Abstract
Features of melanocortin-4 receptor (MC4R) deficiency have been observed to be more pronounced in childhood. Longitudinal data from a population-based study were used to separate the phenotypic effects of MC4R deficiency during childhood and adulthood. The MC4R exon was sequenced in 6,760 individuals of predominantly Pima Indian heritage, and discovered mutations were functionally assessed in vitro. Effects on BMI, height, and slope of BMI change were assessed during childhood (ages 5–20 years) and adulthood (ages 20–45 years). Six mutations affecting MC4R function, including three that may be private to Pima Indians, were found in 159 individuals (2.4%). The slope of BMI increase was greater in individuals carrying an MC4R mutation compared with noncarriers during childhood but not during adulthood. The final adult height obtained was higher in individuals with MC4R deficiency. There was an increased risk for developing type 2 diabetes in individuals with a defective MC4R during childhood and adulthood, but this was only independent of BMI in childhood. The greater rates of body mass accumulation and risk of type 2 diabetes before the age of 20 years in individuals with MC4R deficiency indicate that the effects of these mutations are more apparent during the active growth of childhood.
The melanocortin system in humans integrates opposing signals from the hypothalamic pathways that regulate food intake and energy homeostasis. The major interaction point for the anorexigenic signal, melanocyte-stimulating hormone (α-MSH), and the orexigenic signal, agouti-related peptide, is the melanocortin-4 receptor (MC4R). MC4R haploinsufficiency is recognized as the most common monogenic form of obesity, with a prevalence of 1.7–3.0% in obese individuals (1–6). Children carrying an MC4R mutation have been observed to be obese, hyperphagic, and hyperinsulinemic, with increased linear growth (6–8). Many of these phenotypic features seem to become less prominent with progression into adulthood (3,7–10).
Pima Indians living in Arizona have a high prevalence of obesity (11) and diabetes (12) attributable to genetic (13,14), environmental, and lifestyle influences (12,15). After the first month of life, Pima children have higher BMI z scores than the general U.S. population at all ages (16). Even during childhood, the diabetes that occurs in this population has been shown to consist primarily of type 2 diabetes (17). Previous sequencing of MC4R in severely obese Pima Indians identified the missense change, R165Q, which is known to cause partial loss of function (7,18,19), and an A insertion at nucleotide 100 that causes a frameshift mutation that results in a premature STOP at codon 37 (D37stop) (20) prior to the ligand binding and transmembrane domains. To date, this latter mutation has not been reported in other populations. The prevalence of these two mutations in full-heritage Pima Indians is 1.8% (4). Our aim was to extend these findings by sequencing the entire coding region of MC4R in all participants of our longitudinal survey of health. Knowledge of every MC4R coding mutation in the Gila River Indian community, without prior selection for obese individuals, permitted us to determine the full prevalence and to assess the phenotypic impact of MC4R deficiency during childhood and adulthood. Based on previous observations reported in the literature (6–8), we hypothesized that MC4R deficiency may lead to more pronounced effects during childhood.
RESEARCH DESIGN AND METHODS
Between 1965 and 2007, all members of the Gila River Indian Community aged ≥5 years were invited to participate in the National Institutes of Health longitudinal study of health as previously described (11). Individuals participated as frequently as every 2 years in examinations that included measurements of weight, height, and fasting and 2-h glucose and insulin concentrations during a 75-g oral glucose tolerance test (OGTT). The degree of Pima heritage was determined by the self-reported number of great grandparents of full Pima heritage. The evaluated population was limited to 6,760 people of ≥50% Pima or related Tohono O’odham heritage to reduce confounding from ethnic differences in BMI and risk of type 2 diabetes. Blood pressure measurements were available for 5,957 individuals, and self-reported history of menarche was available for 3,334 female subjects. All adults provided written informed consent; a parent or guardian provided informed consent for children aged ≤18 years, and the child assented. This study was approved by the institutional review board of the National Institute of Diabetes and Digestive and Kidney Diseases.
Direct sequencing of the MC4R exon.
The entire coding region of MC4R was PCR amplified with FideliTaq DNA polymerase (Affymetrix, Santa Clara, CA), using primers 5′-GCAATTTTAGCCTCACAACTT-3′ (forward) and 5′AAATCCACAGTGCCTACAACC-3′ (reverse). Direct sequencing was conducted in all subjects using a Big Dye terminator kit (Applied Biosystems, Carlsbad, CA) and electrophoresed on an automated DNA capillary sequencer (model 3730; Applied Biosystems).
Functional characterization of MC4R variants.
The open reading frame for the common sequence or the variant MC4R DNA was PCR amplified from the genomic DNA of previously sequenced subjects and cloned in the vector pcDNA3.1/V5-His using a TOPO TA expression kit (Invitrogen, Carlsbad, CA). Vector with no insert served as a negative control. All constructs were sequenced as described above to confirm the correct variant. One microgram of each expression construct was transiently cotransfected with a 250-ng mixture of cAMP-responsive element (CRE)-mediated firefly luciferase construct and constitutively expressed Renilla luciferase construct (40:1) (SABioscience, Frederick, MD) using lipofectamine LTX (Invitrogen) into HEK293 (human embryonic kidney) cells maintained in Eagle’s minimum essential medium supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37°C with 5% CO2. Six hours posttransfection, cells were serum starved for 18 h in OPTI-MEM1 plus 0.5% FBS. Graded concentrations of α-MSH (Sigma-Aldrich, St. Louis, MO) were then added to the medium for a final concentration of 10−11 to 10−6 mol. After 6 h, cells were harvested and cAMP-induced luciferase activity, reflecting the level of MC4R activation, was assessed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) on a luminometer (Turner Designs, Sunnyvale, CA). Relative luciferase activity was expressed as a ratio of firefly luciferase activity by cAMP to Renilla luciferase activity to control for transfection efficiency and normalized to a percentage of maximum wild-type activity. Six replicate experiments were performed per construct.
Statistics.
The α level for significance was set at 0.05. Comparisons between the EC50, which is the concentration of α-MSH required to achieve 50% of the maximal effect, and maximum activity of the variants were assessed with one-way ANOVA followed by Dunnett posttest after nonlinear regression fitting of a four-parameter sigmoid dose-response curve using GraphPad Prism 5 (GraphPad Software, La Jolla, CA). If a variant led to either (1) a decrease in the maximum response and/or (2) an increased EC50, it was considered to be a loss-of-function mutation.
Comparison of rates of growth.
For all individuals with two or more nondiabetic, nonpregnant examinations during childhood, defined as aged 5–20 years (n = 3,397; 69 subjects with a loss-of-function MC4R mutation), or adulthood (n = 2,645; 52 subjects with a loss-of-function MC4R mutation), the slope of BMI change was calculated. The age of adults was restricted to >20 and ≤45 years because the number of exams after the age of 45 years was low. To verify that MC4R deficiency was associated with childhood effects independent of early-onset excess adiposity, an analysis (n = 276) was conducted using three control subjects matched by sex, to within 1 year of the initial age, and to within 0.1 of the initial BMI z score for each individual with MC4R deficiency. Linear regression models were used to compare slopes, with sex, degree of Pima heritage, and birth year (to account for secular changes in growth) as covariates.
z Scores and mixed models.
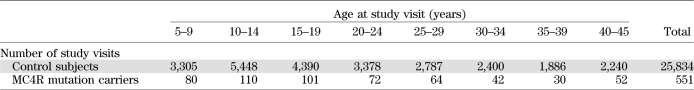
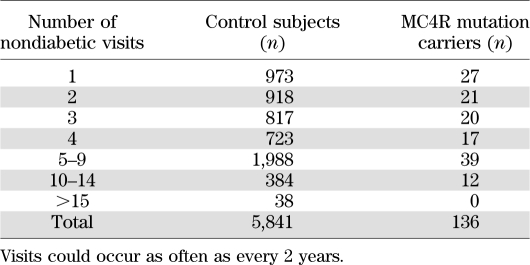
Data from individuals without an MC4R mutation were used to calculate means and SDs for every age by sex for BMI and height. Pima population–specific z scores were calculated for each individual by subtracting the mean from the individual’s value and dividing by the SD. For comparison with reference values, BMI z scores also were calculated from the standards published by the National Center for Health Statistics of the Centers for Disease Control and Prevention (21) using the recommended equation for normalized z scores. The number of nondiabetic, nonpregnant study exams with data for height and weight ranged from 1 to 17 in 5,977 individuals for a total of 26,385 examinations between 1965 and 2007 (Tables 1 and 2). Of these, 13,364 examinations occurred in 4,537 children.
TABLE 1.
Number of study visits per 5-year age range in control subjects (individuals without an MC4R coding mutation) compared with individuals carrying an MC4R loss-of-function mutation
TABLE 2.
Number of times control subjects (individuals without an MC4R mutation) and MC4R loss-of-function mutation carriers were seen for a nondiabetic study visit
Using SAS software (SAS Institute, Cary, NC), mixed models using a first-order autoregressive error structure for repeated measures were used to compare individuals with and without a defective MC4R, controlling for degree of Pima heritage and birth year as fixed effects and sibling relationship as a random effect. Analyses included height and BMI z scores, BMI, final adult height, and systolic and diastolic blood pressure. Excluding the z score analyses, all models also included age and sex as covariates. Blood pressure models were further adjusted for BMI and height. A subset analysis (n = 436) for final adult height with three control subjects matched as above by BMI z score, sex, and age for each individual with MC4R deficiency was conducted to separate the effects of excess adiposity and MC4R deficiency. Analyses were adjusted for multiple comparisons using Dunnett posttest whenever carriers of MC4R variants were considered as separate groups. Linear regression was used to compare the age of menarche between girls with and without MC4R deficiency, controlling for birth year and degree of Pima heritage.
Rates of type 2 diabetes.
Time to development of diabetes was calculated from the first nondiabetic visit to the onset of diabetes determined from the OGTT results (22) or chart review if diabetes was diagnosed between study visits. Cox proportional hazards models were used to determine the hazard rate ratio (HRR) associated with MC4R deficiency for developing diabetes before the age of 20 years with and without BMI as a time-dependent variable and including sex, birth year, and degree of Pima heritage as covariates (n = 4,518; 103 subjects with MC4R deficiency). Similar analyses were conducted to assess the HRR for developing type 2 diabetes between the ages of 20 and 45 years (n = 4,289; 102 subjects with MC4R deficiency).
RESULTS
Functional analysis and prevalence of MC4R mutations.
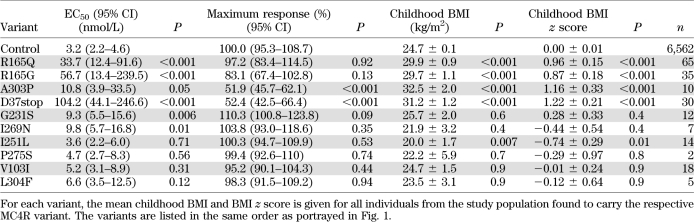
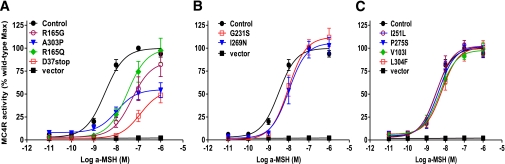
Sequencing of MC4R in 6,760 (44% male) individuals identified one nonsense (D37stop) and nine missense (R165Q, R165G, A303P, G231S, I269N, I251L, P275S, V103I, and L304F) variants. In vitro analysis revealed that 6 of the 10 changes caused decreased MC4R function (see Table 3 and Fig. 1). R165Q, G231S, and I269N were previously known to lead to partial loss of function (1,7,23,24), consistent with our findings. The frameshift mutation, D37stop, predicted termination of the protein prior to the ligand binding domains yet had some detectable activity with exposure to high concentrations of ligand. Two missense mutations, R165G and A303P, also had partial activity. In contrast, I251L, P275S, V103I, and L304F had in vitro activity similar to wild-type MC4R, and individuals with these variants (n = 39) were excluded from all additional analyses.
TABLE 3.
Comparison of the in vitro activity (defined by EC50 and maximum response) for each variant identified by sequencing the MC4R exon
FIG. 1.
Functional characterization of the identified MC4R missense/nonsense variants. MC4R activity (means ± SE) in response to a logarithmic increase of α-MSH is expressed as a percentage of the maximum wild-type MC4R activity. The best-fit estimate of the EC50, the concentration of α-MSH required to achieve 50% of the maximal effect starting from basal activity, and the 95% CIs were obtained from nonlinear regression fitting of the sigmoid dose-response curve using GraphPad Prism 5. A functional MC4R defect was defined as either a statistically significant decrease in the maximum response or a significant increase in the EC50. Those variants showing the greatest in vitro effects are shown in A, variants showing a milder effect are shown in B, and those variants with no detectable difference from wild-type MC4R are shown in C. Vector pcDNA3.1 served as a negative control for background cAMP accumulation.
The prevalence of the six loss-of-function MC4R mutations was 2.4%, with 156 heterozygous individuals and 3 siblings homozygous for the D37stop mutation. To increase the statistical power of additional analyses, all individuals with any of the six loss-of-function MC4R mutations were combined into one group (individual results for the six single nucleotide polymorphisms [SNPs] are presented in the Supplementary Table). By the age of 20 years, 81% of children with an MC4R loss-of-function mutation were considered to be at risk for obesity, defined by a BMI greater than the U.S. population’s 95th percentile, compared with 50% of control subjects (χ2 = 41; P < 0.0001). Of children at risk for obesity, 3.6% had MC4R deficiency. By the age of 45 years, 90% of individuals with MC4R deficiency were obese, defined by a BMI >30 kg/m2, versus 76% of control subjects (χ2 = 9; P < 0.002). Of obese adults, 2.8% carried an MC4R loss-of-function mutation.
Differences in rates of BMI gain.
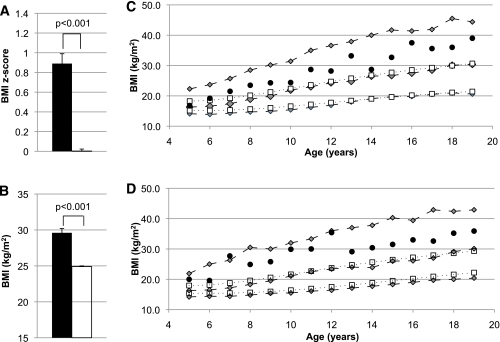
Among the 4,537 individuals with at least one measurement for BMI in childhood, 106 had a loss-of-function MC4R mutation (40 with R165Q, 26 with R165G, 21 with D37stop, 8 with A303P, 8 with G231S, and 3 with I269N). The average age of initial childhood evaluation was similar for individuals with and without an MC4R mutation (aged 10.9 ± 3.7 vs. 11.1 ± 4.2 years; P = 0.6). The ratio of the sexes and the degree of Pima heritage was similar between the two groups. In children with more than one evaluation (n = 3,397), the rate of growth, as measured by the slope of BMI increase, was 1.50 ± 0.08 kg/m2/year in children heterozygous for a loss-of-function mutation compared with 1.18 ± 0.01 kg/m2/year in control subjects (P < 0.001) (Fig. 2). Neither the average number of observations (3.7 ± 1.4 vs. 3.8 ± 1.4; P = 0.6) nor the age range between the first and last observation (aged 8.0 ± 3.4 vs. 8.1 ± 3.6 years; P = 0.9) differed between the two groups. In subset analysis with control subjects matched by BMI z score, age of initial visit, and sex, the degree of difference between the groups was attenuated but still significant (1.55 ± 0.08 vs. 1.35 ± 0.01 kg/m2/year; 95% CI for the difference 0.03–0.37; P = 0.02). Children with a loss-of-function MC4R mutation had a higher adjusted BMI z score over all childhood examinations (0.89 ± 0.10 vs. 0.00 ± 0.02; 0.70–1.09; P < 0.001). Corresponding BMIs were 29.7 ± 0.6 kg/m2 and 24.9 ± 0.1 kg/m2 for those with and without MC4R deficiency, respectively (3.5–5.9; P < 0.001) (Fig. 3). For comparison, U.S. population BMI z scores would be 2.01 ± 0.78 for children with MC4R deficiency and 1.32 ± 0.99 for the rest of the population. The three siblings homozygous for D37stop had an average BMI of 47.2 ± 3.4 kg/m2 (40.5–53.8; P < 0.001) and a BMI z score of 4.5 ± 0.56. As shown in Fig. 3, the average BMI at almost every age for girls and boys with MC4R deficiency was greater than the 50th percentile on the Pima population–specific growth chart and greater than the 95th percentile on the U.S. population growth chart.
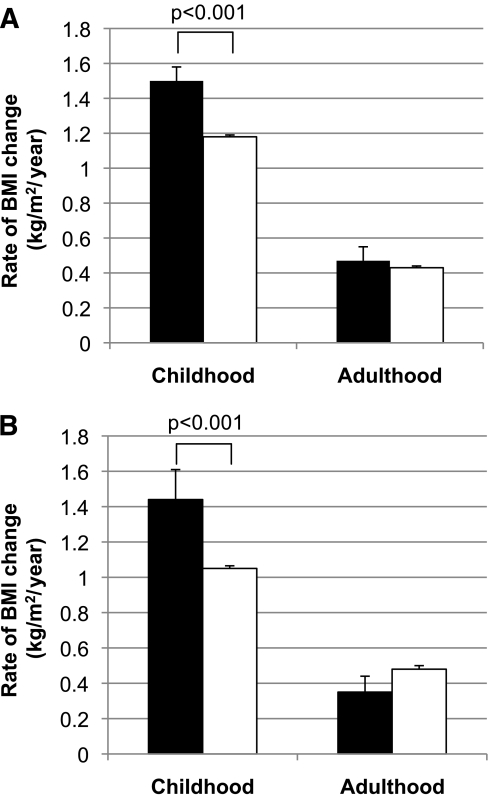
FIG. 2.
A: Rates of BMI change in kilograms per meters squared per year (kg/m2/year) in individuals without diabetes with a loss-of-function MC4R mutation (■) and with normal function (□) during childhood (ages 5–20 years; n = 3,397, 69 subjects with MC4R deficiency) and adulthood (ages 20–45 years; n = 2,695, 52 subjects with MC4R deficiency), adjusted for birth year, degree of Pima heritage, and sex. B: Rates of BMI increase in kilograms per meters squared per year (kg/m2/year) in childhood and adulthood in 1,501 individuals who were present in both the adult and childhood datasets adjusted for birth year, degree of Pima heritage, and sex. Twenty of these individuals had MC4R deficiency (■).
FIG. 3.
A: Pima population–specific BMI z scores in children (age 5–20 years) with (■) and without (□) partial loss-of-function MC4R mutations after adjustment for birth year, sibling relationships, and degree of Pima heritage. B: Mean BMIs of children with (■) and without (□) loss-of-function MC4R mutations. BMI analysis included age, sex, sibling relationships, degree of Pima heritage, and birth year as covariates. C: The BMI growth chart for girls shows that average BMI for individuals with MC4R deficiency (●) was consistently greater than the 50th percentile for the Pima population (gray diamonds, dashed lines) and greater than the 95th percentile for the U.S. population (white boxes, dotted lines) at every age. D: Corresponding boys’ growth chart for BMI. Gray diamonds, dashed lines = 95th, 50th, and 5th percentile for the Pima population; white boxes, dotted lines = 95th and 50th percentile for the U.S. population. (A high-quality color representation of this figure is available in the online issue.)
The degree of difference in the MC4R loss-of-function group stabilized after the age of 20 years, such that the adjusted z score for adults was 0.59 ± 0.11 in people with MC4R deficiency (95% CI for the difference 0.31–0.74; P < 0.001), corresponding to an average BMI of 38.9 ± 0.9 kg/m2 compared with 34.7 ± 0.1 kg/m2 in control subjects (2.5–5.9; P < 0.001). The slope of the BMI increase was no longer greater in adults with a defective MC4R (0.47 ± 0.08 vs. 0.43 ± 0.01 kg/m2/year; P = 0.7) (Fig. 2). We performed subset analyses to verify the differences observed between adults and children. These analyses, which yielded similar results, were (1) to restrict the population to full-heritage Pima Indians (n = 3,188), (2) to exclude children who developed type 2 diabetes (n = 5,838), (3) to include only individuals with multiple visits during both childhood and adulthood (n = 1,501; Fig. 2B), (4) to limit the analysis to individuals with three or more visits (n = 2,650), and (5) to restrict childhood age to between 5 and 16 years (n = 2,877).
Risk of type 2 diabetes.
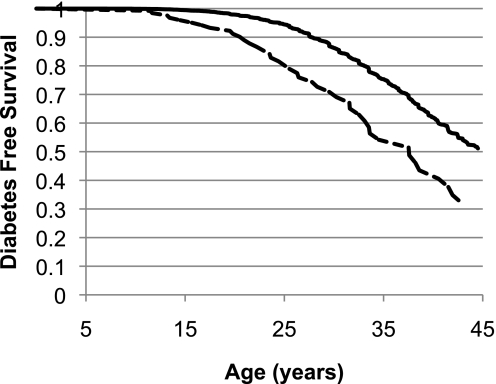
Children carrying an MC4R mutation had higher fasting glucose (5.09 ± 0.04 vs. 4.95 ± 0.01 mmol/L; P < 0.001), 2-h glucose (5.81 ± 0.09 vs. 5.56 ± 0.02 mmol/L; P = 0.009), fasting insulin (279.6 ± 15.6 vs. 231.6 ± 2.4 pmol/L; P < 0.001), and 2-h insulin concentrations (1,129.2 ± 64.8 vs. 962.4 ± 9.6 pmol/L; P < 0.001); however, after accounting for BMI, none of these differences remained statistically significant. Likewise, differences between nondiabetic adults with or without a defective MC4R in fasting or 2-h glucose or insulin concentrations were not significant after adjustment for BMI. Fifteen individuals (10.1%) with MC4R deficiency developed type 2 diabetes before the age of 20 years compared with 154 (2.6%) control subjects. The HRR, adjusted for sex, birth year, and the percentage of Pima heritage, for developing diabetes before the age of 20 years in individuals carrying a loss-of-function MC4R mutation was 4.3 (95% CI 2.3–8.3; P < 0.001) (Fig. 4). The HRR was attenuated but still significant after further adjustment for BMI (3.3 [1.2–9.2]; P = 0.03). MC4R deficiency also increased the risk of developing diabetes between the ages of 20 and 45 years, with an HRR of 2.0 (1.4–2.7; P < 0.001) (Fig. 4). However, after adjustment for BMI, this risk was fully attenuated (1.4 [0.9–2.2]; P = 0.12).
FIG. 4.
Survival curve for time to type 2 diabetes between the ages of 5 and 45 years. By age 20 years, 2.6% of the population without an MC4R mutation (solid line) developed type 2 diabetes compared with 10.1% of those with MC4R deficiency (dashed line). The increased risk for type 2 diabetes in those with MC4R deficiency continued into adulthood, although it was less pronounced. The Cox proportional hazards analysis included the following covariates: degree of Pima heritage, birth year, and sex (P < 0.001).
Height differences.
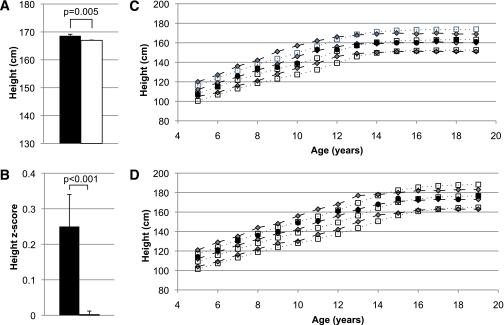
Final adult height was higher in the group with MC4R deficiency (168.8 ± 0.5 vs. 167.2 ± 0.1 cm [95% CI for the difference 0.6–2.6]; P = 0.001). In addition, the average adjusted height z score for those with MC4R deficiency was significantly higher during childhood at 0.25 ± 0.09 (P = 0.01) (Fig. 5). Differences between groups were similar in the subset analysis, with control subjects matched by BMI z score, age, and sex. The three siblings homozygous for the D37stop mutation had a higher average adult height of 175.0 ± 2.9 cm.
FIG. 5.
A: Final adult height in individuals with a loss-of-function MC4R mutation (■) compared with those without an MC4R mutation (□), adjusted for sex, birth year, degree of Pima heritage, and sibling relationships. B: Average height z scores during childhood (ages 5–20 years) for individuals with a loss-of-function MC4R mutation (■) and for those without an MC4R mutation (□), adjusted for birth year, degree of Pima heritage, and sibling relationships. C: The height growth chart for girls shows the average height for individuals with MC4R deficiency at every age (●) in comparison with the remainder of the Pima population (gray diamonds, dashed lines). This growth chart also shows the early increased linear growth seen in the Pima population compared with the U.S. population (white boxes, dotted lines). D: Corresponding boys’ growth chart for height. Gray diamonds, dashed lines = 95th, 50th, and 5th percentile for the Pima population; white boxes, dotted lines = 95th, 50th, and 5th percentile for the U.S. population. (A high-quality color representation of this figure is available in the online issue.)
Blood pressure differences.
Before the age of 20 years, MC4R deficiency was associated with decreased systolic blood pressure in the loss-of-function group even after adjusting for BMI, height, sex, age, and degree of Pima heritage (106.3 ± 0.8 vs. 108.8 ± 0.1 mmHg [95% CI for the difference 0.8–4.1]; P = 0.003) but not with a difference in diastolic blood pressure (P = 0.7). No differences were observed between groups in either systolic (P = 0.4) or diastolic (P = 0.2) blood pressure during adulthood after adjustment for the above confounders.
Differences in menarche.
In the 3,334 women (79 with MC4R deficiency) with data recorded, the presence of an MC4R mutation was associated with a later onset of menses by ~6 months (12.5 ± 0.2 vs. 12.0 ± 0.03 years [95% CI for the difference 0.12–0.83]; P = 0.008), even after adjusting for birth year and degree of Pima heritage. Inclusion of BMI in the model did not alter the results.
DISCUSSION
This study describes a detailed investigation of the prevalence of MC4R coding mutations, assessment of their functionality, and analysis of life course growth patterns in a longitudinal study of health with subjects of predominantly Pima Indian heritage. In this founder population, 10 missense/nonsense mutations were found, of which 6 cause loss of function in vitro. Three of these mutations, D37stop, R165G, and A303P, have not been described in others and may be private to this population. Although each mutation individually was rare, 2.4% of the population was heterozygous for MC4R deficiency, providing support for the hypothesis that accumulation of multiple rare mutations in a population may have a role in susceptibility for a common disease. It is well established that mutations in MC4R that result in decreased cAMP production lead to an increased risk for obesity in both rodents and humans (2,7–10,19), which seems to be attributed to hyperphagia as well as to decreased energy expenditure (4), likely as a result of a reduction in sympathetic nervous system activity (25). Our results show that the phenotypic effects of MC4R deficiency are more pronounced during childhood.
Previous investigators have anecdotally noted that the phenotype of people with MC4R deficiency may differ between children and adults (5,8). For example, the hyperphagia seen in childhood has been reported to be less pronounced in adults, abating sometime in the late teenage years (5,7,8). Our work adds to this previous literature by using data from a large, longitudinal population study to confirm in a more statistically robust way the differential effects of a defective MC4R. Pima children carrying a loss-of-function MC4R mutation had greater rates of weight gain, leading to higher BMIs. After completion of childhood growth, rates of BMI gain stabilized and were no longer different from the rest of the population. Thus, the BMI difference between those with and without MC4R deficiency did not increase further during adulthood but stayed stable at ~5 kg/m2. As this was a population-based study, there was no selection bias for obese individuals. There is, however, a high prevalence of obesity in the Pima population during both childhood and adulthood (11,15,26). Previous reports conflict on the effect of carrying a defective MC4R on the rate of BMI gain in adults, with one study (10) reporting no increased BMI gain per year and another (6) demonstrating an increased penetrance of obesity at age 40 years compared with self-reported BMI at age 20 years. However, no other study has reported prospective standardized weight and height measurements to investigate separate childhood versus adult effects of MC4R deficiency.
The reasons for accelerated weight gain only during childhood, and not adulthood, are not clear. Hyperresponsiveness of the pathways that frame the appetitive response to low peripheral energy stores during childhood may have had greater survival benefit in times of food deprivation. There also may be pathways that interact with the melanocortin system that are less active after the completion of linear growth. In support of the first possibility, a recent study showed that carrying a greater number of SNPs associated with adult BMI decreased the risk of failure to thrive during early infancy (27). In support of the latter possibility, MC4R has been shown to be important in the regulation of the growth hormone axis (28), which is known to decrease in physiologic importance with progression to adulthood. A common SNP near MC4R also has been shown to have a stronger association with BMI before the age of 20 years, which weakened during adulthood (29).
MC4R deficiency has been reported to lead to an early increase in stature (7,8,28), although not every study has confirmed this finding (2,30). Our study found an increase in childhood height z score in individuals with MC4R deficiency as well as a taller final height by ~1.5 cm. The effect on linear growth may be related to the severity of MC4R deficiency, which would explain why our study, with partial loss-of-function mutations, found a relatively small effect and another recent study demonstrated a larger effect on final adult height of 5–7 cm in individuals heterozygous for complete loss-of-function mutations (28). Pima Indian children have a different pattern of linear growth than the general U.S. population and exhibit increased early growth such that, on average, they are taller than the overall U.S. population between the ages of 4 and 14 years. However, final adult height in the Pima population is similar to the general population (16).
Individuals with the I251L genotype had a significantly lower average BMI than the group with a normal MC4R genotype. We did not find any difference in in vitro signaling between this variant and the wild-type MC4R. Previous studies have found that the I251L polymorphism may be protective against obesity (31). Our association results seem to indicate a protective effect as well; however, it should be noted that the group of individuals with this variant included no full-heritage Pima Indians and had an overall lower percentage of Pima heritage than the general population, which also may account, in part, for their lower mean BMI.
The lower blood pressure reported by others in adults (25) was only observed during childhood in our study. Our exclusion of visits after the development of type 2 diabetes or the inclusion of all subjects regardless of obesity status may account for the difference. Our childhood results, as well as the results of others (25,32), indicate that MC4R deficiency dissociates the link connecting hypertension with obesity and raises the possibility that the increased sympathetic nervous system activity seen with excess adiposity may be mediated through MC4R. The delayed menarche observed in this study may be related to a similar phenomenon, as the sympathetic nervous system is implicated in increasing gonadotropin-releasing hormone secretion prior to pubertal onset, and women with familial dysautonomia also have been reported to have delayed menarche (33). The delayed menarche was an unexpected finding because it is contrary to what is usually observed with increased adiposity; however, it is interesting that, whereas other common obesity variants associated with early menarche in a genome-wide association study meta-analysis, a SNP near MC4R did not (34).
Consistent with higher BMIs, children with MC4R deficiency had higher glucose and insulin concentrations during an OGTT. However, these differences did not persist after adjustment for BMI. This is in contrast to the classic description of MC4R deficiency, which describes hyperinsulinemia out of proportion to BMI that improves with age (7,8), although this has not always been confirmed by others (9). We found an increased risk for type 2 diabetes with MC4R deficiency. Before the age of 20 years, this risk was only partially attributable to the increased BMI at a young age. Others have not found an increased prevalence of type 2 diabetes among adults with MC4R deficiency (5,9,25), but we did find a modest risk of developing type 2 diabetes during adulthood that was fully attributable to the increased BMI. The increased risk for type 2 diabetes independent of BMI during childhood was possibly attributable, in part, to the rapidity of the weight gain. Potentially, the elevated risk is only evident in populations with high susceptibility for developing type 2 diabetes. It is unclear why MC4R deficiency would impart additional risk for diabetes beyond that conferred by an increased BMI, although recent studies have implicated MC4R in potentiating downstream insulin signaling (35,36). It also is possible that BMI did not fully represent the effect of excess adiposity in developing insulin resistance and type 2 diabetes in the model.
In conclusion, in this population-based study, the impact of MC4R deficiency on the rate of body mass accumulation was greatest during childhood and became similar to the rest of the population after completion of childhood growth. We also found an increased risk for type 2 diabetes in individuals with an MC4R defect, which was more pronounced during childhood and independent from BMI during childhood. In addition, carrying a partial loss-of-function MC4R mutation was associated with a taller adult height, lower childhood systolic blood pressure, and later menarche. Understanding how monogenic changes cause differing phenotypic expression during childhood and adulthood may help to further insights into common obesity and emphasize the need for prevention efforts during childhood.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
M.S.T. contributed to the study design and discussion, analyzed data, wrote the manuscript, and is the guarantor for the article. Y.L.M. contributed to the discussion, performed experiments, analyzed in vitro data, and contributed to and edited the manuscript. R.L.H., W.C.K., C.B., and J.K. contributed to the study design, discussion, and data collection and reviewed and edited the manuscript. M.M., M.A., and J.T. performed experiments and reviewed the manuscript. L.J.B. contributed to the study design and discussion and reviewed and edited the manuscript.
The authors thank the volunteers who participated in the studies. They also thank the clinical staff of the Phoenix Epidemiology and Clinical Research Branch for conducting the examinations.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0708/-/DC1.
REFERENCES
- 1.Calton MA, Ersoy BA, Zhang S, et al. Association of functionally significant melanocortin-4 but not melanocortin-3 receptor mutations with severe adult obesity in a large North American case-control study. Hum Mol Genet 2009;18:1140–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hainerová I, Larsen LH, Holst B, et al. Melanocortin 4 receptor mutations in obese Czech children: studies of prevalence, phenotype development, weight reduction response, and functional analysis. J Clin Endocrinol Metab 2007;92:3689–3696 [DOI] [PubMed] [Google Scholar]
- 3.Hinney A, Bettecken T, Tarnow P, et al. Prevalence, spectrum, and functional characterization of melanocortin-4 receptor gene mutations in a representative population-based sample and obese adults from Germany. J Clin Endocrinol Metab 2006;91:1761–1769 [DOI] [PubMed] [Google Scholar]
- 4.Krakoff J, Ma L, Kobes S, et al. Lower metabolic rate in individuals heterozygous for either a frameshift or a functional missense MC4R variant. Diabetes 2008;57:3267–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lubrano-Berthelier C, Dubern B, Lacorte JM, et al. Melanocortin 4 receptor mutations in a large cohort of severely obese adults: prevalence, functional classification, genotype-phenotype relationship, and lack of association with binge eating. J Clin Endocrinol Metab 2006;91:1811–1818 [DOI] [PubMed] [Google Scholar]
- 6.Stutzmann F, Tan K, Vatin V, et al. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes 2008;57:2511–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farooqi IS, Yeo GS, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 2000;106:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003;348:1085–1095 [DOI] [PubMed] [Google Scholar]
- 9.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 2000;106:253–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen LH, Echwald SM, Sørensen TI, Andersen T, Wulff BS, Pedersen O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with juvenile-onset obesity. J Clin Endocrinol Metab 2005;90:219–224 [DOI] [PubMed] [Google Scholar]
- 11.Knowler WC, Pettitt DJ, Saad MF, et al. Obesity in the Pima Indians: its magnitude and relationship with diabetes. Am J Clin Nutr 1991;53(Suppl.):1543S–1551S [DOI] [PubMed] [Google Scholar]
- 12.Pavkov ME, Hanson RL, Knowler WC, Bennett PH, Krakoff J, Nelson RG. Changing patterns of type 2 diabetes incidence among Pima Indians. Diabetes Care 2007;30:1758–1763 [DOI] [PubMed] [Google Scholar]
- 13.Hanson RL, Ehm MG, Pettitt DJ, et al. An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am J Hum Genet 1998;63:1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baier LJ, Hanson RL. Genetic studies of the etiology of type 2 diabetes in Pima Indians: hunting for pieces to a complicated puzzle. Diabetes 2004;53:1181–1186 [DOI] [PubMed] [Google Scholar]
- 15.Schulz LO, Bennett PH, Ravussin E, et al. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care 2006;29:1866–1871 [DOI] [PubMed] [Google Scholar]
- 16.Lindsay RS, Cook V, Hanson RL, Salbe AD, Tataranni A, Knowler WC. Early excess weight gain of children in the Pima Indian population. Pediatrics 2002;109:E33. [DOI] [PubMed] [Google Scholar]
- 17.Knowler WC, Bennett PH, Bottazzo GF, Doniach D. Islet cell antibodies and diabetes mellitus in Pima Indians. Diabetologia 1979;17:161–164 [DOI] [PubMed] [Google Scholar]
- 18.Nijenhuis WA, Garner KM, van Rozen RJ, Adan RA. Poor cell surface expression of human melanocortin-4 receptor mutations associated with obesity. J Biol Chem 2003;278:22939–22945 [DOI] [PubMed] [Google Scholar]
- 19.Yeo GS, Lank EJ, Farooqi IS, Keogh J, Challis BG, O’Rahilly S. Mutations in the human melanocortin-4 receptor gene associated with severe familial obesity disrupts receptor function through multiple molecular mechanisms. Hum Mol Genet 2003;12:561–574 [DOI] [PubMed] [Google Scholar]
- 20.Ma L, Tataranni PA, Bogardus C, Baier LJ. Melanocortin 4 receptor gene variation is associated with severe obesity in Pima Indians. Diabetes 2004;53:2696–2699 [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. : CDC growth charts: United States. Adv Data 2000:1–27 [PubMed] [Google Scholar]
- 22.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 23.Kim DH, Shin SW, Baik JH. Role of third intracellular loop of the melanocortin 4 receptor in the regulation of constitutive activity. Biochem Biophys Res Commun 2008;365:439–445 [DOI] [PubMed] [Google Scholar]
- 24.van den Berg L, van Beekum O, Heutink P, et al. Melanocortin-4 receptor gene mutations in a Dutch cohort of obese children. Obesity (Silver Spring) 2011;19:604–611 [DOI] [PubMed] [Google Scholar]
- 25.Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 2009;360:44–52 [DOI] [PubMed] [Google Scholar]
- 26.Price RA, Charles MA, Pettitt DJ, Knowler WC. Obesity in Pima Indians: large increases among post-World War II birth cohorts. Am J Phys Anthropol 1993;92:473–479 [DOI] [PubMed] [Google Scholar]
- 27.Elks CE, Loos RJ, Sharp SJ, et al. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med 2010;7:e1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinelli CE, Keogh JM, Greenfield JR, et al. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab 2011;96:E181–E188 [DOI] [PubMed] [Google Scholar]
- 29.Hardy R, Wills AK, Wong A, et al. Life course variations in the associations between FTO and MC4R gene variants and body size. Hum Mol Genet 2010;19:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinehr T, Hebebrand J, Friedel S, et al. Lifestyle intervention in obese children with variations in the melanocortin 4 receptor gene. Obesity (Silver Spring) 2009;17:382–389 [DOI] [PubMed] [Google Scholar]
- 31.Stutzmann F, Vatin V, Cauchi S, et al. Non-synonymous polymorphisms in melanocortin-4 receptor protect against obesity: the two facets of a Janus obesity gene. Hum Mol Genet 2007;16:1837–1844 [DOI] [PubMed] [Google Scholar]
- 32.Sayk F, Heutling D, Dodt C, et al. Sympathetic function in human carriers of melanocortin-4 receptor gene mutations. J Clin Endocrinol Metab 2010;95:1998–2002 [DOI] [PubMed] [Google Scholar]
- 33.Maayan C, Sela O, Axelrod F, Kidron D, Hochner-Celnikier D. Gynecological aspects of female familial dysautonomia. Isr Med Assoc J 2000;2:679–683 [PubMed] [Google Scholar]
- 34.Elks CE, Perry JR, Sulem P, et al. ; GIANT Consortium Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet 2010;42:1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chai B, Li JY, Zhang W, Wang H, Mulholland MW. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides 2009;30:1098–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chai B, Li JY, Zhang W, Wu X, Zhang C, Mulholland MW. Melanocortin-4 receptor activation promotes insulin-stimulated mTOR signaling. Peptides 2010;31:1888–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]