to be one. This method can be used to study the properties of any ionophore that mediates K+ flux.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Channel formation kinetics of gramicidin A in lipid bilayer membranes. J Membr Biol. 1973;11(2):177–194. doi: 10.1007/BF01869820. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Dankert J. R., Uratani Y. The membrane channel-forming bacteriocidal protein, colicin El. Biochim Biophys Acta. 1983 Mar 21;737(1):173–193. doi: 10.1016/0304-4157(83)90016-3. [DOI] [PubMed] [Google Scholar]
- Cramer W. A., Phillips S. K. Response of an Escherichia coli-bound fluorescent probe to colicin E1. J Bacteriol. 1970 Nov;104(2):819–825. doi: 10.1128/jb.104.2.819-825.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson V. L., Cramer W. A., Bishop L. J., Brunden K. R. Dependence of the activity of colicin E1 in artificial membrane vesicles on pH, membrane potential, and vesicle size. J Biol Chem. 1984 Jan 10;259(1):594–600. [PubMed] [Google Scholar]
- Gutknecht J. Cadmium and thallous ion permeabilities through lipid bilayer membranes. Biochim Biophys Acta. 1983 Oct 26;735(1):185–188. doi: 10.1016/0005-2736(83)90274-2. [DOI] [PubMed] [Google Scholar]
- Hedges A. J. An examination of single-hit and multi-hit hypotheses in relation to the possible kinetics of colicin adsorption. J Theor Biol. 1966 Aug;11(3):383–410. doi: 10.1016/0022-5193(66)90100-7. [DOI] [PubMed] [Google Scholar]
- Kemp G., Wenner C. Solution, interfacial, and membrane properties of gramicidin A. Arch Biochem Biophys. 1976 Oct;176(2):547–555. doi: 10.1016/0003-9861(76)90198-3. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Raftery M. A. Direct spectroscopic studies of cation translocation by Torpedo acetylcholine receptor on a time scale of physiological relevance. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4509–4513. doi: 10.1073/pnas.77.8.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES P. THE ADSORPTION AND KINETICS OF KILLING BY COLICIN CA42-E2. Aust J Exp Biol Med Sci. 1965 Apr;43:191–200. doi: 10.1038/icb.1965.18. [DOI] [PubMed] [Google Scholar]
- Raymond L., Slatin S. L., Finkelstein A. Channels formed by colicin E1 in planar lipid bilayers are large and exhibit pH-dependent ion selectivity. J Membr Biol. 1985;84(2):173–181. doi: 10.1007/BF01872215. [DOI] [PubMed] [Google Scholar]
- Reynolds B. L., Reeves P. R. Kinetics of adsorption of colicin CA42-E2 and reversal of its bactericidal activity. J Bacteriol. 1969 Oct;100(1):301–309. doi: 10.1128/jb.100.1.301-309.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Schwartz S. A., Helinski D. R. Purification and characterization of colicin E1. J Biol Chem. 1971 Oct 25;246(20):6318–6327. [PubMed] [Google Scholar]
- Shannon R., Hedges A. J. Kinetics of lethal adsorption of colicin E-2 by Escherichia coli. J Bacteriol. 1967 Apr;93(4):1353–1359. doi: 10.1128/jb.93.4.1353-1359.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Effect of colicins Ia and E1 on ion permeability of liposomes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6167–6171. doi: 10.1073/pnas.76.12.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Trapane T. L., Prasad K. U. Is the gramicidin a transmembrane channel single-stranded or double-stranded helix? A simple unequivocal determination. Science. 1983 Sep 9;221(4615):1064–1067. doi: 10.1126/science.221.4615.1064. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Mathies R., Eisenberg M., Stryer L. Simultaneous fluorescence and conductance studies of planar bilayer membranes containing a highly active and fluorescent analog of gramicidin A. J Mol Biol. 1975 Nov 25;99(1):75–92. doi: 10.1016/s0022-2836(75)80160-4. [DOI] [PubMed] [Google Scholar]


