Abstract
Myeloproliferative disorders (MPDs), lymphoproliferative disorders (LPDs), acute T-lymphocytic or myeloid leukemia and T-lymphocytic lymphoma were developed in inducible Pten-knockout (Pten−/−) mice. The appearance of these multiple diseases in one animal model provides an opportunity to study the pathogenesis of multiple diseases simultaneously. To study whether Myc function is required for the development of these hematopoietic disorders in Pten−/− mice, we generated inducible Pten/Myc double-knockout mice (Pten−/−/Myc−/−). By comparing the hematopoietic phenotypes of these double-knockout mice with those of Pten−/− mice, we found that both sets of animals developed MPDs and LPDs. However, none of the compound-mutant mice developed acute leukemia or lymphoma. Interestingly, in contrast to the MPDs which developed in Pten−/− mice which are dominated by granulocytes, megakaryocytes predominate in the MPDs of Pten−/−/Myc−/− mice. Our study suggests that the deregulation of PI3K/Akt signaling in Pten−/− hematopoietic cells protects these cells from apoptotic cell death, resulting in chronic proliferative disorders. But due to the differential requirement for Myc in granulocyte as compared to megakaryocyte proliferation, Myc deletion converts Pten−/− MPDs from granulocyte-dominated to megakaryocyte-dominated conditions. Myc is absolutely required for the development of acute hematopoietic malignancies.
Keywords: leukemia, lymphoma, MPD, lymphadenopathy, Pten, c-Myc
INTRODUCTION
Myc is a typical oncoprotein which is involved in controlling a variety of cell behaviors including cell cycle progression, proliferation, differentiation, survival, adhesion, and control of cell size.1,2 In hematopoietic cells, Myc expression can be induced by hematopoietic cytokines and is responsible for mitogen-induced proliferation. Studies have suggested that Myc is a common downstream target for most leukemogenic oncogenes.3 Deregulation of Myc, which induces high levels of proliferation and blocks the full differentiation of hematopoietic progenitor cells (HPCs), might be a key event in the pathogenesis of most human hematopoietic malignancies, including MPDs, acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL) and lymphoma.3–6 Pharmacologic or genetic inhibition of Myc can repress the proliferation and induce the differentiation of leukemic blasts as shown by in vitro studies. Thus, Myc has been recognized as a potential molecular target for anti-leukemia drug development.7Myc transgenic mice develop different types of leukemias depending upon the promoter used to drive Myc expression. Transgenic over-expression of Myc in B and T cells leads to the development of B and T cell leukemias and lymphomas, respectively, in mice.8,9 Over-expression of Myc in HSC/Ps resulted in a significantly increased production of HPCs and excessive mature myeloid cells, a phenotype characteristic of MPDs, which was followed by AML or by T- or B- ALL development.10 Transgenic over-expression of Myc in megakaryocytic/erythroid progenitors (MEPs), which are progenitors for both megakaryocytes and erythrocytes, leads to the development of erythroid leukemia.11 Despite these extensive studies of the Myc protein, due to the co-expression of N-Myc and other potentially functionally redundant members of the Myc protein family in HPCs,12 it remains unclear whether elimination of Myc is sufficient to completely inhibit the development of chronic and acute leukemias.
The PI3K/Akt signaling pathway mediates proliferation, survival and migration signaling in hematopoietic cells stimulated by hematopoietic cytokines. As with Myc deregulation, constitutive activation of PI3K/Akt signaling promotes the uncontrolled growth of hematopoietic cells which is commonly detected in MPDs, AML, ALL and lymphomas, accounting for practically all types of human hematopoietic tumors.13 Mice with transgenic over-expression of a constitutively active form of Akt initially develop MPDs which are followed by AML and/or T-cell lymphoma.14 Because of the significant impact on the pathogenesis of hematopoietic malignancies, molecules that are involved in mediating PI3K/Akt signaling have also been identified as therapeutic targets in the development of anti-leukemic drugs.15 It was known that PI3K/Akt signaling stabilizes the Myc protein by inducing GSK3β-mediated phosphorylation or by inhibiting PP2A-mediated dephosphorylation.16,17 Levels of Pten protein (phosphatase and tensin homolog, deleted on chromosome ten), a negative regulator of PI3K/Akt signaling, are negatively correlated with Myc levels in many T-ALL patient samples.17 However, it is still unknown whether the proliferation and survival of leukemic blasts induced by activated PI3K/Akt signaling is dependent upon Myc activity.
It was found that Pten inactivation is one of the major causes of the constitutive activation of PI3K/Akt signaling in leukemia.18–22 In addition to mutations which were commonly found in T cell leukemia/lymphoma,20,23,24 the expression and activity of Pten are down-regulated in most myeloid and lymphocytic leukemia samples by multiple different mechanisms, such as epigenetic repression25 or post-translational inhibition. It was reported that the phosphatase activity of Pten is negatively regulated by phosphorylation of its c-terminal tail.26 Interestingly, the phosphorylated form of Pten was detected in leukemic blasts from most leukemic samples, including AML, chronic lymphocytic leukemia, and the Pten-expressing T-ALL. Thus, Pten phosphorylation correlates well to the increased PI3K/Akt activity in the same leukemia samples.19,20,22
Pten is expressed in most hematopoietic cells, including hematopoietic stem cells and progenitors (HSC/Ps). Mice with Pten deletion in HSC/Ps develop MPDs, as shown by significantly enhanced granulocyte expansion and tissue infiltration, which progress to acute myelocytic leukemia (AML) or acute T-lymphocytic leukemia (T-ALL) after cells sustain additional mutations.27–29 T cell-specific Pten−/− mice develop LPDs and then a fatal CD4+/CD8− lymphoma after a 3 to 4 month latency period. Additional mutations are required for these lymphoproliferative pre-malignant conditions to progress to full malignancy.30 The Myc gene has been found to be affected by such additional mutations.29 The progression of these diseases observed in Pten−/− mice is reminiscent the progression of human MPD to leukemia and of LPD transformation to lymphoma.
We found that all four types of hematopoietic disorders, MPDs, LPDs, leukemia and lymphoma, developed in our interferon (IFN)-inducible Pten−/− mice depending upon how the mice were maintained. To investigate whether Myc is required for the activated PI3K/Akt-induced proliferation and survival of hematopoietic cells, and to study whether Myc is required for the development of MPDs and polyclonal LPDs, as well as whether Myc is essential for leukemia/lymphoma transformation in Pten−/− mice, we generated IFN-inducible Pten/Myc double-knockout mice (Pten−/−/Myc−/−) in order to assess whether the deletion of Myc is able to prevent the development of these pre-malignancies and malignancies in Pten−/− mice. We found that, as is the case with Pten−/− mice, Pten−/−/Myc−/− mice develop MPDs and LPDs. However none of our Pten−/−/Myc−/− mice developed acute leukemia or lymphoma.
MATERIALS AND METHODS
Generation of inducible Pten−/−/Myc−/− and I/ZEG reporter mice
All experiments were performed in strict accordance with Loyola University IACUC protocol #06-013. Mycfx/fx mice31 were kindly provided by Dr. Frederick W. Alt of the Howard Hughes Medical Institute and Children's Hospital. Ptenfx/fx mice32 were kindly provided by Dr. Tak W. Mak of the Campbell Family Institute for Breast Cancer Research, University of Toronto, Canada. Mycfx/fx mice were first crossed with Mx1Cre mice33 to generate inducible Mx1Cre+Mycfx/fx mice. Mx1Cre+Mycfx/fx mice were then crossed with Ptenfx/fx mice to generate Mx1Cre+Ptenfx/fx/Mycfx/fx mice. In order to generate I/ZEG reporter mice, Mx1Cre mice were crossed with I/ZEG mice34 to produce Mx1Cre+I/ZEG compound transgenic mice. Genotyping was performed by tail biopsy using a PCR assay with primers listed in Supplementary Table 1.
Hematopoietic phenotype analysis
After mice were sacrificed at the indicated times, peripheral blood (PB), bone marrow (BM), spleens, thymus glands and lymph nodes were collected. Cellular components of the PB of mutant mice were examined by CBC. The percentages and absolute numbers of different lineages of hematopoietic cells in BM, spleens, thymuses and lymph nodes were also examined by different combinations of cell-surface marker staining as indicated in the text and by flow cytometric analysis as described.27,35
BM transplantation
To study whether Pten−/−/Myc−/− HSC/Ps can generate MPDs in recipient mice and whether Pten−/−/Myc−/− MPDs would progress to acute malignancies, we transplanted 5×106 BM mononuclear cells (MNCs) from Pten−/−/Myc−/− mice (CD45.2 background) into lethally-irradiated recipient mice (CD45.1 background) with or without support BM cells (CD45.1 background). Mice were monitored for signs of leukemia development. BM MNCs from Pten−/−/Myc+/− mice were transplanted in parallel as controls. Leukemia was diagnosed by detection of leukemic blasts in PB and BM, as well as by observing liver/kidney infiltration. The donor origin of the leukemic cells was confirmed by CD45.2 staining.
q-PCR and qRT-PCR
DNA was extracted by phenol/chloroform extraction. RNA was isolated using RNeasy Plus® Mini kit (Qiagen). cDNA was prepared using SuperScript® First-Strand Synthesis System (Invitrogen). Quantitative PCR for detecting Myc and Pten DNA copies as well as Myc and Pten mRNA expression levels were conducted by using SYBR Green PCR Master® (Applied Biosystems). Information concerning the primers used can be found in Supplementary Table 1. Triplicate RT-PCRs were performed.
Ach-E staining
Fresh-frozen spleen sections were fixed in ice-cold acetone for 5 minutes. After drying at room temperature, tissue sections and colony slides were stained with freshly-prepared acetylcholinesterase staining solution containing 0.5mg/ml acetylthiocholiniodide, 5mM sodium citrate, 3 mM copper sulfate, 0.5 mM potassium ferricyanide in 0.1 M sodium phosphate buffer. After a 3 hour incubation at room temperature, the staining solution was poured off and sections were fixed in 95% ethanol for 10 minutes. Slides were then counterstained with Harris' hematoxylin solution for 30 seconds.
Histologic analysis
WT and mutant mice were sacrificed, and spleens, livers and kidneys were immediately collected. After 2–3 days fixation in zinc formalin, spleen and liver tissues were embedded in paraffin. Sections were cut for H & E staining or antibody immunostaining.
BrdU incorporation
Four hours prior to being sacrificed, mice were injected with BrdU at 50μg/g body weight. The percentages of BrdU+ cells among CD4+ lymphocytes in lymph nodes, Gr1+ granulocytes in BM, and CD41+ megakaryocytes (Mks) in spleens were assessed by cell-surface marker staining followed by cell permeabilization and BrdU antibody staining, as previously reported. BrdU in situ histologic staining was conducted as previously reported.35
Activation-induced cell death of T-lymphocytes
CD3+ T-lymphocytes were purified from spleens of WT, Pten−/−/Myc−/+ and Pten−/−/Myc−/− mice 30 days after polyI:C injections. A total of 2 ×106 cells were cultured at 37°C. in 24-well plates in 2 ml RPMI 1640 medium containing 10% fetal bovine serum, soluble anti-CD3ε (clone 145-2C11; 3 μg/ml), and murine rIL-2 (R&D; 25 U/ml) for 48 hr. to induce activation. Three hours before being harvested, half of the cells were treated with BrdU (10 μM final concentration) for pulse labeling. Activation-induced proliferation was examined by APC-conjugated BrdU antibody staining and flow cytometric analysis. Activation-induced Fas expression was detected by APC-conjugated CD95 antibody staining and flow cytometric analysis. The remaining half of the activated T cells was harvested, washed, and 0.5 × 106/ml of them were replated into 96-well plates with rIL-2, followed by 10ng/ml TNFα (e-Bioscience, Cat.14-8321) or 20ng/ml of FasL (BD Bioscience, clone Jo2, Cat. 554254), together with 2μg/ml of protein G for stimulation of apoptosis. Twenty-four hours post-stimulation, 50 uM propidium iodide (PI) was added to the cells for 15 minutes at room temperature. Cell viability was determined by flow cytometric analysis of the percentage of PI− cells.
Fas-induced granulocyte death
Gr1+ granulocytes were isolated from BM of WT, Pten−/−/Myc−/+ and Pten−/−/Myc−/− mice 30 days after polyI:C injections and incubated in RPMI 1640 medium containing 10% fetal bovine serum and 20ng/ml of GM-CSF with or without 20ng/ml of FasL. After 12 hr. of incubation, cells were stained with PI followed by flow cytometric analysis for cell viability.
Cell migration assay
CD4+ T-lymphocytes were isolated from spleens and Gr1+ granulocytes were isolated from BM of WT, Pten−/−/Myc−/+ and Pten−/−/Myc−/− mice 30 days after polyI:C injections. The migration of these cells was examined in 96-transwell plates by assessing their response to 300ng/ml of SDF1 3 hr. after initiation of incubation.27
Statistical analyses
T-tests were performed to assess the statistical significance of observed changes.
RESULTS
Early development of polyclonal lymphadenopathy in IFN-inducible Pten−/− mice
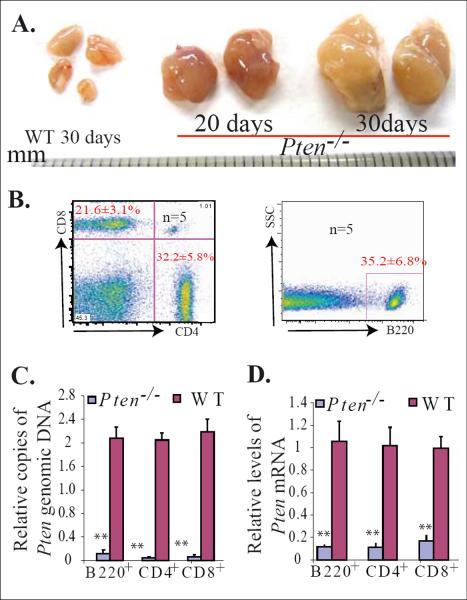
Lymphadenopathy developed in Pten+/− mice and T cell-specific Pten−/− mice at 4 to 8 months and 6–8 weeks of age, respectively.32,36 We have reported that IFN-inducible Pten−/− mice (Mx1Cre+Ptenfx/fx in which Pten was deleted in almost all hematopoietic cells, including HSC/Ps and lymphocytes upon polyI:C injection) develop significant MPDs and die 25 to 40 days after induction of Pten gene deletion.27 By further analysis, we found that these inducible Pten−/− mice also develop the same lymphadenopathy as early as 20 days after the induction of Pten mutation, as demonstrated by enlarged lymph nodes due to lymphocytic hyperplasia (Fig. 1A). Lymphadenopathy affects mainly the submandibular and axillary lymph nodes. At the time of death, most lymph nodes were between 0.4 and 1 cm. in size, the average size being 0.6 ± 0.35 cm. in diameter. The significant increase in both B-lymphocytes (B220+) and T-lymphocytes (CD4+ or CD8+) in the enlarged lymph nodes suggests that it is a polyclonal lymphoproliferative feature (Fig. 1B). Pten deletion in these B- and T-lymphocytes was confirmed by q-PCR to detect Pten copy number and mRNA expression levels (Fig. 1C & D).
Figure 1.
Early development of lymphadenopathy in inducible Pten−/− mice. Mx1Cre+Ptenfx/fx mice and their littermate Mx1Cre−Ptenfx/fx controls (WT) were injected with polyI:C at age 3 weeks, once every other day for a total of five injections. Lymph nodes were collected 20 or 30 days after the last polyI:C injection. A. Significantly enlarged lymph nodes observed in Pten−/− mice. B. The enlarged lymph nodes contain a mixture of T (CD4+ or CD8+) and B (B220+) lymphocytes, as shown by flow cytometric analysis. C & D. Pten deletion in T and B lymphocytes isolated from enlarged lymph nodes was confirmed by q-PCR and qRT (reverse transcription)-PCR to detect Pten genomic DNA (C) and Pten mRNA levels (D), respectively. T and B lymphocytes isolated from lymph nodes of WT mice (Mx1Cre−Ptenfx/fx) were used as controls. The levels of Pten genomic DNA and mRNA in Pten−/− cells were normalized to levels of Pten genomic DNA and mRNA of their WT counterparts. Triplicate experiments were performed. ** indicates statistical significance compared to WT mice.
Spontaneous Pten deletion in a small percentage of hematopoietic cells of Mx1Cre+ Ptenfx/fx mice due to the leakage of Cre expression is sufficient to induce T-cell lymphoma development
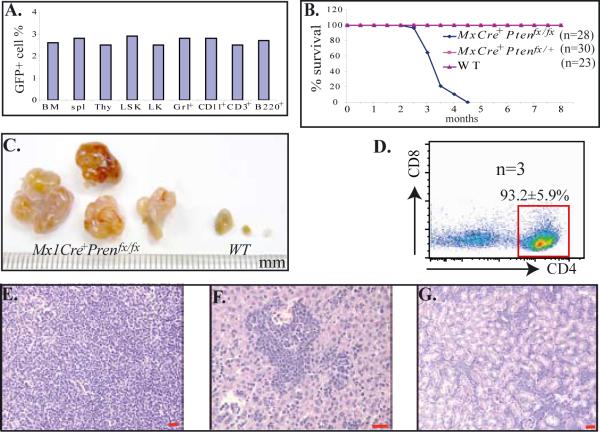
The Mx1Cre mice we used in our studies are an IFN-inducible Cre line.33 Due to the existence of basal background levels of IFN in mice during conditions of normal homeostasis, low levels of leakage were expected when using this mouse line to generate conditional-knockout mice. To examine the percentage of leakage of this Cre line in hematopoietic tissues, we crossed a Cre mouse with an I/ZEG reporter mouse.34 One month after birth, the Mx1Cre+I/ZEG mice were sacrificed and BM, spleens and thymuses were collected. The percentages of GFP+ cells (which represent the percentage of leakage) in LSK-HSCs, LK-HPs and different lineages of differentiated hematopoietic cells were examined by flow cytometry after cell-surface marker staining. We found that almost the same percentage of GFP+ cells (2–3%) could to be detected in MNCs from BM, spleens and thymuses of Mx1Cre+I/ZEG mice, and almost the same percentage (2–3%) of GFP+ cells were distributed in different subsets of hematopoietic cells examined, including HSCs and HPs (Fig. 2A). These data suggested that basal background levels of endogenous IFN can and do induce Mx1Cre expression and loxp site recombination in a small percentage of hematopoietic cells without any appreciable lineage bias.
Figure 2.
Low percentage of leakage of Mx1Cre in hematopoietic cells induces T-cell lymphoma development in Mx1Cre+Ptenfx/fx mice. A. Leakage of Cre expression in a small percentage of hematopoietic cells of Mx1Cre mice, as shown by reporter mouse assay. An Mx1Cre mouse was crossed with an I/ZEG reporter mouse to generate Mx1Cre+I/ZEG, a compound transgenic mouse. One month after birth, Mx1Cre+I/ZEG mice were sacrificed and BM, spleens and thymus glands were collected. Cells isolated from these tissues were stained with cell surface markers as indicated. The GFP+ cell percentages of different populations of hematopoietic cells, including LSK-HSCs and LK-HPCs, were examined by flow cytometry. B. A cohort of Mx1Cre+Ptenfx/fx, Mx1Cre+Ptenfx/+ and littermate WT control mice was maintained without injection of polyI:C and observed for tumor development and survival. C. Enlarged lymph nodes of Mx1Cre+Ptenfx/fx mice collected 4 months after birth. D. Flow cytometric analysis of lymphoblasts isolated from enlarged lymph nodes of Mx1Cre+Ptenfx/fx mice. E–G. Lymphoblast infiltration into lymph nodes (E), livers (F) and kidneys (G) of Mx1Cre+Ptenfx/fx mice, as shown by histologic sectioning and H & E staining. Bars in E–G equal 100μm.
To study whether this low percentage leakage of Mx1Cre is sufficient to induce hematopoietic malignancy in Mx1Cre+Ptenfx/fx (homozygous) mice, we maintained a cohort of 28 Mx1Cre+Ptenfx/fx, 30 Mx1Cre+Ptenfx/+ (heterozygous) and 23 Mx1Cre−Ptenfx/fx or Mx1Cre−Ptenfx/+ (WT control) mice without injecting polyI:C to monitor leukemia and lymphoma development in them. All 28 Mx1Cre+Ptenfx/fx mice died of CD4+CD8− T cell lymphoma within 3 to 4 months, exactly the same type of lymphoma reported in T cell-specific Pten−/− mice (Fig. 2B). All these mice showed significantly enlarged thymuses and lymph nodes (Fig. 2C), as well as splenomegaly, and hepatomegaly (data not shown). As many as 80 to 95% of CD4+ lymphomic blasts could be detected in thymus, spleen, lymph nodes, PB and BM immediately prior to the mouse being euthanized (Fig. 2D). Multiple tissue infiltration of lymphomic blasts was observed, as shown by post mortem analysis (Fig. 2E–G). However, none of the Mx1Cre+Ptenfx/+ nor WT control mice developed significant hematopoietic defects, having been monitored for up to 8 months. These data indicated that even the low percentage of hematopoietic cells showing homozygous Pten deletion in Mx1Cre+Ptenfx/fx mice is sufficient to induce the development of T cell lymphoma.
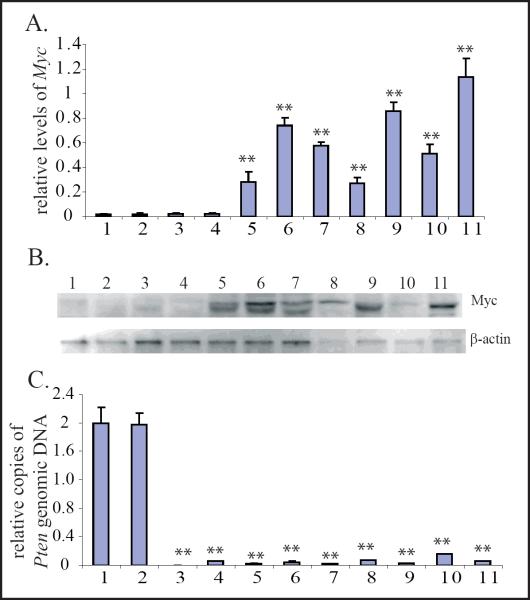
Myc expression is up-regulated in leukemic and lymphomic blasts but not in MPD nor LPD cells of Pten−/− mice
To study whether Myc is involved in MPD development and MPD-to-leukemia transformation, as well as whether it is involved in lymphadenopathy development and T cell lymphoma progression in Pten−/− mice, we examined Myc expression in Gr1+ cells isolated from the BM of WT, Pten−/− MPD and Pten−/− AML mice, as well as in CD4+ cells isolated from lymph nodes of WT, Pten−/− lymphadenopathy, and Pten−/− lymphoma mice and CD4+ blasts from BM of Pten−/− T-ALL mice. We found that both Myc protein and mRNA levels are comparable between Gr1+ cells from WT and Pten−/− MPD mice as well as between CD4+ cells from WT and Pten−/− lymphadenopathy mice, as shown by Western Blot and real-time PCR assays. However, Myc expression levels are significantly increased in Gr1+ AML blasts and CD4+ T-ALL/T-lymphomic blasts in all the samples examined (Fig. 3A & B). These data suggested that deregulation of Myc in the leukemic and lymphomic blasts of Pten−/− mice might be a consequence of additional mutations which may be critical for the development of acute but not chronic hematopoietic malignancies.
Figure 3.
Myc is up-regulated in leukemic and lymphomic blasts but not in cells from MPDs nor LPDs in Pten−/− mice.A & B. Myc mRNA and protein levels were examined in Gr1+ and CD4+ cells isolated from WT, Pten−/− MPD/lymphadenopathy mice, as well as leukemic/lymphomic blasts from Pten−/− mice using qRT-PCR (A) and Western Blot assays (B.) Relative Myc mRNA levels for each sample were normalized to the mRNA levels of its GAPDH gene. Data are a summary of triplicate experiments (A.) C.Pten deletion in Pten−/− cells was confirmed by q-PCR to detect the levels of Pten genomic DNA. Pten DNA levels for each sample were normalized to GAPDH DNA levels first and then normalized to Pten DNA levels of WT Gr1+ granulocytes (Sample 1.) Samples shown in the figure were: 1. Gr1+ granulocytes from BM of WT controls; 2. CD4+ lymphocytes from lymph nodes of WT controls; 3. Gr1+ cells from BM of Pten−/− MPD mice; 4. CD4+ lymphocytes from enlarged lymph nodes of Pten−/−lymphadenopathic mice; 5–7. CD4+ blasts from enlarged lymph nodes of Pten−/− lymphoma mice; 8. Gr1+ leukemic blasts from BM of Pten−/− AML mice; 9–11. CD4+ leukemic blasts from BM of Pten−/− T-ALL mice. ** indicates statistical significance compared to Gr1+ granulocytes from BM of WT controls.
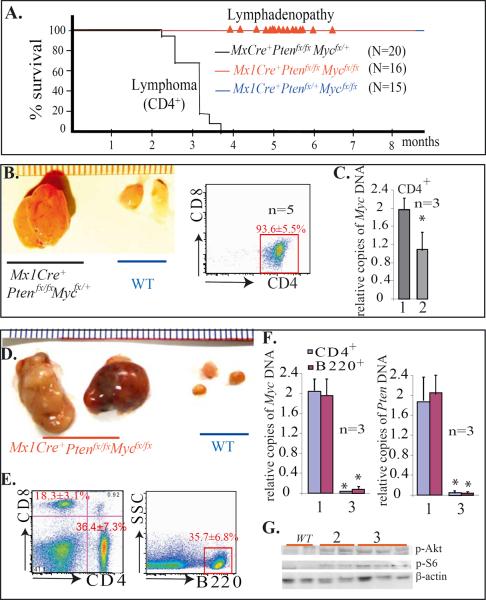
Myc is essential for the pathogenesis of T-cell lymphoma but not for the lymphadenopathy of Pten-mutant mice
To study whether Myc is required for the development of lymphoma in Pten−/− mice, we crossed MxCre+Ptenfx/fx mice with Mycfx/fx mice to generate MxCre+Ptenfx/+/Mycfx/+ and Ptenfx/+/Mycfx/+ mice, which were further crossed to generate MxCre+Ptenfx/fx/Mycfx/fx, MxCre+Ptenfx/fx/Mycfx/+, MxCre+Ptenfx/+/Mycfx/fx and Mx1Cre− WT controls. A cohort of 16 MxCre+Ptenfx/fx/Mycfx/fx mice, 20 MxCre+Ptenfx/fx/Mycfx/+ mice, 15 MxCre+Ptenfx/+/Mycfx/fx mice and 20 Mx1Cre− WT control mice were maintained without injecting polyI:C for long-term observation. All 20 MxCre+Ptenfx/fx/Mycfx/+ mice died of CD4+ lymphoma 3–4 months after birth, just as had occurred with MxCre+/Ptenfx/fx mice (Fig. 4A & B). This suggested that heterozygous deletion of Myc is not sufficient to block lymphoma development in Pten−/− mice. No significant hematopoietic defects were observed in the 15 MxCre+Ptenfx/+/Mycfx/fx mice nor in the 20 Mx1Cre− WT control mice observed over a course of 1 year. However, all 16 MxCre+Ptenfx/fx/Mycfx/fx mice developed lymphadenopathy 5–7 months after birth (Fig. 4A), as demonstrated by enlarged lymph nodes (Fig. 4D), due to the expansion of both T (CD4+ or CD8+) and B (B220+) lymphocytes (Fig. 4E). Lymph nodes were between 1 and 2 cm. in size and averaged 1.6 ± 0.65 cm. in diameter when examined at 8 months of age (Fig. 4D). However, in spite of the high activity of PI3K/Akt signaling in the mutant lymphocytes (Fig. 4G), none of these mice showing LPDs transformed to lymphoma during the course of 12 months of observation. The benign features of LPDs are demonstrated by transplantation studies (Supplementary Data 1). We found that lymphoblasts from MxCre+Ptenfx/fx/Mycfx/+ lymphoma mice were able generate a lethal leukemia-like disease in recipient mice upon transplantation; however, lymphocytes isolated from LPDs of MxCre+Ptenfx/fx/Mycfx/fx mice failed to engraft hematopoietic/lymphoid tissues in recipient mice. These data indicated that Myc is required for lymphoma development but was not necessary for the development of polyclonal LPDs in Pten−/− mice. This notion of the dispensable status of Myc in lymphadenopathy development in Pten−/− mice was further confirmed by studying MxCre+Ptenfx/fx/Mycfx/+ and MxCre+Ptenfx/fx/Mycfx/fx mice after polyI:C injections. We found that, as with MxCre+Ptenfx/fx mice, all MxCre+Ptenfx/fx/Mycfx/+ and MxCre+Ptenfx/fx/Mycfx/fx mice developed lymphadenopathy 20 to 30 days after induction of Pten and Myc gene deletion by polyI:C injection (Supplementary Data 2).
Figure 4.
Myc is essential for the pathogenesis of T-cell lymphoma but not for the LPD of Pten-mutant mice. A. Mx1Cre+Ptenfx/fx/Mycfx/fx, Mx1Cre+Ptenfx/fx/Mycfx/+, and Mx1Cre+Ptenfx/+/Mycfx/fx mice were maintained without polyI:C injection in order to observe for tumor development and overall survival.  Indicates the time of lymphadenopathy development in Mx1Cre+Ptenfx/fx/Mycfx/fx mice. B. CD4+ T cell lymphoma in Mx1Cre+Ptenfx/fx/Mycfx/+ mice. C. Heterozygous deletion of Myc gene in CD4+ lymphomic blasts was confirmed by q-PCR. 1 and 2 represent CD4+ cells isolated from WT lymph nodes and Mx1Cre+Ptenfx/fx/Mycfx/+ lymphoma, respectively. D. Enlarged lymph nodes in Mx1Cre+Ptenfx/fx/Mycfx/fx collected 8 months after birth. E. Polycolonal lymphadenopathy in Mx1Cre+Ptenfx/fx/Mycfx/fx mice as shown by increased mixture of CD4+/CD8+ T lymphocytes and B220+ B lymphocytes in enlarged lymph nodes. F. Homozygous Myc and Pten deletion in CD4+ and B220+ lymphocytes isolated from lymphadenopathy was confirmed by q-PCR assay. 1 and 3 represent CD4+ or B220+ cells isolated from WT lymph nodes and Mx1Cre+Ptenfx/fx/Mycfx/fx lymphadenopathic nodes, respectively. G. p-Akt and p-S6 levels were increased in lymphocytes isolated from the enlarged lymph nodes from both Mx1Cre+Ptenfx/fx/Mycfx/+ (indicated as 2) and Mx1Cre+Ptenfx/fx/Mycfx/fx (indicated as 3) compared to lymphocytes from WT controls.
Indicates the time of lymphadenopathy development in Mx1Cre+Ptenfx/fx/Mycfx/fx mice. B. CD4+ T cell lymphoma in Mx1Cre+Ptenfx/fx/Mycfx/+ mice. C. Heterozygous deletion of Myc gene in CD4+ lymphomic blasts was confirmed by q-PCR. 1 and 2 represent CD4+ cells isolated from WT lymph nodes and Mx1Cre+Ptenfx/fx/Mycfx/+ lymphoma, respectively. D. Enlarged lymph nodes in Mx1Cre+Ptenfx/fx/Mycfx/fx collected 8 months after birth. E. Polycolonal lymphadenopathy in Mx1Cre+Ptenfx/fx/Mycfx/fx mice as shown by increased mixture of CD4+/CD8+ T lymphocytes and B220+ B lymphocytes in enlarged lymph nodes. F. Homozygous Myc and Pten deletion in CD4+ and B220+ lymphocytes isolated from lymphadenopathy was confirmed by q-PCR assay. 1 and 3 represent CD4+ or B220+ cells isolated from WT lymph nodes and Mx1Cre+Ptenfx/fx/Mycfx/fx lymphadenopathic nodes, respectively. G. p-Akt and p-S6 levels were increased in lymphocytes isolated from the enlarged lymph nodes from both Mx1Cre+Ptenfx/fx/Mycfx/+ (indicated as 2) and Mx1Cre+Ptenfx/fx/Mycfx/fx (indicated as 3) compared to lymphocytes from WT controls.
Myc deletion converts Pten−/− MPDs from granulocyte-dominated conditions to megakaryocyte-dominated conditions
We and others reported that Mx1Cre+Ptenfx/fx mice die between 25 to 40 days after polyI:C injection with a typical MPD syndrome, as demonstrated by significantly increased WBCs in PB due to increased granulocytes, as well as splenomegaly/hepatomegaly due to granulocyte infiltration.27–29 We also reported that Myc is required for the proliferation of most lineage-committed hematopoietic progenitors but not megakaryocytic progenitors. Myc knockout mice (Myc−/−) developed a symptom triad of anemia-leukopenia-thrombocytosis due to a significant reduction in the production of granulocytes and erythrocytes and a corresponding significant increase in megakaryopoiesis.37
To study whether Myc is essential for the development of MPDs in Pten−/− mice, we induced deletions of the Pten and Myc genes in a cohort of 18 MxCre+Ptenfx/fx/Mycfx/fx (Pten−/−/Myc−/−), 15 MxCre+Ptenfx/fx/Mycfx/+ (Pten−/−/Myc+/−), and 12 MxCre+Ptenfx/+/Mycfx/fx (Pten+/−/Myc−/−) mice by injection of 250 μg polyI:C/mouse every other day for a total of five injections beginning at the age of 3 weeks. Thirty Mx1Cre− littermate mice were injected and studied in parallel as WT controls. We found that all 18 Pten−/−/Myc+/− mice died 25 to 40 days after the mutations were induced and developed exactly the same MPDs as Pten−/− mice (Fig. 5A–B & Supplementary Data 2–4). This suggested that heterozygous Myc mutations are unable to block MPD development in Pten−/− mice. We also found that Pten heterozygous mutations did not affect the hematopoietic phenotype of the Myc−/− mice. All 12 Pten+/−Myc−/− mice developed the anemia-leukopenia-thrombocytosis triad exactly as the Myc−/− mice had, and survived more than 5 months, as we reported previously. However, all 18 Pten−/−/Myc−/− mice died 25–40 days post-injection with severe anemia (much more so than Myc−/− mice) and thrombocytosis (Fig. 5A & Supplementary Data 2–3). However, WBC counts were comparable to those of WT controls despite the greater range in the counts, as indicated by high standard deviations (Fig. 5A).
Figure 5.
MPDs in Pten−/−/Myc−/− mice are dominated by CD41+ Mks. Three weeks after birth, Mx1Cre+Ptenfx/fx/Mycfx/fx, Mx1Cre+Ptenfx/fx/Mycfx/+, Mx1Cre+Ptenfx/+/Mycfx/fx, and WT littermate control mice at were injected with 250 μg polyI:C every other day for a total of 5 injections to induce gene deletions. PB, spleens, BM and livers were collected for phenotypic analysis 25 to 30 days after polyI:C injections. A. Hemoglobin concentration (Hb), white blood cell count (WBC) and platelet number (Plt) in PB were analyzed by CBC. B. Splenomegaly and hepatomegaly in Pten−/−/Myc−/− mice, as shown by increased mass of spleens and livers. 1, 2 and 3 are spleens from WT, Pten−/−/Myc+/− and Pten−/−/Myc−/− mice, respectively. C–I. Dominant growth of small-sized Mks in Pten−/−/Myc−/− spleens, as shown by flow cytometric analysis (C–E) and Ach-E-stained spleen sections (F–I.) The smaller cell size of CD41+ MKs of Pten−/+/Myc−/−and Pten−/−/Myc−/− mice was shown by flow cytometric forward scatter (E.) J. Individual Mks interspersed among the hepatocytes in the livers of Pten+/−/Myc−/− mice. K–M. Mk infiltration into the livers of Pten−/−/Myc−/− mice, as shown by H & E staining (K) and confirmed by Ach-E staining (L.) Infiltrating Mks are actively proliferating, as shown by BrdU pulse-labeling (M.) ** indicates statistical significance compared to WT mice. $ indicates statistical significance compared to Pten−/−/Myc+/− mice. # indicates statistical significance compared to Pten+/−/Myc−/− mice. Bars equal 100 μm.
In careful post mortem dissections of the mice, we found significant splenomegaly and hepatomegaly in Pten−/−/Myc−/− mice, as demonstrated by increased splenic and hepatic masses, although spleen and liver sizes were not as large as found in Pten−/−/Myc+/− mice (Fig. 5B). Interestingly, in contrast to the case for Pten−/−/Myc+/− mice, which show a significant increase in Gr1+Mac1+ granulocytes in both BM and spleen, we found a slight increase in Gr1+Mac1+ granulocytes and a significant expansion of CD41+ Mks in the spleens of Pten−/−/Myc−/− mice (significantly more than was observed in Pten+/−/Myc−/− mice) (Fig. 5C & D). As was the case with the Mks of Myc−/− mice, the Mks of Pten−/−/Myc−/− mice are also small-sized and of low ploidy (Fig. 5E–I & Supplementary Data 5). Moreover, we found a few individual Mks interspersed among the hepatocytes in the livers of Pten+/−/Myc−/− mice (Fig. 5J), while the Mks (confirmed by Ach-E staining) form clusters in the livers of Pten−/−/Myc−/− mice (Fig. 5K–M). Colonized growth and abnormally high proliferation of Mks in these mice were confirmed by BrdU pulse-labeling (Fig. 5K–M). Small Mks could be observed occasionally in the PB of the double-mutant mice (Supplementary Data 3E). Furthermore, we found significant fibrosis in the spleens of some Pten−/−/Myc−/− mice as shown by reticulin staining (Supplementary Data 6). These data suggested that, in contrast to the MPDs of Pten−/− mice in which granulocytes predominate, Pten−/−/Myc−/− mice develop MPDs in which Mks predominate. This observation is reminiscent of the pathologic changes found in human essential thrombocytosis.
Myc is required for the transformation of MPD to acute leukemia
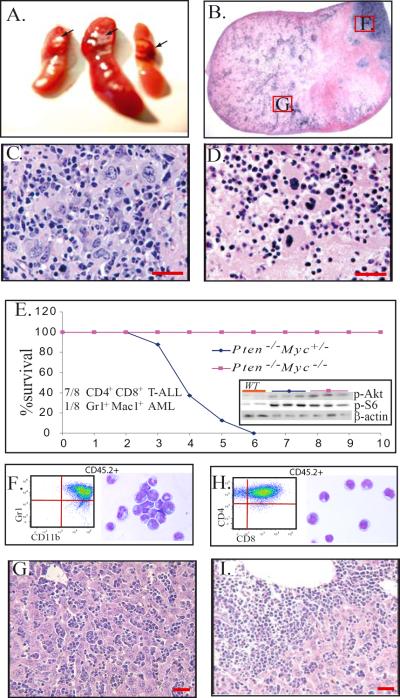
We know that recipient mice which received Pten−/− BM cells developed exactly the same MPDs as the original Pten−/− mice and that these conditions progress to T-ALL or AML when the animals' cells sustain additional mutations within 3 to 4 months after transplantation.27–29 However, we found that the MPDs from Pten−/−/Myc−/− mice were non-transplantable, although increased levels of PI3K/Akt signaling activity were detected in BM cells from Pten−/−/Myc−/− mice comparable to those in BM cells from Pten−/−/Myc+/− mice (Fig. 6E insertion). Lethally-irradiated recipient mice which received 5×106Pten−/−/Myc−/− BM MNCs survived for only 1 to 1.5 months due to failure of hematopoietic reconstitution. The double-gene mutant HSC/Ps generated node-like growths in the spleens of recipient mice, with Mks predominating in these nodes (Fig. 6A–D). Mks located within these nodes were found to be undergoing apoptosis (Fig. 6D). To study whether Myc is required for the transformation of Pten−/− MPDs to acute leukemia, we transplanted BM MNCs from Pten−/−/Myc−/− mice (CD45.2+) into lethally-irradiated recipient mice (CD45.1+), with the support of BM cells from WT mice (CD45.1+). This was done to determine whether or not Myc deletion is able to block leukemic transformation. BM MNCs from Pten−/−/Myc+/− mice (CD45.2+) were transplanted in parallel as positive controls. We found that all mice receiving Pten−/−Myc+/− BM cells die of either CD4+CD8+ T-ALL (7/8) or Gr1+Mac1+ AML (1/8) within 5 months post-transplantation (Fig. 6E–I). However, all 8 recipients that received Pten−/−/Myc−/− BM survived for one year without any signs of leukemia (Fig. 6E), although approximately 1–3% of CD45.2+ cells (Pten−/−/Myc−/− donor origin) could still be detected in the BM of recipient mice at the time of the completion of the experiment (data not shown). These data suggested that Myc is required for the development of acute leukemia in Pten−/− mice.
Figure 6.
Myc is required for acute leukemic transformation of Pten-mutant MPDs. A–D. BM NCs from Pten−/−/Myc−/− mice were transplanted into lethally-irradiated mice. Each recipient mouse received 5×106 mutant BM MNCs without support BM cells. Spleens were collected 1 month after transplantation and analyzed by histologic sections. Node-like growths were observed in the recipient spleens (A arrow and B.) Mks were the major component cells in these nodes (C) and the majority of cells in the middle area of the nodes were undergoing apoptosis (D.) E–I. BM MNCs from Pten−/−/Myc−/+ or Pten−/−/Myc−/− mice (CD45.2+) were transplanted into lethally-irradiated recipient mice (CD45.1+) to examine the leukemic transformation of the mutant cells. Each recipient mouse received 5×106 mutant BM MNCs with the support of 2×105 WT BM cells (CD45.1+). Eight mice were transplanted in each group. Recipient mice were monitored for survival (E) and leukemia development. Insert in Fig. E shows increased p-Akt and p-S6 levels in BM cells from both Pten−/−/Myc−/+ and Pten−/−/Myc−/− mice (3 samples from each) compared to WT controls. F–G. Leukemic blasts from BM of Pten−/−/Myc+/− AML (F) and T-ALL (G) mice were analyzed by flow cytometry by gating on the CD45.2+ donor cells. For morphology studies, CD45.2+ donor derived cells were sorted for cytospins. Representative cytospins of blasts isolated from BM of Pten−/−/Myc+/− AML (F) or T-ALL (G) mice are shown by Wright-Giemsa staining. Figure H–I is a representative liver section from a Pten−/−/Myc+/− AML (H) or T-ALL (I) mouse showing leukemic blast infiltration. Bars equal 100 μm.
Myc is necessary for the proliferation of the Pten−/− granulocytes and lymphocytes but is not essential for their invasiveness nor their resistance to apoptosis
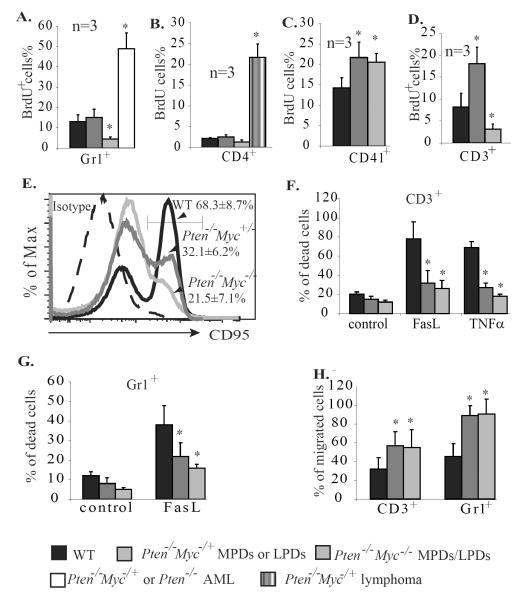
It has been proposed that the high degree of proliferation, increased migration and low apoptotic index of Pten−/− cells might be critical for the development of both pre-leukemic proliferative disorders and leukemia in Pten−/− mice. We wanted to study which of these behavior changes in Pten−/− cells are independent of Myc and are contributory to pre-leukemic proliferative disorders and which changes are Myc-dependent and are essential for leukemic/lymphomic transformation. To do this, we compared the proliferation, apoptosis and migration of Gr1+ cells isolated from the BM of Pten−/− AML, Pten−/−/Myc+/− MPD, Pten−/−/Myc−/− MPD and WT control mice, as well as CD4+ cells from lymph nodes of Pten−/−/Myc+/− lymphoma, Pten−/−/Myc+/− lymphadenopathy, Pten−/−/Myc−/− lymphadenopathy, and WT control mice. We found that the proliferation of Gr1+ and CD4+ cells from Pten−/−/Myc+/− MPDs/LPDs is comparable to that of their counterparts in WT controls, but is significantly increased in the blasts of Pten−/−/Myc+/− AML and lymphomic mice. Homozygous deletion of Myc significantly inhibits the proliferation of both populations of cells, as shown in Pten−/−/Myc−/− MPD and LPD mice (Fig. 7A & B). Interestingly, compared to WT controls, Gr1+ and CD4+ cells from Pten−/− mice are resistant to Fas-induced apoptosis (Fig. 7F & G) due to decreased Fas expression (Fig. 7E), and are more sensitive to SDF1-induced migration (Fig. 7H) independent of the status of the Myc gene. In addition, consistent with our previous reports that the proliferation of Mks is less dependent upon Myc, we found that the proliferation of CD41+ Mks is promoted by Pten deletion even in a Myc−/− background (Fig. 7C and Supplementary Data 7). However the increased apoptosis and low ploidy of Myc−/− Mks is not reversed by Pten deletion (Supplementary Data 5).
Figure 7.
Myc is required for the proliferation but not the survival nor migration of granulocytes and lymphocytes. A–C. All mice were injected with BrdU 4 hr. before being sacrificed. Three mice were injected in each group (for the AML group, 1 mouse with Pten−/−/Myc+/− genotype and 2 with Pten−/− genotype were used). Percentages of cells incorporating BrdU (BrdU+%) in Gr1+ granulocytes from BM (A), CD4+ lymphocytes from lymph nodes (B), and CD41+ Mks from spleens (C) of the mice were analyzed to determine the proliferation of these cells. D–F. CD3+ lymphocytes were isolated from spleens of WT, Pten−/−/Myc+/−, and Pten−/−/Myc−/− mice 25 days after polyI:C injection and were treated with anti-CD3ε and IL-2 to induce activation. Activation-induced proliferation (D) and Fas expression (E) were examined by BrdU pulse-labeling and CD95 antibody staining, respectively. Activation-related cell deaths of CD3+ T cells were analyzed by PI staining 12 hr. after TNFα or FasL stimulation (F.) G. Gr1+ granulocytes were isolated from BM of WT, Pten−/−/Myc+/− and Pten−/−/Myc−/− mice 25 days after polyI:C injection and treated with 20ng/ml FasL for 12 hr. Cell death was analyzed by PI staining and flow cytometry. H. Migration of the CD3+ and Gr1+ cells was analyzed 3 hr. after SDF1 induction. * indicates significant difference compared to WT controls.
DISCUSSION
Inactivation of Pten and/or constitutive activation of PI3K/Akt are commonly found in both human chronic and acute hematopoietic malignancies. Transgenic and knockout animal model studies demonstrated that constitutive activation of PI3K/Akt signaling alone is sufficient to induce MPDs and LPDs, two chronic hematopoietic proliferative disorders. Just as is the case with human MPDs and LPDs, after certain period of latency, AML/ALL or lymphoma develops in the mutant mice upon sustaining addition mutations.27–29,38–40
It was found that PI3K/Akt signaling prevents the degradation of Myc protein, suggesting that Myc might be required for PI3K/Akt signaling-induced cell proliferation and leukemia development.16,17 Interestingly, we found that activation of PI3K/Akt signaling by Pten gene deletion was unable to elevate the levels of Myc protein in hematopoietic cells. In fact, Myc expression is only significantly elevated after acute malignant transformation of Pten−/− cells. Consistent with this observation, our genetic studies demonstrated that Myc protein is absolutely required for acute malignant progression but is not essential for the chronic hyperplasia seen in Pten−/− mice. Consistent with the results of our previous studies, we found that Myc deletion converts Pten−/− MPDs from granulocyte-dominated conditions to Mk-dominated conditions due to the selective requirement for Myc in the proliferation of most lineages of hematopoietic progenitors but not for the proliferation of Mk progenitors.37 In addition, despite the fact that N-Myc is expressed in hematopoietic cells, its levels are not increased in Pten−/− cells at either the chronic proliferative lesion or acute leukemia/lymphoma stages (data not shown). The fact that Pten−/−/Myc−/− mice failed to develop leukemia/lymphoma indicated that endogenous N-Myc cannot replace c-Myc activity during leukemia development in Pten−/− mice. Our studies suggest that PI3K/Akt signaling mainly stimulates a survival signal in hematopoietic cells. The apoptotic resistance of Pten-mutant cells is the major cause of chronic proliferative disorders. Our studies support the notion that Myc is the common target of additional mutations that lead to the progression of chronic proliferative disorders toward the development of acute leukemia/lymphoma.29
The unbiased leakage of Mx1Cre in hematopoietic cells provides a useful tool for leukemia and lymphoma research
The Mx1Cre mouse is an IFN-inducible Cre line. Because it is nearly 100% efficient in inducing loxp site-mediated recombination in hematopoietic cells, including HSCs, upon polyI:C injection, the Mx1Cre mouse has been routinely used to study gene functions in adult hematopoiesis.27–29,35 However, in normal housing conditions, low levels of leakage are expected even in non-polyI:C-injected animals due to basal levels of interferon expression. Approximately 2–3% leakage was detected in this Cre line in all lineages of hematopoietic cells without cell-type bias. By crossing this Cre line with different loxp mouse lines, spontaneous gene recombination event(s) induced by Mx1Cre can be used to turn on or turn off expression of specific gene(s) in a small percentage of hematopoietic cells without any particular cell lineage bias. In fact, this scenario might better reflect the spontaneous nature of actual gene mutations in human hematopoietic cells. Only the mutations that induce the dominant growth of certain populations of hematopoietic cells due to the activation of certain oncogenes or inactivation of certain tumor suppressors will result in hyperplasia or leukemia/lymphoma. By using this Cre line, we generated a Pten-mutant animal model which develops T cell lymphoma under normal housing conditions due to the dominant growth of mutant CD4+ lymphocytes.
Myc is not essential for the development of lymphadenopathy nor MPDs, but is absolutely required for the transformation to acute leukemia and lymphoma in Pten−/− mice
β-catenin is a critical mediator of Wnt signaling, the activity of which is higher in HSCs and might play important roles in HSC self-renewal.41–43 Abnormal activation of β-catenin has been detected in leukemic stem cells (LSCs) in human and murine CML at crisis phase (CML-BC),43,44 as well as in murine Pten−/− T-ALL and AML.29 Heterozygous β-catenin mutation did not block MPD development in Pten−/− mice but significantly inhibited T-ALL/AML transformation in these same mice. These studies suggested that β-catenin might be critical for the transformation of MPD to leukemia and plays critical roles in the self-renewal of LSCs in CML-BC.29,43 Interestingly, Zhao et al. demonstrated that β-catenin is required for the self-renewal of LSCs of Bcr/Abl-induced CML but is not essential for the self-renewal of LSCs of Bcr/Abl-induced acute B lymphocytic leukemia (B-ALL). Homozygous deletion of β-catenin in murine HSC/Ps is able to repress Bcr/Abl fusion protein-induced CML development,42,44 but cannot prevent fusion protein-induced B-ALL development.42 The authors proposed that CML stem cells might originate from normal HSCs which require β-catenin for their long-term self-renewal, whereas B-ALL stem cells might originate from pro-B cells which are independent of β-catenin for their self-renewal. The molecular mechanism for this differential requirement for β-catenin in MPD-leukemia syndromes in Pten−/− mice and CML-B ALL syndromes in Bcr/Abl transgenic mice merits further investigation.
Myc has been identified as a direct target of Wnt-β-catenin signaling. Inactivation of Myc prevents tumorigenesis induced by abnormal Wnt-β-catenin activation in APC-mutant mice. In hematopoietic cells, Myc expression increases rapidly in response to growth factor stimulation,45,46 which is required for the proliferation of the majority hematopoietic cells but not HSCs nor megakaryocytes.37 Mice with Myc-deleted HSCs develop a thrombocytosis-anemia-leukopenia triad of symptoms due to a significant increase in megakaryocytosis and a concomitant reduction in erythropoiesis and myelopoiesis.37 Studies of T cell-specific knockout mice suggested that Myc is required for cell growth and proliferation in immature thymocytes at the pre-TCR checkpoint,47,48 in stage 0 progenitors of invariant Vα14-bearing natural killer T (iNKT) cells,49,50 and in memory CD8+ T cells.51 However, the differentiation and survival of these thymocytes seems not to be impaired in Myc-deficient mice. T lymphocytes with Myc deletion fail to expand in response to T cell receptor signaling, although most active markers are expressed.47
The development of lymphadenopathy in Pten−/−/Myc−/− mice suggested that the polyclonal lymphocytic hyperplasia seen in Pten-mutant animals was not due to increased Myc-dependent proliferation and subsequent increased lymphocyte generation but was a consequence of resistance to apoptosis in these mutant peripheral lymphocytes (peripheral tolerance). We found that thymocyte development in the thymus glands of Pten−/−/Myc−/− mice was exactly the same as was seen in Myc single gene-knockout mice (Supplementary Data 8).37,47 The conversion of Pten−/− MPDs from granulocyte-dominated to Mk-dominated syndromes by Myc deletion supports our previous finding that Mks are less dependent upon Myc for their proliferation than are granulocytes.37 The fact that ALL/AML or lymphoma do not develop in Pten−/−/Myc−/− mice indicates the essential role of Myc in the transformation of these lympho-myeloid hyperplasias to acute malignancies. However, it remains to be determined whether or not Myc is still required for the progression of such acute malignancies after they have developed. In addition, the fact that Pten−/−/Myc−/− MPDs resemble essential thrombocytosis suggests that Mks are significantly expanded. However, none of the recipients receiving Pten−/−/Myc−/− BM developed acute megakaryocytic leukemia due to increased apoptosis of the double-mutant Mks. We are actively investing whether protecting double-gene mutant Mks from apoptosis by introducing pro-survival signals is able to induce acute megakaryocytic leukemia.
Furthermore, germline mutations of the Pten gene cause a group of inherited autosomal dominant disorders called Pten hamartoma-tumor syndrome, including Cowden syndrome (CS), Lhermitte-Duclos disease and Bannayan-Zonana syndrome, all characterized by developmental defects and benign growths in multiple organs (hyperplastic lesions such as hamartomatous polyps of the gastrointestinal tract, mucocutaneous lesions, and trichilemmomas). These hyperplastic lesions show significantly increased risk for malignant transformation.52,53 Studies using tissue-specific Pten-knockout mice (Pten−/−) suggested that inactivation of Pten alone results in benign tumor growth of multiple tissue cells, such as intestinal polyps and prostatic intraepithelial neoplasias.54,55 Additional mutations that result in gain-of-function of oncogenes and/or loss-of-function of other tumor suppressor genes might be absolutely required for the full development of malignancies.27–30,32,54,55 It will be interesting to ascertain whether Myc is also required for the malignant transformation of these Pten-mutant benign tumors.
Supplementary Material
Acknowledgements
We appreciate Dr. Ignacio Moreno de Alborán of the Department of Immunology and Oncology, Centro Nacional de Biotecnología/CSIC, Universidad Autónoma de Madrid, Madrid, Spain for his kindness in providing the Mycfx mouse line. We appreciate Dr.
Tak W. Mak of the Campbell Family Institute for Breast Cancer Research, University of Toronto, Toronto, Canada, for his kindness in providing the Ptenfx mouse line. We appreciate the excellent animal care services provided by the staff of the Department of Comparative Medicine of Loyola University Medical Center. This work was supported by the NIH–R01 5R01HL95896-2, the NSFC Project 81071774, the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, the grant from Science and Technology Commission of Shanghai Municipality (10540503400), Shanghai Normal University Leading Disciplines Project (DZL808), and the Shanghai Leading Academic Discipline Project (S30406), as well as by a grant from the Jimmy Burns Foundation. The authors have no conflicting financial interests.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
Authorship contribution statement: Jun Zhang, Yechen Xiao, Yinshi Guo and Zhou Zhang performed most of the research and analyzed the data. Shubin Zhang and Wei Wei performed some of the research. Peter Breslin analyzed the data and wrote the paper. Jiwang Zhang designed the research, analyzed the data and wrote the paper.
None of the authors has any conflict of interest to disclose.
REFERENCES
- 1.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 2.Eilers M, Eisenman RN. Myc's broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman B, Amanullah A, Shafarenko M, Liebermann DA. The proto-oncogene c-myc in hematopoietic development and leukemogenesis. Oncogene. 2002;21:3414–3421. doi: 10.1038/sj.onc.1205400. [DOI] [PubMed] [Google Scholar]
- 4.Weng AP, Millholland JM, Yashiro-Ohtani Y, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang ZH, Dong CL, Chen Z, et al. Transcriptional regulation of survivin by c-Myc in BCR/ABL-transformed cells: implications in antileukemic strategy. J Cell Mol Med. 2009;13:2039–2052. doi: 10.1111/j.1582-4934.2008.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiner S, Birke M, Garcia-Cuellar MP, Zilles O, Greil J, Slany RK. MLL-ENL causes a reversible and myc-dependent block of myelomonocytic cell differentiation. Cancer Res. 2001;61:6480–6486. [PubMed] [Google Scholar]
- 7.Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol. 2006;16:318–330. doi: 10.1016/j.semcancer.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Smith DP, Bath ML, Harris AW, Cory S. T-cell lymphomas mask slower developing B-lymphoid and myeloid tumours in transgenic mice with broad haemopoietic expression of MYC. Oncogene. 2005;24:3544–3553. doi: 10.1038/sj.onc.1208399. [DOI] [PubMed] [Google Scholar]
- 9.Harris AW, Pinkert CA, Crawford M, Langdon WY, Brinster RL, Adams JM. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988;167:353–371. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo H, Li Q, O'Neal J, Kreisel F, Le Beau MM, Tomasson MH. c-Myc rapidly induces acute myeloid leukemia in mice without evidence of lymphoma-associated antiapoptotic mutations. Blood. 2005;106:2452–2461. doi: 10.1182/blood-2005-02-0734. [DOI] [PubMed] [Google Scholar]
- 11.Skoda RC, Tsai SF, Orkin SH, Leder P. Expression of c-MYC under the control of GATA-1 regulatory sequences causes erythroleukemia in transgenic mice. J Exp Med. 1995;181:1603–1613. doi: 10.1084/jem.181.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurenti E, Varnum-Finney B, Wilson A, et al. Hematopoietic stem cell function and survival depend on c-Myc and N-Myc activity. Cell Stem Cell. 2008;3:611–624. doi: 10.1016/j.stem.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steelman LS, Abrams SL, Whelan J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 14.Kharas MG, Okabe R, Ganis JJ, et al. Constitutively active AKT depletes hematopoietic stem cells and induces leukemia in mice. Blood. 2010;115:1406–1415. doi: 10.1182/blood-2009-06-229443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCubrey JA, Steelman LS, Abrams SL, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22:708–722. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 16.Gustafson WC, Weiss WA. Myc proteins as therapeutic targets. Oncogene. 2010;29:1249–1259. doi: 10.1038/onc.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnet M, Loosveld M, Montpellier B, et al. Posttranscriptional deregulation of MYC via PTEN constitutes a major alternative pathway of MYC activation in T-cell acute lymphoblastic leukemia. Blood. 2011;117:6650–6659. doi: 10.1182/blood-2011-02-336842. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez A, Sanda T, Grebliunaite R, et al. High frequency of PTEN, PI3K, and AKT abnormalities in T-cell acute lymphoblastic leukemia. Blood. 2009;114:647–650. doi: 10.1182/blood-2009-02-206722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheong JW, Eom JI, Maeng HY, et al. Phosphatase and tensin homologue phosphorylation in the C-terminal regulatory domain is frequently observed in acute myeloid leukaemia and associated with poor clinical outcome. Br J Haematol. 2003;122:454–456. doi: 10.1046/j.1365-2141.2003.04452.x. [DOI] [PubMed] [Google Scholar]
- 20.Silva A, Yunes JA, Cardoso BA, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maser RS, Choudhury B, Campbell PJ, et al. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature. 2007;447:966–971. doi: 10.1038/nature05886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shehata M, Schnabl S, Demirtas D, et al. Reconstitution of PTEN activity by CK2 inhibitors and interference with the PI3-K/Akt cascade counteract the antiapoptotic effect of human stromal cells in chronic lymphocytic leukemia. Blood. 2010;116:2513–2521. doi: 10.1182/blood-2009-10-248054. [DOI] [PubMed] [Google Scholar]
- 23.Larson Gedman A, Chen Q, Kugel Desmoulin S, et al. The impact of NOTCH1, FBW7 and PTEN mutations on prognosis and downstream signaling in pediatric T-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. Leukemia. 2009;23:1417–1425. doi: 10.1038/leu.2009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barata JT. The impact of PTEN regulation by CK2 on PI3K-dependent signaling and leukemia cell survival. Adv Enzyme Regul. 2011;51:37–49. doi: 10.1016/j.advenzreg.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Yoshimi A, Goyama S, Watanabe-Okochi N, et al. Evi1 represses PTEN expression and activates PI3K/AKT/mTOR via interactions with polycomb proteins. Blood. 2011;117:3617–3628. doi: 10.1182/blood-2009-12-261602. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol Cell Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Grindley JC, Yin T, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 28.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 29.Guo W, Lasky JL, Chang CJ, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue L, Nolla H, Suzuki A, Mak TW, Winoto A. Normal development is an integral part of tumorigenesis in T cell-specific PTEN-deficient mice. Proc Natl Acad Sci U S A. 2008;105:2022–2027. doi: 10.1073/pnas.0712059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Alboran IM, O'Hagan RC, Gartner F, et al. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity. 2001;14:45–55. doi: 10.1016/s1074-7613(01)00088-7. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A, Yamaguchi MT, Ohteki T, et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 33.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 34.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 35.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 36.Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. Impaired Fas response and autoimmunity in Pten+/− mice. Science. 1999;285:2122–2125. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 37.Guo Y, Niu C, Breslin P, et al. c-Myc-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Blood. 2009;114:2097–2106. doi: 10.1182/blood-2009-01-197947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwabi-Addo B, Giri D, Schmidt K, et al. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci U S A. 2001;98:11563–11568. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knobbe CB, Lapin V, Suzuki A, Mak TW. The roles of PTEN in development, physiology and tumorigenesis in mouse models: a tissue-by-tissue survey. Oncogene. 2008;27:5398–5415. doi: 10.1038/onc.2008.238. [DOI] [PubMed] [Google Scholar]
- 41.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 42.Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Chen Y, Douglas L, Li S. beta-Catenin is essential for survival of leukemic stem cells insensitive to kinase inhibition in mice with BCR-ABL-induced chronic myeloid leukemia. Leukemia. 2009;23:109–116. doi: 10.1038/leu.2008.262. [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki T, Liu ZJ, Kawahara A, et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. doi: 10.1016/0092-8674(95)90332-1. [DOI] [PubMed] [Google Scholar]
- 46.Waters CM, Littlewood TD, Hancock DC, Moore JP, Evan GI. c-myc protein expression in untransformed fibroblasts. Oncogene. 1991;6:797–805. [PubMed] [Google Scholar]
- 47.Dose M, Khan I, Guo Z, et al. c-Myc mediates pre-TCR-induced proliferation but not developmental progression. Blood. 2006;108:2669–2677. doi: 10.1182/blood-2006-02-005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douglas NC, Jacobs H, Bothwell AL, Hayday AC. Defining the specific physiological requirements for c-Myc in T cell development. Nat Immunol. 2001;2:307–315. doi: 10.1038/86308. [DOI] [PubMed] [Google Scholar]
- 49.Dose M, Sleckman BP, Han J, Bredemeyer AL, Bendelac A, Gounari F. Intrathymic proliferation wave essential for Valpha14+ natural killer T cell development depends on c-Myc. Proc Natl Acad Sci U S A. 2009;106:8641–8646. doi: 10.1073/pnas.0812255106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mycko MP, Ferrero I, Wilson A, et al. Selective requirement for c-Myc at an early stage of V(alpha)14i NKT cell development. J Immunol. 2009;182:4641–4648. doi: 10.4049/jimmunol.0803394. [DOI] [PubMed] [Google Scholar]
- 51.Bianchi T, Gasser S, Trumpp A, MacDonald HR. c-Myc acts downstream of IL-15 in the regulation of memory CD8 T-cell homeostasis. Blood. 2006;107:3992–3999. doi: 10.1182/blood-2005-09-3851. [DOI] [PubMed] [Google Scholar]
- 52.Wanner M, Celebi JT, Peacocke M. Identification of a PTEN mutation in a family with Cowden syndrome and Bannayan-Zonana syndrome. J Am Acad Dermatol. 2001;44:183–187. doi: 10.1067/mjd.2001.110390. [DOI] [PubMed] [Google Scholar]
- 53.Liaw D, Marsh DJ, Li J, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 54.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.