Abstract
Objectives
The cortisol awakening response (CAR) is related with psychosocial factors and health in potentially significant ways, suggesting that it may be a distinctive marker of hypothalamic-pituitary-adrenal (HPA) axis function and dysfunction. This sought to expand upon previous work that examined the association between CAR and ratings of laboratory-evoked acute pain stimulation. In addition to evoked pain ratings, this study also tested whether CAR was prospectively related with salivary cortisol and soluble tumor necrosis factor-α receptor II (sTNFαRII) responses to acute pain stimulation.
Methods
This study included 36 healthy, pain-free volunteers of both sexes recruited via posted study flyers. Prior to completion of laboratory pain testing, salivary cortisol samples were obtained at home over the course of a single morning according to the following time frame: upon awakening, and 15, 30, and 60 min after awakening. Following collection of saliva, study participants brought their home saliva samples to the laboratory for assay and subsequently completed acute experimental pain testing procedures.
Results
Cluster analysis of CAR revealed two distinct groups with similar patterns of cortisol response to awakening; increased and flattened. Relative to flattened CAR, increased CAR was associated with greater ratings of pain intensity and unpleasantness. Salivary cortisol was significantly increased and sTNFαRII significantly decreased following pain testing, but neither of these responses differed as a function of increased versus flattened CAR.
Discussion
CAR may be a marker for stress sensitivity and/or the anticipation of impending stress, which could explain why the increased CAR cohort reported greater acute pain ratings.
Keywords: Cortisol awakening response, acute pain, inflammation, HPA axis, stress
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis in an important endocrine system that is involved in human adaptation to physical and emotional challenges including the experience of pain.1,2 The glucocorticoid cortisol is the hormonal end product of the HPA axis and plays an important role in efforts to adjust to such challenges. Normal basal HPA axis activity follows a distinct diurnal rhythm with the highest cortisol levels during the early morning hours; thereafter, cortisol steadily decreases throughout the day until it reaches its nadir during the first half of the night.3 In addition to this reliable diurnal rhythm, there is a brisk increase in cortisol that occurs in relation to awakening in the morning. This cortisol awakening response (CAR) is characterized by a rise in cortisol upon awakening that is distinct from the circadian rise in HPA axis activity in the morning hours, and it occurs in people of all ages.4,5 CAR generally reaches its peak during the initial 30 – 45 minutes post-awakening, but may continue to increase up to an hour after awakening.4 The magnitude of the increase in cortisol upon awakening has been reliably associated with psychosocial factors and health in potentially significant ways, suggesting that it may be a distinctive marker of HPA function and dysfunction.4,6
Previous literature reviews have suggested that HPA axis dysfunction may be implicated in the onset of chronic pain conditions like fibromyalgia and rheumatoid arthritis.7,8 Accordingly, the number of studies examining the relation between CAR and pain is anticipated to grow. Recent evidence has shown that CAR is associated with several disorders known to consistently co-occur with many painful conditions; these disorders include depression and anxiety,6,9,10 sleep disturbance,11 and chronic fatigue12 among others. However, the exact role of CAR in the development and perpetuation of these disorders remains unclear. The existing literature specifically addressing the relation between CAR and pain is scant and mixed. In a study that compared healthy controls to patients with chronic whiplash-associated disorder (WAD) who experienced pain, WAD patients demonstrated an overall flattened CAR compared to healthy controls.13 Conversely, another study revealed that men with chronic pelvic pain syndrome had significantly increased awakening cortisol responses compared to healthy controls.14 Furthermore, it has been suggested that there are no meaningful differences in CAR between healthy controls and patients with chronic osteoarthritis.15 Recently, our group has shown that differences in CAR were significantly related with pain ratings made in response to a cold pressor test in healthy young adults.16 In this study, individuals with flattened CAR (i.e., minimal cortisol increase to awakening) reported greater mean pain intensity and pain unpleasantness ratings to noxious cold compared to individuals with an increased CAR; these results currently await replication and expansion.
Bouts of clinical pain and laboratory-evoked noxious stimulation represent acute stressors that have previously been shown to provoke changes in autonomic (e.g., catecholamines), endocrine (e.g., cortisol), and immune (e.g., inflammatory cytokines) processes.17–21 These physiological changes are interactive and generally comprise what is commonly referred to as “the stress-response.” Activation of the stress-response to an acute bout of pain can lead to behavioral and peripheral changes that improve the ability of the organism to make allostatic adjustments for the maintenance of homeostasis.22,23 On balance, the physiological changes (i.e., stress response) often observed during acute bouts of clinical and evoked pain are vastly considered adaptive, and meant to coordinate allostasis while protecting the organism during times of threat or challenge. It is not until these adaptive responses become excessive, fail to return to normalcy, or otherwise function in a dysregulated manner that pathology may ensue. To date, no study has examined whether the stress response to an acute pain stimulus varies as a function of differences in CAR. Examination of differences in the physiological responses to acute pain may help to identify mechanisms that explain how CAR is associated with the pain experience, as well as other parameters of health and well-being.
The current study sought to expand upon our group’s previous investigation of the association between CAR and pain responses to laboratory-evoked acute pain stimulation in a non-clinical sample. In addition to experimentally-evoked pain responses, this study also tested whether CAR was prospectively related with HPA axis and inflammatory responses to acute pain stimulation. Specifically, salivary cortisol and the soluble receptor for tumor necrosis factor-α (sTNFαRII) were examined. We chose to assess sTNFαRII because it is more stable and reliably measured than TNF-α.24 Healthy participants who exhibited an increased cortisol response to awakening were compared to participants who demonstrated a flattened cortisol response to awakening on pain relevant outcomes. The following questions were tested: 1) Are reports of pain intensity and unpleasantness significantly different for individuals with an increased CAR compared to individuals with a flattened CAR? 2) Are the salivary cortisol and sTNFαRII responses to acute pain significantly different between the increased and flattened CAR groups?
Materials and Methods
Participants
The present study includes a portion of self-report, HPA axis, and inflammatory data collected from individuals who took part in a larger investigation examining patterns of biological marker responses to painful and non-painful quantitative sensory testing. For this study, healthy and pain-free volunteers (N = 36) of both sexes (47% women) with a mean age of 21 years (SD = 2.5) were recruited from an urban university setting in the mid-Atlantic United States. Potential participants completed a screening battery to determine health status and eligibility. Individuals were unable to participate if they met any of the following criteria: (a) age less than 18 or over 45 years; (b) ongoing chronic pain problems; (c) diagnosed with hypertension or taking medication for blood pressure; (d) circulatory disorders; (e) history of cardiac events; (f) history of metabolic disease or neuropathy; (g) pregnant; (h) currently using prescription analgesics, tranquilizers, antidepressants, or other centrally acting agents; (i) use of nicotine, (j) use of prescription medication (e.g., corticosteriods, oral contraceptives), (k) psychiatric disorders (e.g., depression), or (l) chronic or acute health problems that affect the HPA axis or immune system. Informed consent was obtained in accordance with approved protocol guidelines of the University Institutional Review Board. All participants were compensated $20 for their participation.
Experimental protocol
At the first visit, participants were provided with detailed verbal and written instructions regarding home collection of saliva samples (for CAR) and a suitable day for the collection of the saliva samples was selected. Participants received a batch of saliva sampling devices (salivettes; Sarstedt) to assess their cortisol levels after awakening (see below for a more detailed description of the sampling procedure). Participants returned these saliva samples to our laboratory within one week prior to their second scheduled visit. Given concerns with adherence to home cortisol sampling,25 participants were provided with detailed take-home instruction regarding how and when to collect saliva samples. They also completed a checklist indicating their adherence to the saliva sampling procedures, which included no food, drink, or brushing of teeth within the first hour of awakening. None of the participants reported any difficulty adhering to the saliva collection procedures.
On the second visit, participants were successively assigned to complete one of three potential pain tasks including a cold pressor task (CPT; N = 10), a hot water task (HWT; N = 10), and an ischemic pain task (IPT; N = 16). All pain testing sessions occurred between the hours of 4P.M. and 7P.M. Participants were presented with audio-taped instructions for how to complete the pain testing procedures. Cortisol and the soluble receptor for TNF-α (sTNFαRII) were collected from oral fluid before, immediately following and during recovery from pain testing per the sampling procedures discussed below. The Perceived Stress Scale (PSS)26 was also completed during the second visit. Perceived stress was considered as a potential factor to be controlled in this study because prior reports have suggested that associations between CAR and various health-related outcomes can be influenced by the amount of stress reportedly experienced by individuals during the approximate time of CAR assessment.6
CAR assessment
Four salivary cortisol samples were obtained at home over the course of a single morning. It has been suggested that an individual’s CAR is consistent over days and even weeks making it a reliable biological marker of adrenocortical functioning;27 therefore, assessment of CAR on a single day seemed justified. Upon awakening, and 15, 30, and 60 min after awakening, participants placed a cotton salivette in their mouth for 2 min 30 s. Cortisol obtained in this way is not influenced by saliva flow rate and is in its unbound, biologically active state.3 Once collected, participants returned the samples to our laboratory where they were stored at −80°C until batched assayed using high sensitivity enzyme immunoassay kits (Salimetrics, State College, PA).
Laboratory pain testing and pain reports
Cold Pressor Task (CPT)
The CPT procedure involved a NESLAB RTE-10 liter water bath (Thermo Electron Corporation, Portsmouth, New Hampshire) filled with circulating cold water maintained at approximately 4°C (± 0.2°C). Participants were instructed to place their dominant hand into the cold water up to their wrist. Standardized instructions indicated that participants should keep their hands immersed “for the entire duration of the procedure or until the pain becomes intolerable”, which is a typical measure of cold pain tolerance. Unbeknownst to participants, the maximum allowable duration of the CPT was 300 seconds (though participants were permitted to terminate the task at any time if the sensations become intolerable). While prior research has used different cutoff times, our 300 second cutoff is consistent with many previous studies.28
Hot Water Task (HWT)
The hot water task is a tonic pain model similar in procedure to the CPT, but the temperature of the water is increased to 47°C.29 A NESLAB RTE-10 liter water bath (Thermo Electron Corporation, Portsmouth, New Hampshire) was filled with circulating hot water maintained at approximately 47°C (± 0.2°C). Just as with the CPT, participants were instructed to place their dominant hand into the hot water up to their wrist and to keep their hands immersed “for the entire duration of the procedure or until the pain becomes intolerable”. The maximum allowable duration of the HWT was 300 seconds (though participants were permitted to terminate the task at any time if the sensations become intolerable).
Ischemic Pain Task (IPT)
The IPT consisted of a modified submaximal effort tourniquet procedure that involved exercising the hand as blood flow to the arm was occluded, evoking ischemic pain. As is the standard IPT procedure, maximum grip strength of the dominant hand was determined using a Lafayette hand-held dynamometer (Lafayette Instrument Co., Lafayette, IN). Participants then elevated the dominant arm above heart level for 30 seconds to drain blood from the arm. The arm was then occluded with a standard blood pressure cuff positioned proximal to the elbow and inflated to 240 mm Hg using a Hokanson E20 rapid cuff inflator (D.E. Hokanson, Inc., Bellevue, WA). Participants then performed 20 handgrip exercises of 2 seconds duration with 4 second intervals at 50% of their maximum grip strength. Participants were instructed to say “stop” if they felt that the pain had become intolerable; otherwise, the task was carried out to 900 seconds. A duration of 900 seconds for the IPT is common and consistent with previous research.30
Throughout each pain task (CPT, HWT, IPT), separate ratings of pain intensity and pain unpleasantness were collected at 30 second intervals following task initiation, or until the task was discontinued due to pain intolerance. Ratings of worst pain were also obtained at the exact time the task was terminated. A description of the difference between pain intensity (“How strong the pain feels”) and pain unpleasantness (“How unpleasant or disturbing the pain is for you”) was read to all subjects. Following this, pain intensity and pain unpleasantness were assessed by asking subjects for verbal self-reports on 0–100 scales, with 0 = “No pain” (or “Not at all unpleasant”) and 100 = “Pain as intense as I can imagine” (or “Pain as unpleasant as I can imagine). Numeric rating scales of pain intensity and pain unpleasantness have demonstrated validity through their ability to detect treatment effects, as well as their strong association to other measures of pain intensity and unpleasantness.31 Pain tolerance was measured as the time (seconds) from pain onset to discontinuation. Mean pain intensity and unpleasantness ratings, in addition to ratings of worst pain intensity and unpleasantness, were examined in statistical analyses.
Cortisol and sTNFαRII responses to pain testing
Consistent with the procedures incorporated by Dickerson and colleagues,32 the biological parameters in this study were obtained from oral fluids, which provide an established method for assessing cortisol3 and has been validated for assessing certain inflammatory products.33 Particularly, it has been demonstrated that levels of sTNFαRII in oral fluids are significantly and highly correlated with those obtained from plasma.33 Therefore, oral fluid collection seemed to be a less reactive and invasive means for reliably measuring HPA axis and immune activity relative to a needle stick with blood draws.
An OraSure collection device (Epitope, Beaverton, OR) was placed into the mouth, between the lower cheek and gum, for 2.5 minutes per sampling time-point; this placement collects samples mainly containing oral mucosal transudate (OMT). Along with the OraSure device, salivettes (Sarsted, Leicester, UK) were concurrently placed into the mouth, on top of the tongue, for saliva collection. OMT and saliva are both oral fluids; OMT is a filtrate of blood plasma while saliva contains enzymes and other particles from the parotid and salivary glands. Cortisol in saliva is in its unbound, biologically active form, and its concentration is independent of saliva flow rate. OMT was collected for sTNFαRII rather than saliva because sTNFαRII is more readily available in OMT.
After obtaining the OMT and saliva samples, the samples were immediately refrigerated before being transferred and stored at −80°C until batch assayed. Cortisol was measured using high sensitivity salivary cortisol immunoassay kits (Salimetrics, State College, PA). Intra- and interassay variability was less than 8%. sTNFαRII was measured using Quantikine Human sTNFαRII enzyme immunoassay kits (R&D Systems, Minneapolis, MN). The Bradford method34 was used to quantify protein in the oral fluids, using the Bio-Rad protein assay kit with bovine plasma albumin as the standard. The sTNFαRII results are reported using the ratio of the experimental value for the analyte to the protein concentration in the test sample. This ratio controls for changes in salivary flow rate, which can be altered by experimental procedures or vary between individuals. The ratio values are more reliable than the analyte values alone.33 The intra- and interassay variability was less than 5%.
Saliva and OMT samples were collected at baseline (approximately 5 minutes prior to pain task initiation), immediately following termination of the pain task, and at pre-determined intervals during recovery from the pain testing procedure (+10, 15, 20, 25 and 35 minutes post-pain task). These sampling time-points were chosen based on a meta-analysis of prior research showing that peak changes in cortisol occur at approximately 30 minutes following stressor onset.35 Additionally, a recent study has reported that sTNFαRII responses to acute stress have been found to occur at that same time point.32
Questionnaires
Perceived Stress Scale (PSS)
Participants’ level of perceived stress was measured by means of the PSS scale.26 This scale is a self-report instrument that evaluates the level of perceived stress during the last month. Originally, the scale was comprised of 14 items, but more recently a 10 item short-version was created following the guidelines put forth by the authors of the original version.36 Respondents are asked to rate each item on a 5-point scale ranging from 0 (never) to 4 (very often). The total score of the PSS is obtained by reversing the scores of the four positive items and then summing across all 10 items. A higher score indicates a higher level of perceived stress. In the current study, internal consistency for the PSS scale was very good (α = .82)
Data analysis
A significant amount of skew in the distributions of all cortisol and sTNFαRII data was discovered. Accordingly, these data were subjected to logarithmic transformation using a log10 function, which was effective for reducing skew according to Shapiro-Wilk’s tests (p’s > 0.05). Subsequent statistical analysis of cortisol and sTNFαRII responses was completed using transformed values; however, study figures show anti-log mean values for easier interpretation of results. Area under the cortisol response to awakening curve was calculated with respect to increase (AUCI CAR) and ground (AUCG CAR) to capture increase in salivary cortisol across the selected CAR time points (i.e., upon awakening, 15, 30, and 60 min post-awakening). As previously described by Pruessner and colleagues,37 AUCG is assumed to be a measure more related to total hormone output, whereas AUCI is a parameter that emphasizes the changes over time and is more related to sensitivity of the system. In this study we specifically examined AUCI CAR because it has previously been suggested that the increase in CAR may be the most appropriate measure for assessing HPA activation following waking in relation to biopsychosocial factors.6
The AUCI CAR variable was then subjected to cluster analysis using k-means38 with the objective to define two distinct groups of participants with similar patterns of cortisol response to awakening. Cluster analysis was completed rather than a median split to reduce the arbitrary nature of the grouping and to better ensure that the two groups of participants were as similar as possible for CAR. Since the cluster analysis method makes no restrictions in terms of group size, numbers of participants in each group are usually not balanced. The cluster analysis produced a group of participants with flattened AUCI CAR (N = 24) and a group demonstrating increased AUCI CAR (N = 12). Repeated measures analysis of variance (RM-ANOVA) was computed to detect differences in cortisol responses after awakening between the groups defined by the cluster analysis, with Greenhouse-Geisser corrections applied for repeated measures. Significant interaction and main effects were further analyzed by Bonferroni adjusted post-hoc tests.
Differences in the distribution of participants’ sex and the type of laboratory pain task completed as a function of the flattened and increased AUCI CAR groupings were examined using Chi-square. The flattened and increased AUCI CAR groups were compared on reports of average and worst pain intensity and pain unpleasantness using multivariate analysis of variance (MANOVA). Additional RM-ANOVAs were completed to examine differences between the flattened and increased CAR groups in cortisol and sTNFαRII responses assessed before, immediately following and during recovery from laboratory pain testing. Analyses include the partial η2 as a measure of effect size where appropriate. Following the conventions of Cohen,39 partial η2 = 0.01 is considered a small effect, partial η2 = 0.06 a medium-sized effect and partial η2 = 0.14 a large effect.
Results
Cortisol response to awakening
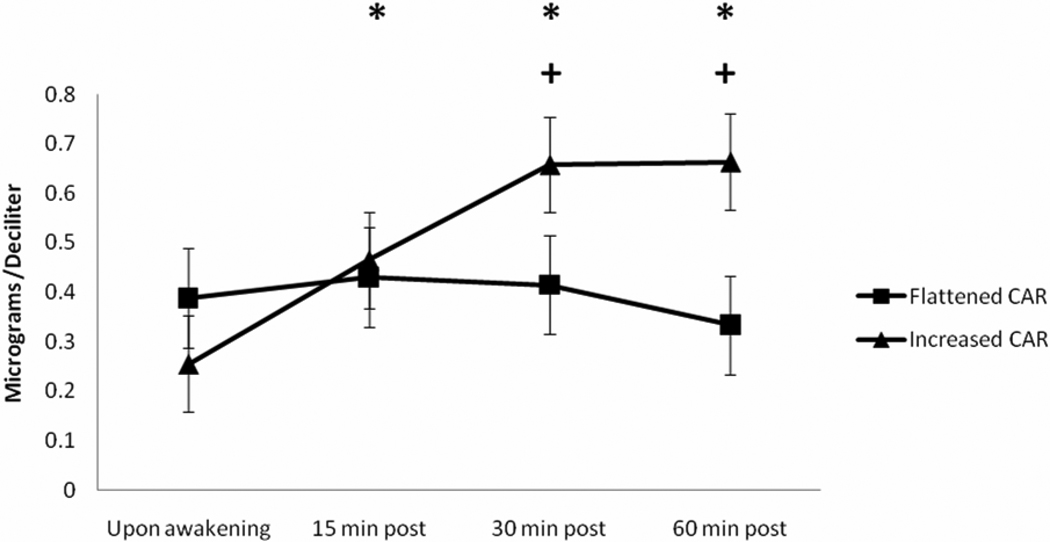
The patterns of cortisol response to awakening for the two groups (flattened and increased AUCI CAR) produced by cluster analysis were distinctly different as shown in Figure 1. Indeed, RM-ANOVA with a Greenhouse-Geisser correction revealed a significant AUCI CAR group by time interaction for the cortisol response to awakening (F(3,102) = 15.25, p < 0.001, η2 = 0.310, observed power = 0.99). From waking levels, cortisol was significantly increased by approximately 49% at 15 min, 66% at 30 min, and 59% at 60 min for the increased AUCI CAR group (p’s < 0.001 with Bonferroni adjustment). The rise in cortisol was significantly greater at 30 and 60 min for the AUCI CAR group relative to the flattened AUCI CAR group (p’s < 0.01 with Bonferroni adjustment). The flattened AUCI CAR group demonstrated non-significant change in cortisol response over the 60 min observation period when compared to waking levels (p’s > 0.05). Results for AUCI CAR do not appear to have been meaningfully influenced by heterogeneity of covariance or variance according to Box’s test and Levene’s tests, respectively (p’s > 0.05).
Figure 1.
Anti-log mean values of cortisol response to awakening for the increased (N = 12) and flattened (N = 24) AUCI CAR groups. * = significant increase in cortisol from baseline for the increased AUCI CAR group. + = significant differences between increased and flattened AUCI CAR groups.
CAR group comparisons and examination of covariates
The flattened and increased AUCI CAR groups did not significantly differ in their reports of perceived stress during the month preceding study participation (F(1,34) = .31, p = .58); perceived stress was not included in any subsequent study model as a statistical covariate. The two AUCI CAR groups were compared for differences in total cortisol output (AUCG CAR) during the CAR relevant cortisol sampling time points. The overall output of cortisol was found to be marginally greater for the increased AUCI CAR group relative to the flattened AUCI CAR group (F(1,34) = 3.51, p = .07). Total cortisol output during CAR assessment (AUCG CAR) was subsequently included in all study models to better examine unique relations among increase in cortisol (AUCI CAR) and study outcomes. Results from Chi-Square tests showed that AUCI CAR group distributions did not significantly differ by sex (χ2(1) = 1.39, p = 0.24) or by the type of laboratory pain task completed (χ2(2) = 0.90, p = 0.64). Further, the flattened and increased AUCI CAR groups did not significantly differ in their pain tolerance (F(1,34) = 1.66, p = .21). Despite the lack of significant AUCI CAR group differences for pain tolerance or distribution of the type of pain task completed, conservative efforts were still employed to minimize any potential influence on the results due to differences in pain testing. Outcome data for pain and physiological responses were collapsed and examined across the three pain tasks, but all subsequent analyses statistically controlled for the type of pain task completed.
CAR and acute pain reports
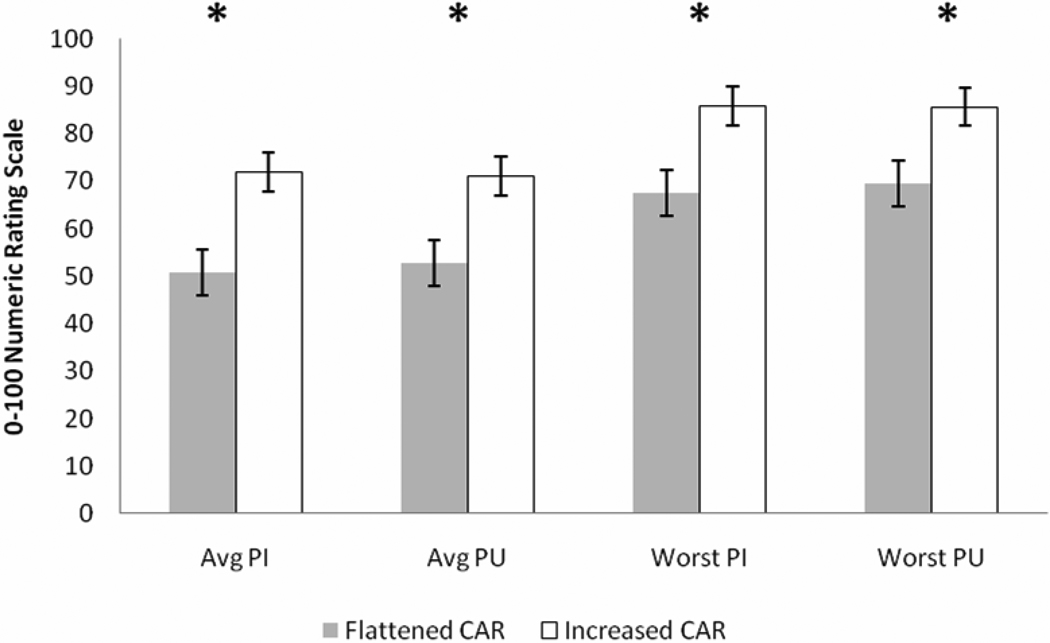
Results of MANOVA demonstrated a marginally significant multivariate effect of AUCI CAR group on pain responses (F(4,29) = 2.37, p = .07, η2 = 0.247, observed power = 0.45); however, further probing of the multivariate effect revealed very significant univariate effects (Figure 2). Interestingly, the increased AUCI CAR group reported greater average pain intensity (F(1,32) = 9.21, p < .01, η2 = 0.223, observed power = 0.69), average pain unpleasantness (F(1,32) = 7.82, p < .01, η2 = 0.196, observed power = 0.62), worst pain intensity (F(1,32) = 5.79, p = .02, η2 = 0.153, observed power = 0.57), and worst pain unpleasantness (F(1,32) = 4.60, p = .04, η2 = 0.126, observed power = 0.50) compared to the flattened AUCI CAR group (Table 1). Box’s test and Levene’s tests were non-significant (p’s > 0.05).
Figure 2.
Ratings of average and worst pain intensity and pain unpleasantness. * = ratings of increased AUCI CAR group (N = 12) significantly greater than ratings of flattened AUCI CAR group (N = 24).
Table 1.
Comparison of participants with increased CAR (N = 12) versus flattened CAR (N = 24)
| Increased AUCI CAR | Flattened AUCI CAR | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Average Pain Intensity | 72.1 | 17.9 | 50.5 | 24.1 |
| Average Pain Unpleasantness | 71.2 | 19.4 | 52.7 | 21.1 |
| Worst Pain Intensity | 85.8 | 11.8 | 67.5 | 30.2 |
| Worst Pain Unpleasantness | 85.7 | 15.8 | 69.5 | 27.1 |
| Pain Tolerance (sec) | 355.3 | 202.3 | 478.8 | 298.5 |
| AUCG CAR | 33.6 | 13.3 | 23.7 | 15.2 |
| Perceived Stress | 16.7 | 5.2 | 16.1 | 7.7 |
| Sex | 33.3% women | 54.2% women | ||
| Type of Pain Task Completed | 33.3% CPT, 33.3% HWT, 33.3% IPT | 25% CPT, 25% HWT, 50% IPT | ||
Note: CAR = cortisol awakening response; AUCI = area under the curve, increase; AUCG = area under the curve, ground; CPT = cold pressor task; HWT = hot water task; IPT = ischemic pain task; pain tolerance measured in seconds.
CAR and physiological responses to acute pain
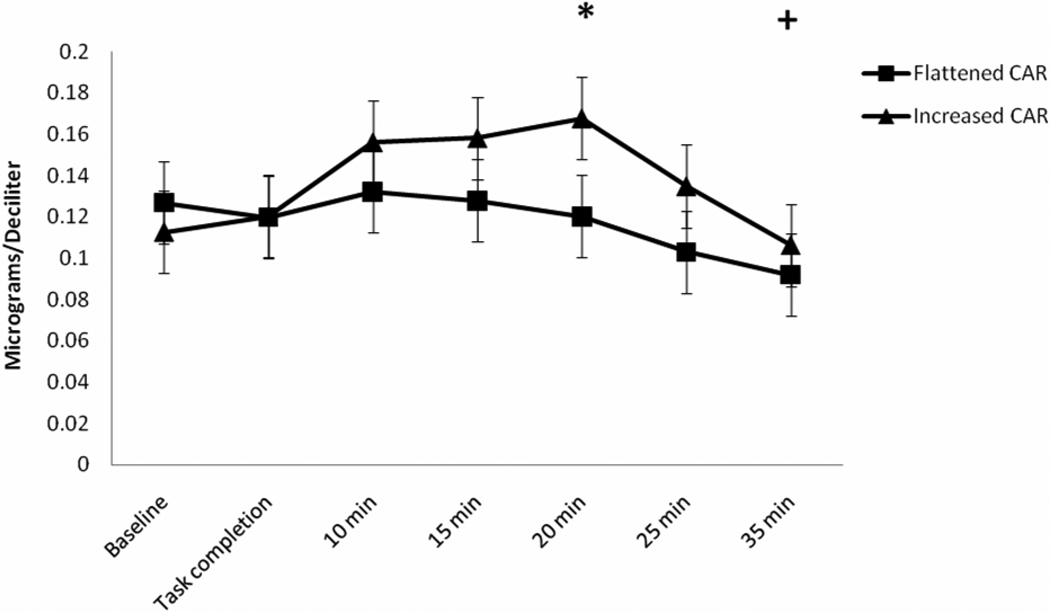
Cortisol responses to acute pain are shown in Figure 3. Results of RM-ANOVA with Greenhouse-Geisser correction demonstrated a significant main effect of time (F(6,192) = 6.22, p < 0.01, η2 = 0.155, observed power = 0.65), suggesting that all participants (irrespective of increased versus flattened AUCI CAR status) demonstrated a cortisol response that was significantly increased from baseline levels at 20 minutes and then significantly below baseline levels at 35 minutes following completion of the acute pain tasks (p’s < 0.025 with Bonferroni adjustment). Contrary to our hypothesis, a significant AUCI CAR group by time interaction failed to emerge (F(6,192) = 0.83, p = 0.45, η2 = 0.066, observed power = 0.19), suggesting that cortisol responses to acute pain did not significantly differ over time as a function of AUCI CAR status. It may be important to mention that the effect size for this interaction fell within the moderate range; however, the observed power for detecting this effect was quite low. Further, examination of Figure 3 shows a tendency for the post-pain task cortisol responses of the increased AUCI CAR group to be consistently greater than those of the flattened AUCI CAR group. Box’s test and Levene’s tests were non-significant (p’s > 0.05).
Figure 3.
Anti-log mean values of cortisol response to acute pain. * = cortisol significantly increased from baseline irrespective of CAR group. + = cortisol significantly decreased from baseline irrespective of CAR group.
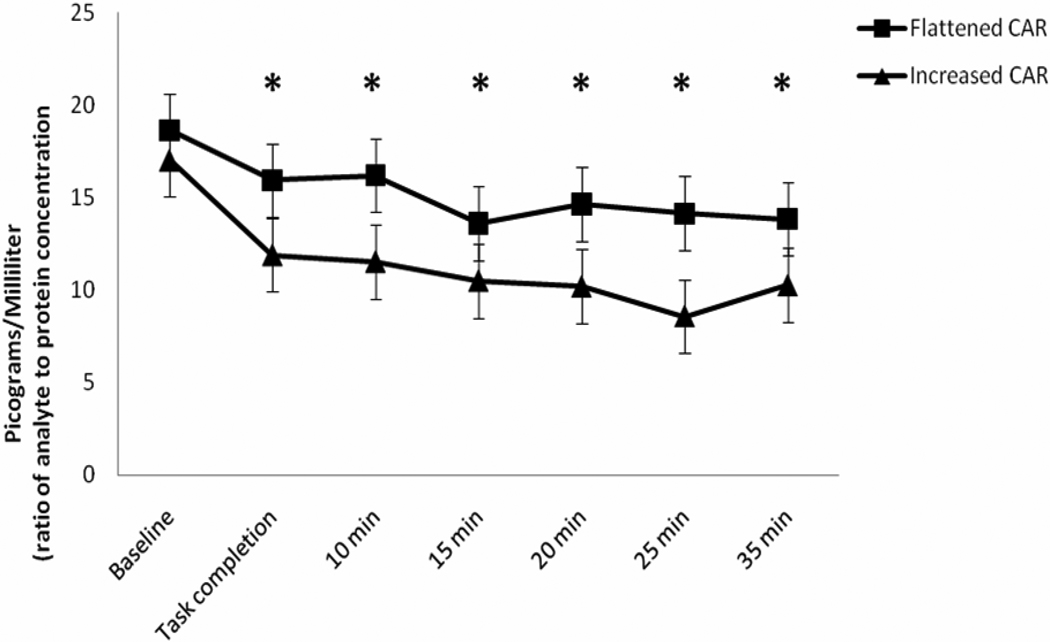
Analysis of sTNFαRII responses to acute pain (Figure 4) revealed another significant main effect of time (F(6,192) = 2.59, p = 0.03, η2 = 0.075, observed power = 0.64), whereby all participants (irrespective of AUCI CAR status) demonstrated a sTNFαRII response that was significantly decreased from baseline levels immediately following completion of the pain tasks and remained significantly below baseline levels through each successive time point (p’s < 0.001 with Bonferroni adjustment). The interaction between AUCI CAR group and time was not found to be statistically significant for the sTNFαRII response (F(6,192) = 1.34, p = 0.25, η2 = 0.050, observed power = 0.45). Thus, it appears that the patterns of sTNFαRII response were not statistically different across the AUCI CAR groups. Again, it can be pointed out that the effect size for this interaction was in the small to moderate range; however, the observed power was arguably insufficient. Examination of Figure 4 shows a tendency for the post-pain task sTNFαRII responses of the increased AUCI CAR group to be consistently more diminished than those of the flattened AUCI CAR group. Box’s test and Levene’s tests were also non-significant for this analysis (p’s > 0.05).
Figure 4.
Anti-log means values of sTNFαRII response to acute pain. * = sTNFαRII significantly decreased from baseline irrespective of CAR group.
Discussion
In the sample of healthy adults reported here, experimentally-evoked acute pain sensitivity differed as a function of the cortisol increase upon awakening. Self-reports of average and worst pain intensity and unpleasantness were significantly greater for the group of individuals with an increased cortisol response to awakening compared to the group of individuals with a flattened response. These findings are a direct contrast to the results of the study previously reported by our group..16 In our previous study, individuals with a flattened CAR reported greater pain ratings. Our contradictory findings from healthy, non-clinical samples are not unlike the mixed findings reported from studies that examined CAR function in clinical samples of individuals with chronic pain conditions.13,14,40 It has been suggested that CAR function may vary with the duration of the chronic pain condition. Whereas chronic pain is initially associated with increased HPA axis activity, it is hypothesized, that some individuals develop a hypoactive HPA axis when chronic pain persists over a long period of time.8 This hypothesis is consistent with the theory of allostatic load,41,42 which has been described as a physiological process representing the cumulative wear and tear on the body resulting from chronic or inadequate adaptation to stress.43 This explanation may account for conflicting results regarding CAR in chronic pain conditions; however, the application of this hypothesis to healthy, non-clinical samples may be more tenuous. Two potential explanations for the differential results between the present study and our group’s previous study merit mentioning.
First, no clinically-validated guidelines exist to define normal and dysregulated cortisol responses to awakening. Accordingly, the use of statistical approaches to designate group membership (i.e., flattened versus increased CAR) are warranted. The current study used a k-means cluster analysis to differentiate increased CAR from flattened CAR, whereas our previous study used median split procedures. Cluster analysis may be more effective than the median split for differentiating CAR because median split procedures impose an arbitrary structure on data by creating groups of approximately equal size. Cluster analysis methods go beyond median split procedures to identify structural groupings that provide a satisfactory fit with the data set irrespective of sample size.44,45 Despite the potential differences between cluster analysis and median split, the use of a median split did not drastically alter the relations between CAR and pain reports in the current study (data not shown). It remains unknown whether the use of cluster analysis, as opposed to median split, in our previous study might have produced different patterns of association between CAR and pain ratings. It is important to note that the superiority of cluster analysis to median spilt procedures, or vice versa, remains a topic of debate. A second explanation might be related to the time frame for when CAR is assessed in relation to laboratory pain testing. In the current study CAR was assessed prior to completion of the laboratory pain testing procedures; however, CAR was assessed following completion of pain testing in our groups’ previous investigation. This is a potentially important difference in methodology because CAR samples collected prior to pain testing would seemingly be more effective than samples collected after pain testing for capturing the influence of individuals’ anticipation of their research involvement and any resulting distress. Recently, it has been speculated that anticipation of daily social demands may be essential in regulating the CAR magnitude for that particular day.4 It may be that an enhanced cortisol response to awakening acted as a marker for stress sensitivity and/or the stressful anticipation of the impending experimental pain task that was experienced later that day. Anticipatory stress could explain, at least in part, why individuals with increased CAR reported greater ratings of experimentally-evoked acute pain relative to those with a flattened CAR. It is unlikely that pain ratings were meaningfully impacted by differences across CAR groups for chronic stress history or by differences attributable to the three different pain tasks. This is because reports of perceived stress over the past month were virtually identical between the two CAR groups and the type of experimental pain task completed was statistically controlled during data analysis.
The role of the CAR in modulating physiological responses to stress has not been clearly defined and no previous study, to our knowledge, has examined the association of CAR with HPA axis or inflammatory (re)activity following acute pain. In this study, salivary cortisol was found to be significantly increased from baseline following acute pain stimulation, while the sTNFαRII response was significantly decreased. The finding of an enhanced cortisol response to acute painful stimulation is consistent with previous studies;46,47 however, the concomitant suppression of the inflammatory cytokine receptor (i.e., sTNFαRII) response contrasts previous studies demonstrating elevated markers of inflammation following experimental pain.48,49 In these studies,48,49 the most significant enhancements in inflammatory cytokines following experimental pain induction were demonstrated 60 minutes post-pain and beyond. Unfortunately, nothing can be discerned about sTNFαRII responses beyond the 35 minute mark in the present study. Contrary to our hypothesis, the patterns of physiological response were not found to significantly differ as a function of increased versus flattened CAR. However, the cortisol and sTNFαRII responses for the increased CAR cohort were generally more pronounced compared to the responses of the flattened CAR cohort. Despite the lack of support in the current study, it cannot be fully concluded that CAR is unassociated with cortisol and sTNFαRII responses to acute pain at this time. Particularly because the effect sizes for the CAR group by time interaction were found to be moderate for cortisol (η2 = 0.066) and sTNFαRII (η2 = 0.050); however, the current study appears to have been insufficiently powered to detect these effects (observed power = 0.19 and 0.45, respectively). The lack of statistical power for examining interaction effects is most likely related to the present study’s small sample size (N = 36). Also because of the small sample size, it was not feasible to examine the associations (i.e., zero-order correlations) among perceived stress, pain reports, and physiological responses within each CAR cohort. It will be important for future studies attempting to expand upon our work to include larger samples sizes with appropriate statistical power in order to further elucidate the relations among CAR and physiological response to painful stressors.
The present study cannot answer but merely speculate on the potential psychosocial and behavioral factors that might help to explain the association of CAR with experimental pain responses as observed here. For example, several studies have shown that higher levels of depressive symptoms were associated with a greater CAR,9 particularly for participants in the nonclinical range of depression.50 Furthermore, CAR and depressive symptoms were significantly positively correlated with measures of chronic and acute stress perception.50 Negative mood and depression have previously been established as reciprocally linked with more severe experiences of pain in clinical and experimental studies.7 Taken together, it seems plausible that the group of individuals with increased CAR in the current study may have also experienced greater depressive symptoms (albeit in the nonclinical range) compared to the flattened CAR group. This possibility may help explain why the increased CAR group rated their pain experiences as more severe. Another potential factor that may affect the association of CAR with pain is sleep behaviors. Several past studies have reported that early morning awakening, perhaps as a proxy for shortened sleep time, was associated with a larger CAR;51,52 however, a minority of studies have disagreed with this association.53 Much like depressive symptoms, sleep disturbances and poor sleep quality have also been established as bi-directionally linked with greater experimental and clinical pain severity.54 Therefore, it stands to reason that poor sleep may represent an underlying mechanism affecting the relationship between increased CAR and greater pain ratings observed in the current study; however, this hypothesis currently awaits investigation.
The current study possesses several limitations that should be considered when interpreting the findings. First, this cross-sectional study, with observational data, was not designed to establish causal relations; however, it does preliminarily associate greater experimental pain ratings with increased CAR. The current study does not invalidate our group’s previous study, which demonstrated an association between flattened CAR and pain ratings. Rather, these mixed results underscore the need for additional research to clarify the association of CAR with pain in healthy samples. Another potential limitation is that the current study did not account or control for the menstrual cycle phase of female participants. Recently, it has been shown that CAR was significantly greater during ovulation compared to the luteal and follicular phases.55 This suggests that the association of CAR and pain ratings in the current study may have possibly been affected by female participants’ menstrual cycle. Although this possibility was unknown to our group at the time of the data collection (2007–2008), future CAR studies may wish to account for the menstrual cycle. Lastly, this study was only able to sample a single soluble receptor of an inflammatory cytokine (sTNFαRII). Simultaneous assessment of many actual cytokines in a biological sample would provide more comprehensive information rather than assessing the receptor of a single cytokine.56 The systemic measurement of multiple cytokines in oral fluids, plasma, and/or serum may provide a more detailed indication of the associations among CAR and inflammatory responses following acute painful stimulation.57
In conclusion, this study suggests that a greater cortisol response to awakening, measured prior to acute pain stimulation, is associated with greater intensity and unpleasantness ratings. However, support is not provided for the association of CAR with cortisol or sTNFαRII responses to acute pain. Future research should attempt to replicate the current study with particular focus on HPA axis and inflammatory responses to painful stimulation. This is because CAR may actually be associated with physiological responses to painful stimulation, yet the current study simply lacked an adequate sample size and statistical power to detect such associations. Future studies of CAR and pain may also wish to expand upon the present findings by concurrently examining health-related psychosocial and behavioral factors such as depressed mood and disturbed sleep. Teasing apart the inter-relations among CAR, pain, mood, and sleep may help to further characterize the role of CAR in the experience of pain.
Acknowledgments
Funding and support for this study and manuscript preparation was provided by NIH/NCCAM Grant R21AT003250-01A1 (L.McGuire) and NIH Training Grant T32NS045551-06 (B. Goodin).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsigos C, Chrousos GP. Hypothalmic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9(2):122–145. doi: 10.1016/j.jpain.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirschbaum C, Hellhammer D. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 4.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Hucklebridge F, Clow A, Rahman H, et al. The cortisol response to normal and nocturnal awakening. J Psychophysiol. 2000;14:24–28. [Google Scholar]
- 6.Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Blackburn-Munro G, Blackburn-Munro R. Chronic pain, chronic stress and depression: coincidence or consequence? J Neuroendocrinol. 2001;13:1009–1023. doi: 10.1046/j.0007-1331.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 8.Blackburn-Munro G, Blackburn-Munro R. Pain in the brain: are hormones to blame? Trend Endocrinol Met. 2003;14(1):20–27. doi: 10.1016/s1043-2760(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 9.Bhagwagar Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology (Berl) 2005;182:54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- 10.Huber TJ, Issa K, Schik G, et al. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology. 2006;31:900–904. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology. 2004;29:1184–1191. doi: 10.1016/j.psyneuen.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Roberts AD, Wessely S, Chalder T, et al. Salivary cortisol response to awakening in chronic fatigue syndrome. Br J Psychiatry. 2004;184:136–141. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- 13.Gaab J, Baumann S, Budnoik A, et al. Reduced reactivity and enhanced negative feedback sensitivity of the hypothalamus-pituitary-adrenal axis in chronic whiplash-associated disorder. Pain. 2005;119:219–224. doi: 10.1016/j.pain.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Anderson RU, Orenberg EK, Chan CA, et al. Psychometric profiles and hypothalamic-pituitary-adrenal axis function in men with chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2008;179:956–960. doi: 10.1016/j.juro.2007.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoromi S, Muniyappa R, Nackers L, et al. Effects of chronic osteoarthritis pain on neuroendocrine function in men. J Clin End Metab. 2006;91:4313–4318. doi: 10.1210/jc.2006-1122. [DOI] [PubMed] [Google Scholar]
- 16.Fabian LA, McGuire L, Page GG, et al. The association of the cortisol awakening response with experimental pain ratings. Psychoneuroendocrinology. 2009;34:1247–1251. doi: 10.1016/j.psyneuen.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland-Fischer P, Greisen J, Grofte T, et al. Increased energy expenditure and glucose oxidation during acute nontraumatic skin pain in humans. Eur J Anaesthesiol. 2009;26(4):311–317. doi: 10.1097/EJA.0b013e328324b5e9. [DOI] [PubMed] [Google Scholar]
- 18.Isowa T, Ohira H, Murashima S. Reactivity of immune, endocrine and cardiovascular parameters to active and passive acute stress. Biol Psychol. 2004;65:101–120. doi: 10.1016/s0301-0511(03)00115-7. [DOI] [PubMed] [Google Scholar]
- 19.Sjors A, Larsson B, Karlson B, et al. Salivary cortisol response to acute stress and its relation to psychological factors in women with chronic trapezius myalgia-a pilot study. Psychoneuroendocrinology. 2010;35(5):674–685. doi: 10.1016/j.psyneuen.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Kronfli T, Haythornthwaite JA, et al. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140(1):135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X-M, Hamza M, Wu T-X, et al. Upregulation of IL-6, IL-8, and CCL2 gene expression after acute inflammation: correlation to clinical pain. Pain. 2009;142(3):275–283. doi: 10.1016/j.pain.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korte SM, Kookhaas JM, Wingfield JC, et al. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 24.Diez-Ruiz A, Tilz GP, Zangerle R, et al. Soluble receptors for tumor necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 25.Thorn L, Hucklebridge F, Evans P, et al. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31:1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 27.Lasikiewicz N, Hendrickx H, Talbot D, et al. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: associations with sleep quality and metabolic parameters. Psychoneuroendocrinology. 2008;33:143–151. doi: 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Walsh N, Schoenfeld L, Ramamurth S, et al. Normative model for cold pressor test. Am J Phys Med Rehab. 1989;68:6–11. doi: 10.1097/00002060-198902000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Greenspan JD, Roy EA, Caldwell PA, et al. Thermosensory intensity and affect throughout the perceptible range. Somatosens Mot Res. 2003;20:19–26. doi: 10.1080/0899022031000083807. [DOI] [PubMed] [Google Scholar]
- 30.Fillingim RB, Ness TJ, Glover TL, et al. Morphine responses and experimental pain: Sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Breivik H, Borchgrevnik PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 32.Dickerson SS, Kemeny ME, Aziz N, et al. Immunological effects of induced shame and guilt. Psychosom Med. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- 33.Nishanian P, Aziz N, Chung J, et al. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clin Diagn Lab Immunol. 1998;5:507–512. doi: 10.1128/cdli.5.4.507-512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health. Newbury Park, CA: Sage; 1988. pp. 31–67. [Google Scholar]
- 37.Pruessner JC, Kirschbaum C, Meinlschmid G, et al. Two formulas for computation of area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 38.Jain AK. Data clustering: 50 years beyond k-means. Pattern Rec Lett. 2010;8(1):651–666. [Google Scholar]
- 39.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum; 1988. [Google Scholar]
- 40.Geiss A, Varadi E, Steinbach K, et al. Psychoneuroimmunological correlates of persisting sciatic pain in patients who underwent discectomy. Neurosci Lett. 1997;237:65–68. doi: 10.1016/s0304-3940(97)00810-0. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 42.Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: integrating models of stress from the social and life sciences. Psychol Rev. 2010;117(1):134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52 supp 2:10–16. doi: 10.1016/s0026-0495(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 44.Jain AK, Duin RPW, Mao J. Statistical pattern recognition: a review. IEEE Trans Pattern Anal Mach Intell. 2000;22(1):4–37. [Google Scholar]
- 45.Williams DA, Keefe FJ. Pain beliefs and the use of cognitive-behavioral coping strategies. Pain. 1991;46:185–190. doi: 10.1016/0304-3959(91)90074-8. [DOI] [PubMed] [Google Scholar]
- 46.al'Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- 47.Gluck ME, Geliebter A, Hung J, et al. Cortisol, hunger, and desire to binge eat following a cold stress in obese woman with binge eating disorder. Psychosom Med. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- 48.Edwards RR, Kronfli T, Haythornthwaite JA, et al. Association of catastrophizing with interleukin-6 responses to acute pain. Pain. 2008;140:135–144. doi: 10.1016/j.pain.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roupe van der Voort C, Heijnen CJ, Wulffraat N, et al. Stress induces increases in IL-6 production by leucocytes of patients with the chronic inflammatory disease juvenile rheumatoid arthritis: a putative role for alpha(1)-adrenergic receptors. J Neuroimmuno. 2000;110:223–229. doi: 10.1016/s0165-5728(00)00328-3. [DOI] [PubMed] [Google Scholar]
- 50.Pruessner M, Hellhammer D, Pruessner J, et al. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- 51.Wust S, Wolf J, Hellhammer DK, et al. The cortisol awakening response – normal values and confounds. Noise Health. 2000;2(7):79–88. [PubMed] [Google Scholar]
- 52.Federenko I, Wust S, Hellhammer D, et al. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 53.Hucklebridge FH, Clow A, Abeyguneratne T, et al. The awakening cortisol response and blood glucose levels. Life Sci. 1999;64:931–937. doi: 10.1016/s0024-3205(99)00019-3. [DOI] [PubMed] [Google Scholar]
- 54.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–132. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 55.Wolfram M, Bellingrath S, Kudielka BM. The cortisol awakening response (CAR) across the female menstrual cycle. Psychoneuroendocrinology. 2011;36:905–912. doi: 10.1016/j.psyneuen.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 56.Prabhakar U, Eirikis E, Reddy M, et al. Validation and comparative analysis of a multiplexed assay for the simultaneous quantitative measurement of Th1/Th2 cytokines in human serum and human peripheral blood mononuclear cell culture supernatants. J Immunol Meth. 2004;291:27–38. doi: 10.1016/j.jim.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Sachdeva N, Yoon HS, Oshima K, et al. Biochip array-based analysis of plasma cytokines in HIV patients with immunological and virological discordance. Scand J Immunol. 2007;65:549–554. doi: 10.1111/j.1365-3083.2007.01906.x. [DOI] [PubMed] [Google Scholar]