Abstract
Two immunization procedures were compared for their ability to yield monoclonal antibodies that react with plasma membrane-bound differentiation antigens of Dictyostelium. In the first method, hybridomas prepared from BALB/c mice immunized with aggregating amoebae produced monoclonal antibodies that recognized antigens present on both growing and aggregating Dictyostelium amoebae. None of the monoclonal antibodies reacted with only the injected aggregation-stage cell type. In contrast, monoclonal antibodies that reacted with differentiation antigens were easily obtained by primary immunization of BALB/c mice with living aggregation-stage cells, followed by secondary immunization with a preparation of plasma membrane from aggregating cells or intact aggregating cells mixed with polyclonal BALB/c antiserum raised against undifferentiated cells. By this method, approximately 20% of all anti-Dictyostelium monoclonal antibodies obtained in a fusion are specific for differentiation antigens. The properties and developmental regulation of several of these antigens are described. The possible uses of this immunological method to detect unique determinants on other kinds of cells and the likely immune mechanisms that make it successful are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay S. L., Henderson E. J. Thermosensitive development and tip regulation in a mutant of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1982 Jan;79(2):505–509. doi: 10.1073/pnas.79.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie C., Klein C., Swierkosz J. Monoclonal antibodies: use to detect developmentally regulated antigens on D. discoideum amebae. Cell. 1983 Apr;32(4):1115–1123. doi: 10.1016/0092-8674(83)90295-7. [DOI] [PubMed] [Google Scholar]
- Gregg J. H., Krefft M., Haas-Kraus A., Williams K. L. Antigenic differences detected between prespore cells of Dictyostelium discoideum and Dictyostelium mucoroides using monoclonal antibodies. Exp Cell Res. 1982 Nov;142(1):229–233. doi: 10.1016/0014-4827(82)90427-x. [DOI] [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Lymphocyte-mediated cytotoxicity and blocking serum activity to tumor antigens. Adv Immunol. 1974;18:209–277. doi: 10.1016/s0065-2776(08)60311-9. [DOI] [PubMed] [Google Scholar]
- Horst M. N., Basha S. M., Baumbach G. A., Mansfield E. H., Roberts R. M. Alkaline urea solubilization, two-dimensional electrophoresis and lectin staining of mammalian cell plasma membrane and plant seed proteins. Anal Biochem. 1980 Mar 1;102(2):399–408. doi: 10.1016/0003-2697(80)90174-8. [DOI] [PubMed] [Google Scholar]
- Hutchins B. L., Frazier W. A. Purification and characterization of a membrane-associated cAMP-binding protein from developing Dictyostelium discoideum. J Biol Chem. 1984 Apr 10;259(7):4379–4388. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- KALISS N. The survival of homografts in mice pretreated with antisera to mouse tissue. Ann N Y Acad Sci. 1957 Mar 22;64(5):977-90; discussion, 990-3. doi: 10.1111/j.1749-6632.1957.tb52489.x. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L. Lysosomal enzymes possess a common antigenic determinant in the cellular slime mold, Dictyostelium discoideum. J Biol Chem. 1981 Apr 10;256(7):3564–3575. [PubMed] [Google Scholar]
- Krefft M., Voet L., Gregg J. H., Mairhofer H., Williams K. L. Evidence that positional information is used to establish the prestalk-prespore pattern in Dictyostelium discoideum aggregates. EMBO J. 1984 Jan;3(1):201–206. doi: 10.1002/j.1460-2075.1984.tb01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew W. D., Patterson P. H. The production of a monoclonal antibody that blocks the action of a neurite outgrowth-promoting factor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):625–631. doi: 10.1101/sqb.1983.048.01.066. [DOI] [PubMed] [Google Scholar]
- Milstein C., Lennox E. The use of monoclonal antibody techniques in the study of development cell surfaces. Curr Top Dev Biol. 1980;14(Pt 2):1–32. doi: 10.1016/s0070-2153(08)60187-8. [DOI] [PubMed] [Google Scholar]
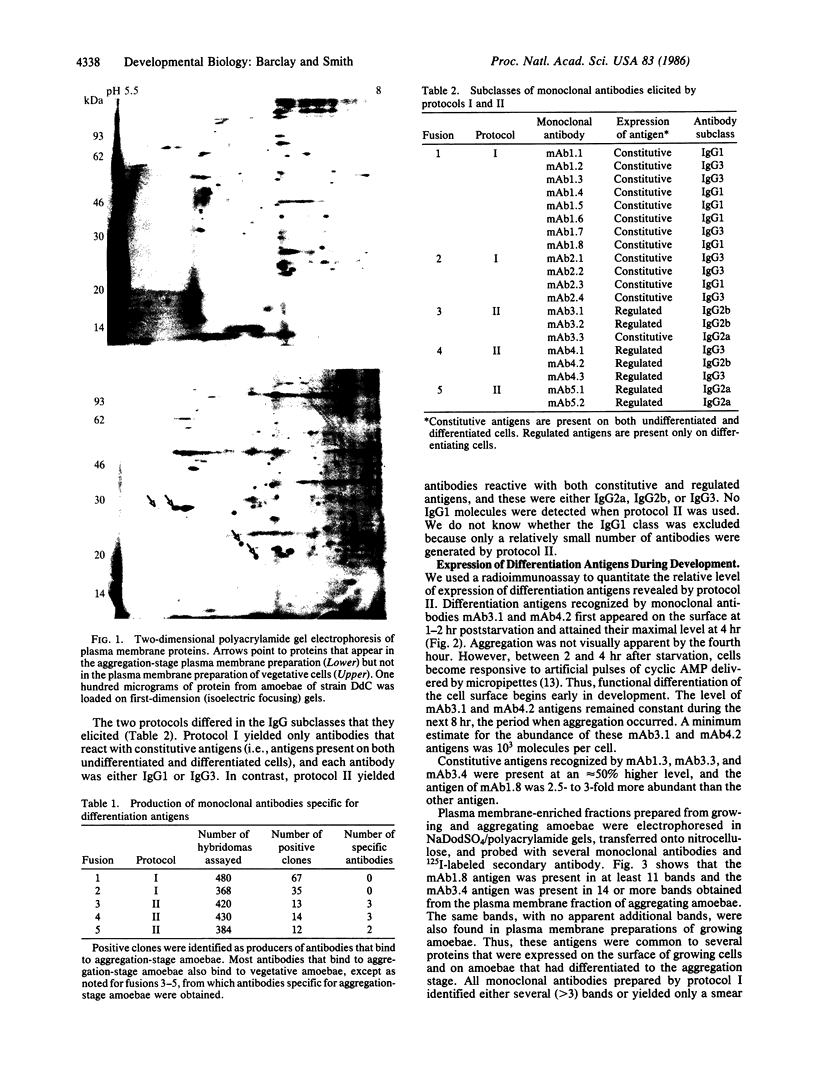
- Prem Das O., Henderson E. J. Developmental regulation of Dictyostelium discoideum plasma membrane proteins. J Cell Biol. 1983 Nov;97(5 Pt 1):1544–1558. doi: 10.1083/jcb.97.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintáns J., Quan Z. S. Idiotype shifts caused by neonatal tolerance to phosphorylcholine. J Immunol. 1983 Feb;130(2):590–595. [PubMed] [Google Scholar]
- Robertson A., Drage D. J., Cohen M. H. Control of Aggregation in Dictyostelium discoideum by an External Periodic Pulse of Cyclic Adenosine Monophosphate. Science. 1972 Jan 21;175(4019):333–335. doi: 10.1126/science.175.4019.333. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle W. O. Immunological unresponsiveness. Adv Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]