Abstract
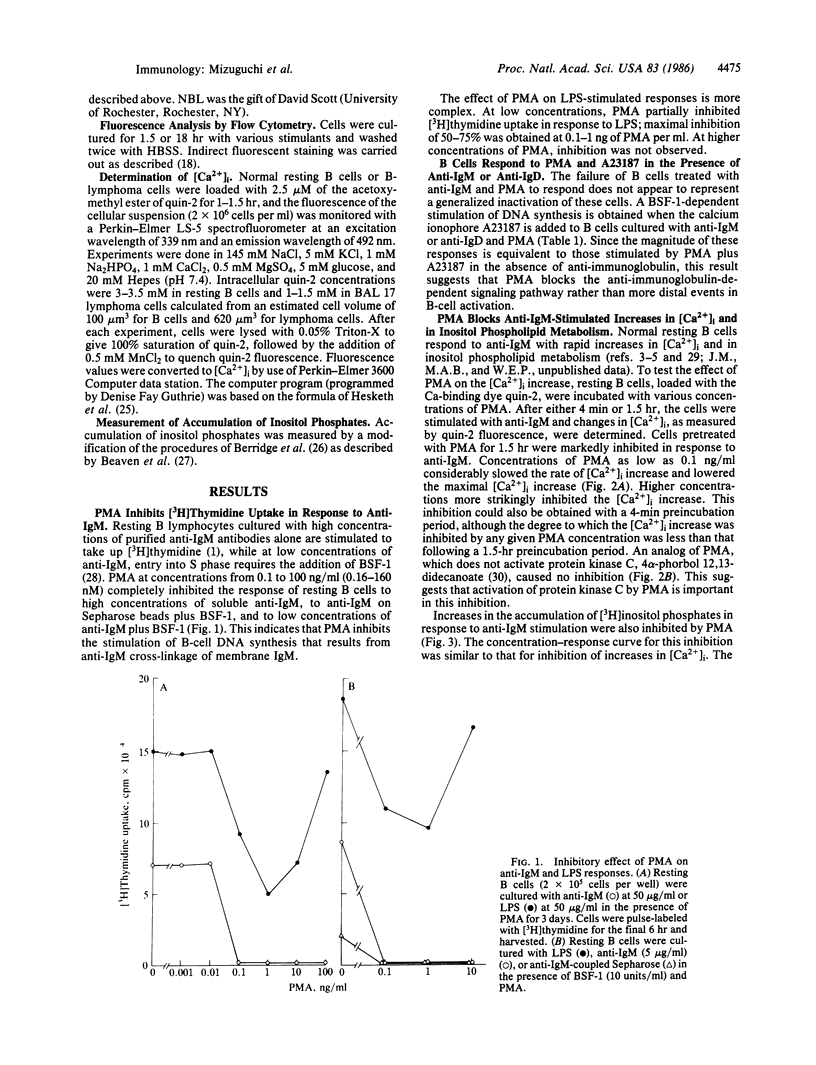
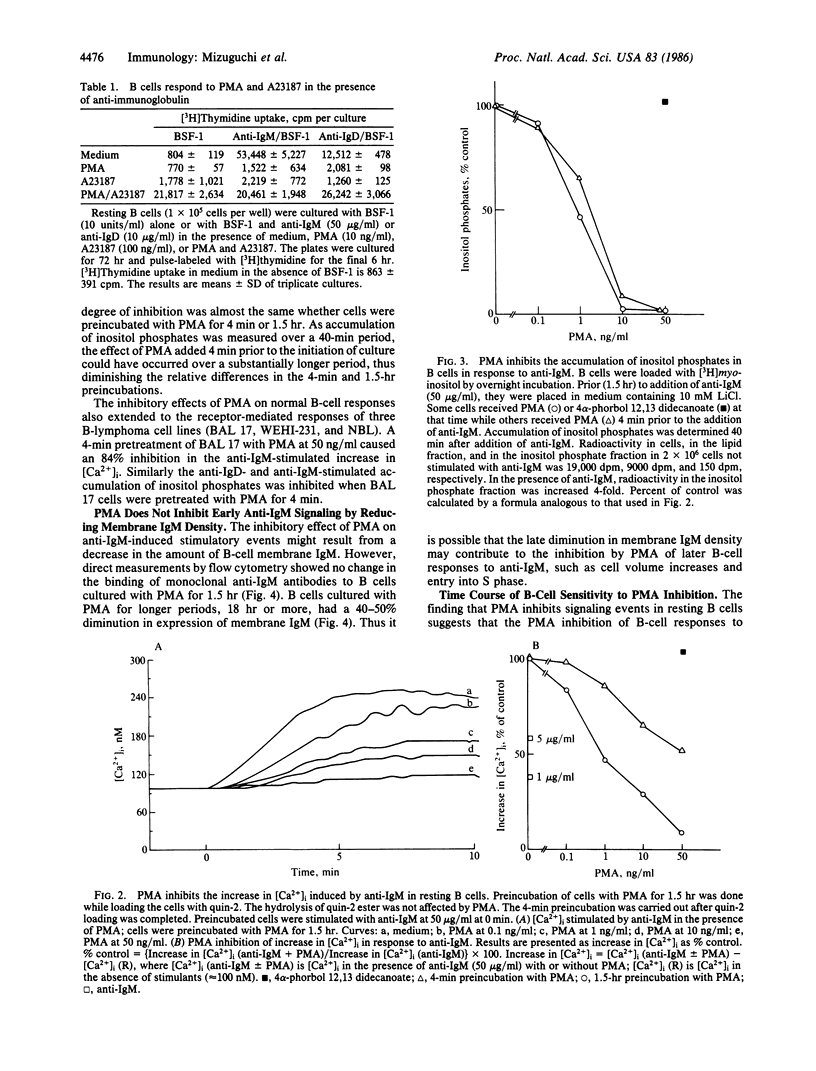
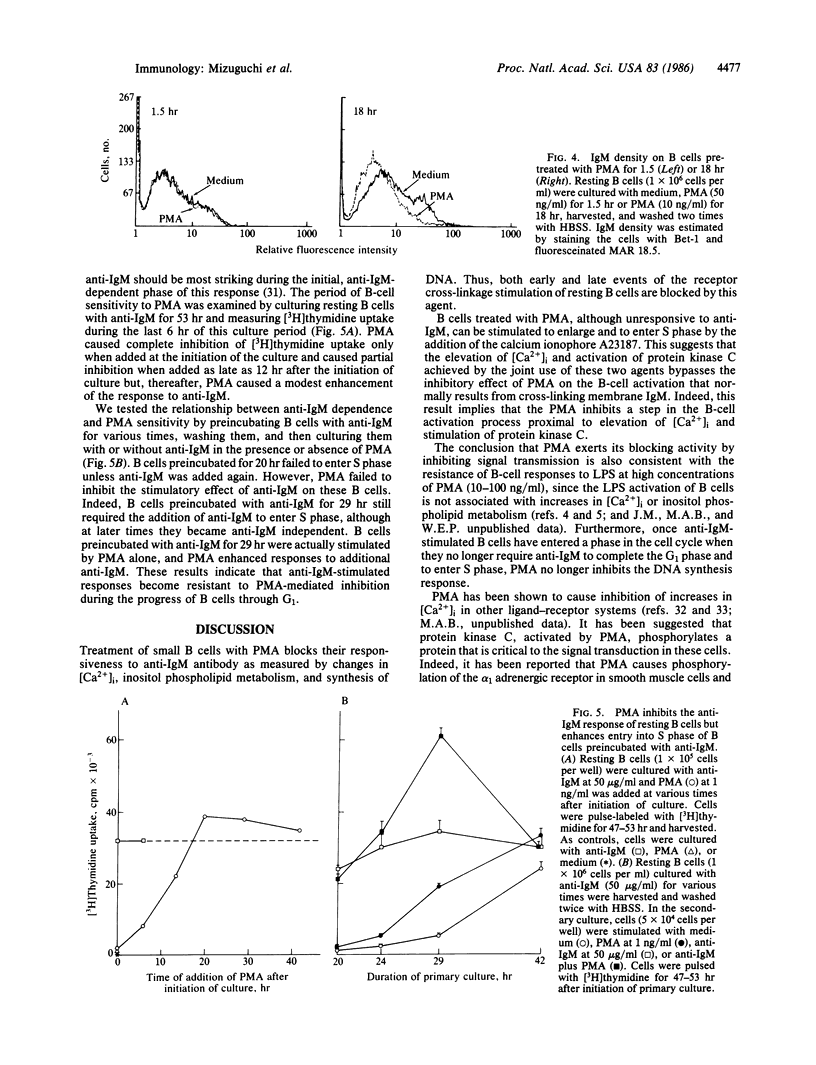
Cross-linking the membrane immunoglobulins of resting B cells leads to activation as judged by increased inositol phospholipid metabolism, intracellular free calcium concentration ([Ca2+]i), and cell volume. Such activated B cells enter S phase in the presence of B-cell stimulatory factor 1. Phorbol myristate acetate (PMA) is a potent inhibitor of anti-IgM- and anti-IgD-stimulated B-cell responses. In B cells concentrations of PMA ranging from 0.1 to 100 ng/ml completely inhibit anti-IgM-stimulated DNA synthesis and block anti-IgM-stimulated increases in inositol phospholipid metabolism and in [Ca2+]i. Preincubation periods as short as 4 min block these effects although longer preincubations are somewhat more effective in inhibiting increases in [Ca2+]i. Preincubation with PMA for 1.5 hr does not diminish expression of membrane IgM. This strongly suggests that PMA inhibits responses of resting B cells to anti-IgM by interrupting signal transmission rather than by diminishing cross-linking of membrane immunoglobulin on B cells. In contrast to resting B cells, B cells activated in vitro for 29 hr show enhanced responses to anti-IgM in the presence of PMA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abb J., Bayliss G. J., Deinhardt F. Lymphocyte activation by the tumor-promoting agent 12-O-tetradecanoylphorbol-13-acetate (TPA). J Immunol. 1979 May;122(5):1639–1642. [PubMed] [Google Scholar]
- Aman P., Gordon J., Klein G. TPA (12-O-tetradecanoyl-phorbol-13-acetate) activation and differentiation of human peripheral B lymphocytes. Immunology. 1984 Jan;51(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Beaven M. A., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7137–7142. [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoglio J. H. Monocyte-independent stimulation of human B lymphocytes by phorbol myristate acetate. J Immunol. 1983 Nov;131(5):2279–2281. [PubMed] [Google Scholar]
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Goding J. W., Schrader J. W. The regulation of growth and differentiation of a murine B cell lymphoma. I. Lipopolysaccharide-induced differentiation. J Immunol. 1981 Jun;126(6):2461–2465. [PubMed] [Google Scholar]
- Cambier J. C., Monroe J. G. B cell activation. V. Differentiation signaling of B cell membrane depolarization, increased I-A expression, G0 to G1 transition, and thymidine uptake by anti-IgM and anti-IgD antibodies. J Immunol. 1984 Aug;133(2):576–581. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Coggeshall K. M., Cambier J. C. B cell activation. VIII. Membrane immunoglobulins transduce signals via activation of phosphatidylinositol hydrolysis. J Immunol. 1984 Dec;133(6):3382–3386. [PubMed] [Google Scholar]
- DeFranco A. L., Raveche E. S., Paul W. E. Separate control of B lymphocyte early activation and proliferation in response to anti-IgM antibodies. J Immunol. 1985 Jul;135(1):87–94. [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp S. A., Harmony J. A. Increased phosphatidylinositol metabolism is an important but not an obligatory early event in B lymphocyte activation. J Immunol. 1985 Jun;134(6):4087–4094. [PubMed] [Google Scholar]
- Hara T., Fu S. M. Human T cell activation. I. Monocyte-independent activation and proliferation induced by anti-T3 monoclonal antibodies in the presence of tumor promoter 12-o-tetradecanoyl phorbol-13 acetate. J Exp Med. 1985 Apr 1;161(4):641–656. doi: 10.1084/jem.161.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylowicz C. M., Keeler K. D., Klaus G. G. Activation and proliferation signals in mouse B cells. I. A comparison of the capacity of anti-Ig antibodies or phorbol myristic acetate to activate B cells from CBA/N or normal mice into G1. Eur J Immunol. 1984 Mar;14(3):244–250. doi: 10.1002/eji.1830140308. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz C. M., Klaus G. G. Effects of tumour promoter phorbol myristate acetate on mouse lymphocytes: selective inhibition of B cell activation by mitogens and antigens. Immunology. 1984 Feb;51(2):327–332. [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kim K. J., Kanellopoulos-Langevin C., Merwin R. M., Sachs D. H., Asofsky R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J Immunol. 1979 Feb;122(2):549–554. [PubMed] [Google Scholar]
- Kishimoto A., Takai Y., Mori T., Kikkawa U., Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980 Mar 25;255(6):2273–2276. [PubMed] [Google Scholar]
- Koretzky G. A., Daniele R. P., Nowell P. C. A phorbol ester (TPA) can replace macrophages in human lymphocyte cultures stimulated with a mitogen but not with an antigen. J Immunol. 1982 Apr;128(4):1776–1780. [PubMed] [Google Scholar]
- Kung J. T., Sharrow S. O., Sieckmann D. G., Lieberman R., Paul W. E. A mouse IgM allotypic determinant (Igh-6.5) recognized by a monoclonal rat antibody. J Immunol. 1981 Sep;127(3):873–876. [PubMed] [Google Scholar]
- Lanier L. L., Gutman G. A., Lewis D. E., Griswold S. T., Warner N. L. Monoclonal antibodies against rat immunoglobulin kappa chains. Hybridoma. 1982;1(2):125–131. doi: 10.1089/hyb.1.1982.1.125. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg L. M., Cotecchia S., Lomasney J. W., DeBernardis J. F., Lefkowitz R. J., Caron M. G. Phorbol esters promote alpha 1-adrenergic receptor phosphorylation and receptor uncoupling from inositol phospholipid metabolism. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5651–5655. doi: 10.1073/pnas.82.17.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling M., Livnat D., Pillai P. S., Scott D. W. Lymphoma models for B cell activation and tolerance. I. Conditions for the anti-mu-dependent stimulation of growth in NBL, a nude B cell lymphoma. J Immunol. 1985 Mar;134(3):1449–1454. [PubMed] [Google Scholar]
- Monroe J. G., Kass M. J. Molecular events in B cell activation. I. Signals required to stimulate G0 to G1 transition of resting B lymphocytes. J Immunol. 1985 Sep;135(3):1674–1682. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Lahet S., Inman J., Paul W. E. Partial purification of murine B cell stimulatory factor (BSF)-1. J Immunol. 1985 Oct;135(4):2518–2523. [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Rabin E. M., Ohara J., Paul W. E. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Mizel S. B. Signal requirements for T lymphocyte activation. I. Replacement of macrophage function with phorbol myristic acetate. J Immunol. 1979 Oct;123(4):1749–1754. [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Lieman H., Pecht I. Protein kinase C regulation of the receptor-coupled calcium signal in histamine-secreting rat basophilic leukaemia cells. Nature. 1985 Jan 3;313(5997):59–60. doi: 10.1038/313059a0. [DOI] [PubMed] [Google Scholar]
- Sieckmann D. G., Asofsky R., Mosier D. E., Zitron I. M., Paul W. E. Activation of mouse lymphocytes by anti-immunoglobulin. I. Parameters of the proliferative response. J Exp Med. 1978 Mar 1;147(3):814–829. doi: 10.1084/jem.147.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Butler J. L., Cooper M. D. Human B cell responsiveness to B cell growth factor after activation by phorbol ester and monoclonal anti-mu antibody. J Immunol. 1985 Apr;134(4):2470–2476. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Zavoico G. B., Halenda S. P., Sha'afi R. I., Feinstein M. B. Phorbol myristate acetate inhibits thrombin-stimulated Ca2+ mobilization and phosphatidylinositol 4,5-bisphosphate hydrolysis in human platelets. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3859–3862. doi: 10.1073/pnas.82.11.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]


