Abstract
Blood-brain barrier (BBB) dysfunctions have been implicated in the development and progression of Alzheimer's disease. Cerebral endothelial cells (CECs) and astrocytes are the main cell components of the BBB. Although amyloid-β oligomers (Aβ42) have been reported to mediate oxidative damage to the CECs and astrocytes and trigger the downstream MAPK/ERK pathway, the cell surface binding site for Aβ42 and exact sequence of these events have yet to be elucidated. In this study, the receptor for advanced glycation endproducts (RAGE) was postulated to function as a signal transducing cell surface receptor for Aβ42 to induce ROS generation from NADPH oxidase and trigger downstream pathways for the phosphorylation of extracellular-signal-regulated kinases (ERK1/2) and cytosolic phosphorilase A2 (cPLA2). We found that Aβ42 competed with the anti-RAGE antibody (AbRAGE) to bind to RAGE on the surfaces of CECs and primary astrocytes. In addition, AbRAGE abrogate Aβ42-induced ROS production and the co-localization between the cytosolic (p47-phox) and membrane (gp91-phox) subunits of NADPH oxidase in both cell types. AbRAGE, as well as NADPH oxidase inhibitor and ROS scavenger suppressed Aβ42-induced ERK1/2 and cPLA2 phosphorylation in CECs. At the same time, only AbRAGE, but not NADPH oxidase inhibitor or ROS scavenger, inhibited the ERK1/2 pathway and cPLA2 phosphorylation in primary astrocytes. Therefore, this study demonstrates that NADPH oxidase complex assembly and ROS production are not required for Aβ42 binding to RAGE at astrocytic surface leading to sequential phosphorylation of ERK1/2 and cPLA2, and suggests the presence of two different RAGE-dependent downstream pathways in the CECs and astrocytes.
Keywords: Aβ42, RAGE, NADPH oxidase, ERK, cPLA2
1. Introduction
Alzheimer's disease (AD) is a chronic neurodegenerative disorder, which affects approximately 10% of the population at age 65 and older. The pathology of AD is characterized by an increased deposition of amyloid-β peptide (Aβ) in the brain, and a progressive impairment of cognition and memory of affected individuals. Blood Brain Barrier (BBB) dysfunction is observed in all of the stages of AD, and may even precede neuron degeneration in AD brains (Hofman et al., 1997, Iadecola, 2003, Ruitenberg et al., 2005, de la Torre, 2006, Girouard and Iadecola, 2006, Deane and Zlokovic, 2007, Zlokovic, 2008, Bell and Zlokovic, 2009). During the early stages of AD, microvasculature deficiencies and hypertrophy of astrocytes are commonly observed (Farkas and Luiten, 2001, Borroni et al., 2002). Numerous in vivo and in vitro studies have demonstrated that the vascular deposition of Aβ induces oxidative stress in cerebral vasculature and astrocytes (Cai et al., 2003, Abramov and Duchen, 2005). Aβ-induced oxidative stress in cells, in turn, initiates a cascade of redox reactions leading to apoptosis and neurovascular inflammation (Emmanuelle et al., 1997, Suo et al., 1998, Tan et al., 1999, Xu et al., 2001, Yin et al., 2002, Hsu et al., 2007, Vukic et al., 2009)
Aβ-induced oxidative stress is associated with overproduction of reactive oxygen species (ROS) (Park et al., 2005, Girouard and Iadecola, 2006, Callaghan et al., 2008, Park et al., 2008). ROS can be generated by several enzymatic systems, but there is evidence that the superoxide-producing enzyme, NADPH oxidase, is a major source of ROS in CECs and astrocytes (Cai et al., 2003, Abramov and Duchen, 2005, Park et al., 2005, Qing et al., 2005, Park et al., 2008, Zhu et al., 2009). Although these studies demonstrate that Aβ mediates oxidative damage to astrocytes and CECs mainly through the activation of NADPH oxidase, how Aβ activates NADPH oxidase has yet to be elucidated.
Aβ-induced cytotoxic effects are also associated with the activation of MAPK/ERK1/2 cascade and the phosphorylation of cytosolic phospholipase A2 (cPLA2) (Stephenson et al., 1996, McDonald et al., 1998, Dineley et al., 2001, Moses et al., 2006, Zhu et al., 2006, Shelat et al., 2008, Young et al., 2009). The ERKs (extracellular-signal-regulated kinases) are widely expressed protein kinases, and part of a signal transduction system through which extracellular stimuli are transduced. Activation of ERKs occurs in response to growth factor stimulation, cytokines, virus infection, transforming agents, carcinogens, and after the activation of high-affinity IgG receptors (McDonald et al., 1998). Phospholipases A2 (PLA2s) are ubiquitously distributed enzymes that catalyze the hydrolysis at the sn-2 position of phospholipids to produce lysophospholipids and release arachidonic acid (Murakami and Kudo, 2002, Sun et al., 2004). PLA2s are classified into three major families: calcium-dependent cytosolic PLA2 (cPLA2), secretory PLA2 (sPLA2) and calcium-independent PLA2 (iPLA2). cPLA2 has been implicated in diverse cellular responses such as mitogenesis, differentiation, inflammation and cytotoxicity, and overproduction of this enzyme is involved in many neurodegenerative diseases, including AD (Stephenson et al., 1996, Sun et al., 2007).
Recent studies have indicated that the receptor for advanced glycation endproducts (RAGE) is a binding site for Aβ (Yan et al., 1996, Lue et al., 2001, Sasaki et al., 2001, Arancio et al., 2004, Chaney et al., 2005). RAGE is a multi-ligand cell surface receptor which is normally expressed in brain endothelium and, at low levels, in microglia and neurons (Lue et al., 2001, Sasaki et al., 2001, Zlokovic, 2008). However, in AD brains, RAGE expression is increased by several-fold in cerebral endothelial cells, astrocytes, microglia, and neurons (Lue et al., 2001, Sasaki et al., 2001). Aβ binding to RAGE has been demonstrated to regulate Aβ transport across BBB, upregulate pro-inflammatory cytokines and adhesion molecules in CECs, and contribute to the transport of Aβ from the cell surface into the intracellular space in cortical neurons (Giri et al., 2000, Lue et al., 2001, Takuma et al., 2009). Since RAGE has been postulated to function as a signal transducing cell surface receptor for Aβ, it is reasonable to hypothesize that binding of Aβ1–42 oligomers (Aβ42) to surface RAGE results in activating of NADPH oxidase to induce ROS generation, and activate downstream pathways, including phosphorylation of ERK1/2 and cPLA2.
2. Experimental Procedures
2.1 Cell cultures and treatment
Mouse bEnd3 line of cerebral endothelial cells (CECs) was purchased from Fisher Scientific. Rat primary cortical astrocytes were purchased from Invitrogen (Carlsbad, CA). Purity of astrocyte culture was verified by double immunostaining with astrocytic marker (primary mouse monoclonal antibody to S100, Abcam Inc, Cambridge, MA) and microglia marker (primary rabbit polyclonal to antibody Coronin 1a, Neuromics, Edina, MN). The purity of astrocytes obtained from Invitrogen is 100%.
Aβ42 and scrambled sequence peptide (Aβ42–1) from American Peptide (Sunnyvale, CA) were prepared by diluting 5mM Aβ in DMSO to 100 μM in ice-cold culture Ham's medium, and sonicated. The oligomeric form of Aβ42 in cell culture was verified by Western blot analysis. Menadione (Sigma, St. Louis, MO) was dissolved in DMSO (1mg/ml) and then diluted in cell culture medium to final concentration of 50 μM; rabbit polyclonal antibody against RAGE (AbRAGE, Abcam Inc), the specific NADPH oxidase inhibitor, gp91ds-tat (AnaSpec, Fremont, CA), and ROS scavenger, Tiron (Sigma), were diluted in DMEM. Experimental groups: control (cells without any treatment); cells treated with 2 and 5 μM of Aβ42 for 1 and 2 hr; cells treated with AbRAGE for 4 hr; cells treated with AbRAGE followed by Aβ42 (5 μM for 2 hr) treatment; cells treated with gp91ds-tat (1 μM for 1 hr) and tiron (5 mM for 1 hr) followed by Aβ42. Cells treated with menadione (50 μM for 30 min) and Aβ42–1 (5 μM for 2 hr) served as a positive and negative controls.
2.2 Immunofluorescent staining for RAGE
Cells were grown on cover slips until confluence. After treatment with Aβ42 (2 and 5 μM for 1 hr), cells were fixed immediately using 3.7% paraformaldehyde solution for 30 min. To block non-specific binding, 5% sheep serum in PBS was applied to the cells for 1 hr. RAGE at the cell surface was labeled with its primary antibody (Abcam) without permeabilization at 4°C overnight, followed by sheep secondary antibody (Abcam) labeling at 25°C for 1 hr. To confirm the specificity of the primary antibodies, cells were labeled by secondary antibodies alone. Secondary antibodies did not show immunostaining in the absence of the primary antibody.
2.3 Measurement of ROS production
DHE (dihydroethidium) staining was applied to determine superoxide anion production (Zanetti et al., 2005). DHE reacts with O•-2 to produce oxyethidium (oxy-E), a highly fluorescent product, which binds to DNA and causes an increased fluorescent intensity in cell nuclei. For ROS measurements, cells were starved for 4 hr, rinsed twice with warm phenol free DMEM, and incubated with DHE (20μM) for 2 hr.
2.4 Quantitative Immunofluorescence microscopy (QIM)
Bright-field illumination and fluorescence microscopy were performed with Nikon TE-2000 U fluorescence microscope and 40X, NA 0.95 objective. Images were acquired using a cooled CCD camera controlled with a computer and uses MetaView imaging software. The typical exposure time for fluorescence image acquisition was 400 msec. Background was subtracted for all images prior to analysis. Relative expression of RAGE was quantified by calculating the intensity of secondary antibody fluorescence per cell. The intensity was then normalized by the control cells (without any treatment). A similar approach was applied to quantify the oxy-E fluorescence.
2.5 Double immunofluorescent labeling of gp91-phox and p47-phox
Cells were grown on cover slips until confluence. After treatment cells were fixed immediately using 3.7% paraformaldehyde solution for 30 min. To block non-specific binding, 5% mixture of sheep and donkey serum in PBS was applied to the cells for 1 hr. Plasma membrane gp91-phox was labeled with its primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) without permeabilization at 4°C overnight. For p47-phox labeling, cells were permeabilzed by 0.1% Triton X-100 in PBS for 5 min and labeled with its primary antibody (Santa Cruz Biotechnology) at 4°C overnight, followed by sheep and donkey polyclonal secondary antibodies (Abcam) labeling at room temperature for 1 hr. The emission spectra of the secondary antibodies were 528 nm (for gp91-phox) and 620 nm (for p47-phox). To confirm the specificity of the primary antibodies, cells were labeled by secondary antibodies alone. Secondary antibodies did not show immunostaining in the absence of the primary antibody.
2.6 Confocal immunofluorescence microscopy
Confocal immunofluorescence microscopy was performed with Olympus FV1000 confocal microscope. Confocal images were acquired with a 60 X, numerical aperture 1.2 oil immersion objective lens for colocalization studies of the cellular surface gp91-phox and p47-phox. The colocalization images were obtained by suppressing all colors, except yellow, in superimposed images using Matlab and Adobe Photoshop. A z-series at 1 μm intervals were captured to determine the spatial co-localization characteristics of gp91-phox or p47-phox staining within individual cells. The colocalization (the area of coincident intensity) between two channels was quantified by normalizing the coincident intensity (yellow) to the total intensity of the corresponding channel.
2.7 Western blot analysis
Following the treatments, primary astrocytes and CECs were harvested in 300 μL sample buffer containing 50 mM Tris-HCl, pH 7.4 1 mM EDTA, 100 mM NaCl, 0.1% sodium dodecyl sulfate, 1 mM phenylmethylsulfonyl fluoride, 1mM sodium o-vanadate, 1μg/mL leupeptin, 1 μg/mL pepstatin, and 10 μg/mL aprotinin. Lysates were collected, sonicated, and equivalent amounts of each sample (40 μL) were resolved in 11.55% Tris-HCl gel electrophoresis. After electrophoresis, proteins were transferred to nitrocellulose membranes. Membranes were incubated in Tris-buffered saline, pH 7.4, with 0.5% Tween 20 (TBS-T) containing 5% non-fat milk for 1 hr at room temperature. The blots were then washed and reacted with either rabbit anti-phospho-cPLA2 (1:600; Cell Signaling Technology, Boston, MA) or rabbit anti-cPLA2 (1:600; Cell Signaling Technology) or mouse anti-phospho-ERK1/2 (1:4000, Cell Signaling Technology) and rabbit anti-ERK1/2 (1:500; Cell Signaling Technology) overnight at 4°C. After washing with TBS-T, they were incubated with goat anti-rabbit and goat anti-mouse IgG – horseradish peroxidase (1:5000; Santa Cruz) for 1 hr at room temperature. The blots then were washed 3 times with TBS-T. Immunolabeling was detected by chemiluminescence (SuperSignal West Pico and West Fempto). For quantification, blots were scanned and intensity of protein bands was measured as optical density using the Quantity One program (BioRAD, Hercules, CA, USA). Phospho-ERK1/2 and ERK1/2 were detected at 42/44 kDa, and phospho-cPLA2 and cPLA2 were detected at 110 kDa. Ratios of phospho- ERK1/2 to ERK1/2 and phospho-cPLA2 to cPLA2 were calculated for each sample and normalized to the control.
2.8 Statistical analysis
Data from at least three independent experiments are reported as mean ± SD. Mean differences between experimental groups were tested with unpaired t-test. Values were considered significantly different at the p ≤ 0.05 level. Statistical analyses were performed on the Sigma Plot 8.0 software.
3. Results
3.1 Oligomeric Aβ42 interacts with the RAGE
It has been reported that the binding of soluble Aβ to soluble RAGE inhibits further aggregation of Aβ peptides, while membrane bound RAGE-Aβ interactions elicit activation of the NF-κB transcription factor and promote sustained chronic neuroinflamation (Chaney et al., 2005). To further provide evidence of the Aβ42-RAGE binding, we examined with the immunofluorescence microscopy of RAGE for primary astrocytes and CECs pretreated with 2 and 5 μM of oligomeric Aβ42 at 37°C and 4°C (in which condition the internalization of surface receptors is suppressed). A lower fluorescent intensity of labeled RAGE was observed for cells pretreated with oligomeric Aβ42 in both temperature conditions (Fig. 1), suggesting that Aβ42 oligomers compete with AbRAGE to bind to RAGE at the surface of the astrocytes as well as CECs.
Fig.1. Oligomeric Aβ42 reduced the fluorescent intensity of RAGE staining at the primary astrocytes and CECs surface.
Cells were treated with 2 and 5 μM of Aβ42 for 1 hr at +4°C and 37°C, fixed and incubated with rabbit polyclonal to RAGE (AbRAGE) overnight. Control cells were kept in the same conditions, but without any treatment. *- p ≤ 0.05, ** - p ≤ 0.01, *** - p ≤ 0.001 compared to the control
3.2 Polyclonal antibody to RAGE and NADPH inhibitor suppress Aβ42-induced ROS production in astrocytes and CECs
To investigate ROS production in cells, we applied fluorescent microscopy of DHE, which reacts with O•-2 to produce oxyethidium (oxy-E) with a higher quantum yield. Fig. 2 shows the images of DHE-stained CECs (A) and astrocytes (B) treated with Aβ42 oligomers, Aβ42-1, AbRAGE, ROS scavenger (tiron), and NADPH oxidase inhibitor (gp91ds-tat). Quantitative analysis was accomplished by integration of fluorescent intensity for each cell. Fig. 2 C, D show that 5 μM of Aβ42 increased DHE fluorescence in both primary astrocytes as well as in CECs by ~ 75% as compared to the control. Since menadione has been reported previously to induce ROS generation in astrocytes (Zhu et al., 2009), results from the treatment of menadione served as a positive control (Fig. 2A, C). As a negative control, reversed Aβ42-1 did not increase ROS generation. At the same time, Aβ42 stimulated ROS production in CECs and astrocytes was attenuated by blocking the cell surface RAGE with its antibody (Fig. A, C), or by the pretreatment with gp91ds-tat, the specific NADPH oxidase inhibitor (Fig. B, D). AbRAGE, or the inhibitor alone had no effect on DHE intensity. As a control, scrambled sequence peptide sr-gp91ds-tat (sr-gp) did not suppress Aβ42-induced ROS overproduction. To verify this technique of measurement for superoxide anions, we demonstrated that ROS scavenger suppressed an Aβ42-mediated increase in DHE intensity. This data suggest that Aβ42 oligomers induce ROS production through their binding to RAGE leading to NADPH oxidase activation.
Fig.2. Representative images of dihydroethidium (DHE)-stained CECs (A) and astrocytes (B) treated with Aβ42 oligomers, polyclonal antibody to RAGE (AbRAGE), ROS scavenger (tiron), and NADPH oxidase inhibitor (gp91 ds_tat). Relative DHE fluorescent intensity in primary astrocytes (C) and CECs (D).
Polyclonal antibody to RAGE, NADPH oxidase inhibitor and tiron significantly suppressed oxidative stress induced by Aβ42 in primary astrocytes and CECs. *- p≤0.05; ** - p ≤ 0.01; *** - p ≤ 0.001 compared to the control; ºº - p ≤ 0.01 compared to the Aβ treatment
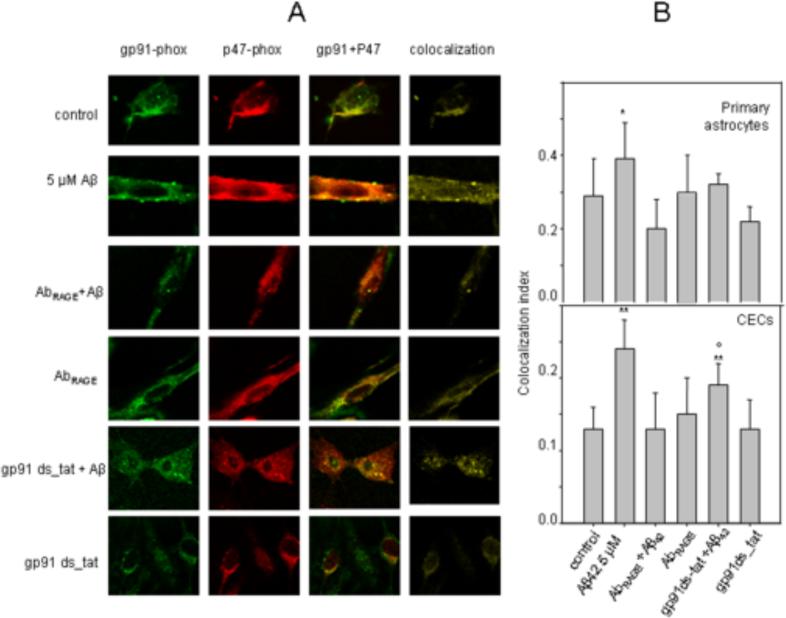
3.3 Polyclonal antibody to RAGE suppresses Aβ42-induced colocalization of cytosolic subunit p47-phox of NADPH oxidase with its membrane subunits gp91-phox
AbRAGE as well as NADPH oxidase inhibitor suppressed Aβ42-induced ROS production in CECs and astrocytes (Fig. 2C, D). NADPH oxidase is a membrane-bound enzyme that catalyzes the production of ROS from oxygen and NADPH. NADPH oxidase is a complex system consisting of two membrane-bound elements (gp91-phox and p22-phox), three cytosolic components (p67-phox, p47-phox and p40-phox), and a low-molecular-weight G protein (Babior, 1999). Activation of NADPH oxidase is associated with the migration of the cytosolic components to the cell membrane and assembling with its membrane subunits. To confirm the role of Aβ42-RAGE interactions in NADPH oxidase activation and subsequent ROS generation, we quantified the effect of Aβ42 and AbRAGE on the colocalization of p47-phox with gp91-phox by analyzing confocal images of double immunofluorescent-labeled gp91-phox and p47-phox in astrocytes and CECs (Fig. 3 A,B). Our results indicate that Aβ42 significantly increased the colocalization of cytosolic subunit p47-phox of NADPH oxidase with its membrane subunits gp91-phox, suggesting that Aβ42 enhances NADPH oxidase complex assembling. At the same time, pre-treatment with AbRAGE significantly suppressed the colocalization of p47-phox with gp91-phox induced by Aβ42. To validate the fluorescent confocal microscopy method for measurement of the colocalization between these two subunits, we demonstrated that NADPH oxidase inhibitor (gp91ds-tat) suppressed Aβ42-mediated increase in colocalization (Fig. 3 A,B). The inhibitor alone, as well as AbRAGE, had no effect on the colocalization. This data indicated that Aβ42 oligomers induced colocalization of cytosolic subunit p47-phox of NADPH oxidase with its membrane subunits gp91-phox and subsequent ROS generation through binding to RAGE.
Fig.3. Polyclonal antibody to RAGE and NADPH oxidase inhibitor suppressed Aβ42 induced colocalization of p47-phox to gp91-phox.
Representative confocal images of gp91-phox and p47-phox in astrocytes (A). The images of colocalization (yellow) were obtained from merged confocal images of corresponding p47-phox (red) and gp91-phox (green) by suppressing all colors except yellow in Matlab. (60X, NA 0.95 objective). Quantitative analysis of colocalization between gp91-phox and p47-phox (B). * - p ≤ 0.05; ** - p ≤ 0.01compared to the control; º - p ≤ 0.05 compared to the Aβ treatment
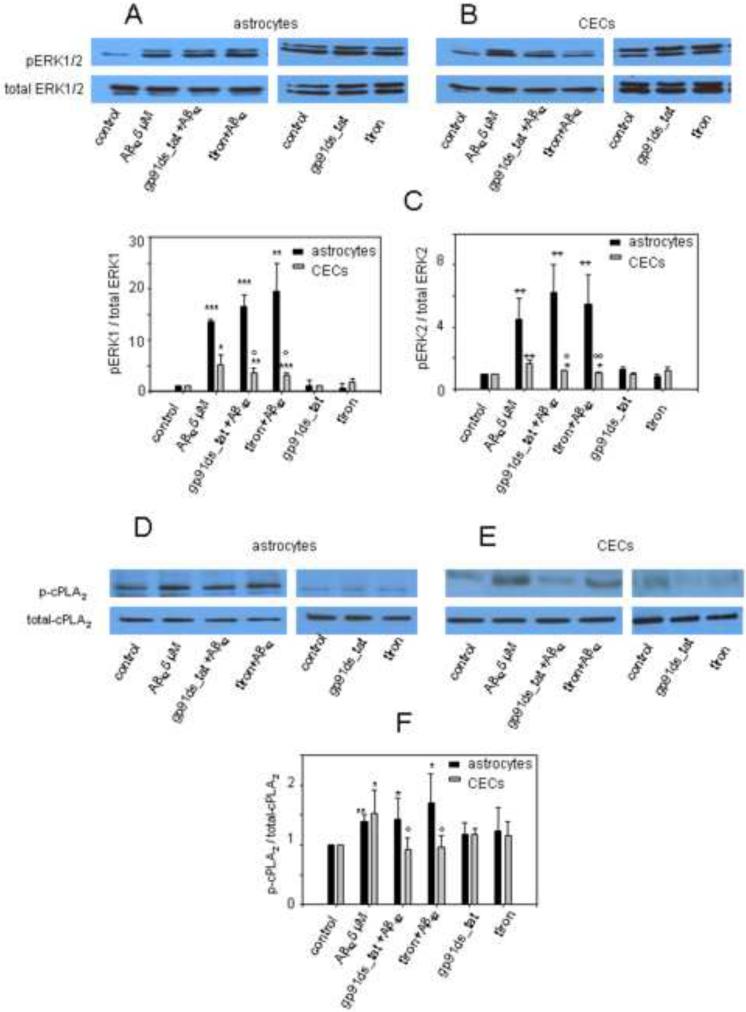
3.4 Polyclonal antibody to RAGE inhibit Aβ42-induced phosphorylation ERK1/2 and cPLA2 in primary astrocytes and CECs
Aβ42 has been shown to induce MAPK/ERK downstream signaling pathways, including the activation of extracellular-signal-regulated kinases (ERK), which further leads to phosphorylation of cPLA2 (Emmanuelle et al., 1997, Xu et al., 2001, Yin et al., 2002, Hsu et al., 2007, Shelat et al., 2008). Here, we tested if Aβ42 binding to RAGE induced ERK1/2 activation and phosphorylation of cPLA2 in CEC and astrocytes. Fig. 4 shows that Aβ42 significantly increased phosphorylation of ERK1/2 and cPLA2 in astrocytes and CECs, which were suppressed by the pretreatment with AbRAGE. Additionally, AbRAGE alone had no effect on phosphorylation of ERK1/2 and cPLA2 in both cell types. Our data suggested that Aβ42 binding to RAGE is required for the activation of ERK1/2 and further phosphorylation of cPLA2 induced by Aβ42.
Fig.4. Polyclonal antibody to RAGE inhibits ERK1/2 and cPLA2α phosphorylation in primary astrocytes and CECs.
Aβ42-induced phosphorylation of ERK1/2 (A,B) and cPLA2α (C,D) was suppressed by the pretreatment with polyclonal antibody to RAGE (AbRAGE). *- p≤0.05;**- p ≤ 0.01, *** - p ≤ 0.001 compared to the control; º - p ≤ 0.05, ºº - p ≤ 0.01, ººº - p ≤ 0.001 compared to the Aβ treatment.
3.5 Effects of NADPH oxidase inhibitor and ROS scavenger on ERK1/2 and cPLA2 phosphorylation in CECs and primary astrocytes
ROS from NADPH oxidase has been shown to induce the activation of ERK1/2 and phosphorylation of cPLA2 in primary neurons (Shelat et al., 2008). Our data suggest that Aβ42 oligomers induce ROS production through binding to RAGE which activates NADPH oxidase, and thus blocking cell surface RAGE with its antibody suppresses Aβ-induced phosphorylation of ERK1/2 and cPLA2 in CECs and primary astrocytes. Therefore, we hypothesized that Aβ42 binding to RAGE induces phosphorylation of ERK1/2 and cPLA2 through activation of NADPH oxidase and ROS production.
In support of our hypothesis, Fig. 5 B,C,E,F show that pretreatment of CECs with NADPH oxidase inhibitor or ROS scavenger could significantly suppress Aβ42-induced phosphorylation of ERK1/2 and cPLA2. Surprisingly, only AbRAGE, but neither NADPH oxidase inhibitor nor ROS scavenger, inhibited Aβ42-induced ERK1/2 and cPLA2 phosphorylation in primary astrocytes (Fig. 4 A,B and Fig.5 A,C,D,F). NADPH oxidase inhibitor alone, as well as ROS scavenger, had no effect on phosphorylation of ERK1/2 and cPLA2 in both cell types (Fig. 3.5 A–F).
Fig.5. Effects of NADPH oxidase inhibitor and ROS scavenger on ERK1/2 and cPLA2α phosphorylation in primary astrocytes and CECs.
Aβ42-induced phosphorylation of ERK1/2 and cPLA2α was suppressed by the pretreatment with NADPH oxidase inhibitor (gp91ds_tat) and ROS scavenger (tiron) in CECs (B, C, E, F), but not in primary astrocytes (A, C, D, F). *- p≤0.05;**- p ≤ 0.01, *** - p ≤ 0.001 compared to the control; º - p ≤ 0.05, ºº - p ≤ 0.01, ººº - p ≤ 0.001 compared to the Aβ treatment
These data suggest a presence of two different downstream pathways in the CECs and astrocytes. Although Aβ42 binding to RAGE is required to induce phosphorylation of ERK1/2 and cPLA2 in both CECs and primary astrocytes, NADPH oxidase inhibition does not suppress phosphorylation of ERK1/2 and cPLA2 in primary astrocytes.
4. Discussion
Results from this study provided support for the involvement of RAGE in Aβ42-mediated oxidative stress and downstream MAPK/ERK pathway in CECs and astrocytes. Specifically, we demonstrated that Aβ42 oligomers directly bind to RAGE at the surface of the cells to induce colocalization between NADPH oxidase subunits gp91-phox and p47-phox, and subsequently leading to generation of ROS, and phosphorylation of ERK1/2 and cPLA2 in CECs. Interestingly, our data also showed that activation of NADPH oxidase and increased ROS production were not required for Aβ-induced phosphorylation of ERK1/2 and cPLA2 in astrocytes.
In agreement with impairments of BBB structure and functions in AD, many studies have indicated a decrease in cerebral blood flow, reduced microvascular density, and low immunoreactivity of endothelial markers CD34 and CD31 in AD brains (Kalaria and Pax, 1995, Luc et al., 1997, Berzin et al., 2000, Bailey et al., 2004, Ervin et al., 2004). Although there is evidence that oxidative stress is a major factor leading to a BBB cells dysfunction in AD, the mechanism for production of Aβ-induced ROS has not been clearly elucidated (Smith et al., 1991, Behl et al., 1994, Mecocci et al., 1994, Cini and Moretti, 1995, Simonian and Coyle, 1996, Behl and Holsboer, 1998, Heneka and O'Banion, 2007). Recently, RAGE has been postulated to function as a signal transducing cell surface acceptor for Aβ (Yan et al., 1996, Lue et al., 2001, Sasaki et al., 2001, Arancio et al., 2004, Chaney et al., 2005). In fact, the possible links between RAGE and oxidative stress has been reported (Yan et al., 1996, Lue et al., 2001, Sasaki et al., 2001, Arancio et al., 2004, Chaney et al., 2005). ROS has been demonstrated to be generated by the advanced glycation end products (AGE)-RAGE interaction in human endothelial cells (Wautier et al., 2001). Inhibition studies have indicated that anti-RAGE IgG significantly suppressed oxidative stress induced by Aβ both in vascular and neuronal cell (Yan et al., 1996); however, the relationship between Aβ-RAGE interactions and NADPH oxidase has yet to be elucidated.
It is suggested that NADPH oxidase is the primary source of superoxide in astrocytes and CECs (Cai et al., 2003, Abramov and Duchen, 2005, Park et al., 2005, Qing et al., 2005, Park et al., 2008, Zhu et al., 2009). We have previously reported that Aβ42 oligomers induce ROS generation through activation of NADPH oxidase in primary astrocytes (Zhu et al., 2006). It has been shown that inhibition of NADPH oxidase significantly suppressed ROS released in rat astrocytes (Qing et al., 2005). Aβ-induced ROS overproduction and mitochondrial depolarization were absent in astrocytes cultured from gp91phox knockout transgenic mice (Abramov and Duchen, 2005). In a model of AD, inhibition of NADPH oxidase has been found to abrogate Aβ-induced ROS production and alteration of cerebrovascular functions (Park et al., 2005). APP transgenic mice lacking the NADPH oxidase subunits gp91-phox or phagocytic NADPH oxidase (Nox2) did not develop oxidative stress, cerebrovascular dysfunction, and behavioral deficits (Park et al., 2005, Park et al., 2008). Our results suggest that Aβ42 oligomers induce ROS production through binding to RAGE and activation of NADPH oxidase (Fig. 2, 3).
Aβ-induced ROS generation, in turn, triggers the downstream pathway of cPLA2 (Shelat et al., 2008). It has been reported that the immunoreactivity of cPLA2 (group IVA) increased in reactive astrocytes in severe AD brains (Stephenson et al., 1996, 1999). In vitro, treatment of astrocytes with Aβ increased ROS production from NADPH oxidase and activated cPLA2 (Zhu et al., 2006, Hicks et al., 2008). Furthermore, cPLA2 was shown to cause a decrease in mitochondrial membrane potential (Δψm) and resulted in more ROS production (Zhu et al., 2006). In primary rat cortical astrocytes, menadione induced ROS production mediated by NADPH oxidase (Zhu et al., 2009). In neurons, Aβ-induced ROS generation led to phosphorylation of cPLA2 and arachidonic acid (AA) release (Shelat et al., 2008). In turn, hydrolytic products of cPLA2 was shown to enhance NADPH oxidase activity, forming a viscous cycle (Cherny et al., 2001, Levy, 2006). Our data showed that Aβ42 induced phosporylation of cPLA2 was totally suppressed by AbRAGE (Fig.4), suggesting the involvement of RAGE in this signaling pathway.
There are several cell-specific mechanisms identified for activation of cPLA2. In primary astrocytes, phosphorylation of cytosolic cPLA2 and the subsequent release of AA can be activated by G-protein-coupled receptor agonists (Yin et al., 2002, Sun et al., 2007). In cortical neurons, protein kinase C (PKC), extracellular signal-regulated kinases (ERK1/2), and p38 mitogen-activated protein kinase have been involved in activation of cPLA2 and ROS production (Sun et al., 2007). In turn, there is a link between Aβ, RAGE, and MAPKs. Several studies have indicated that Aβ induced apoptosis signal-regulating kinase 1 (ASK1) dephosphorylation and p38 MAPK activation in CECs (Emmanuelle et al., 1997, Xu et al., 2001, Yin et al., 2002, Hsu et al., 2007). Similarly, stimulation of RAGE has also been demonstrated to mediate alterations in the phosphorylation state of MAPKs (Origlia et al., 2009). Our data provided a link between these studies and demonstrated that binding of Aβ42 to RAGE mediated alterations in the phosphorylation state of ERK1/2 and cPLA2 both in primary astrocytes as well as in CECs (Fig.4).
Although Aβ42 binding to RAGE is required to induce phosphorylation of ERK1/2 and cPLA2 in both cell types, we found the existence of two slightly different downstream pathways for CECs and astrocytes. In CECs, NADPH oxidase inhibitor gp91ds-tat and ROS scavenger Tiron attenuated Aβ42-induced ROS production, ERK1/2 activation, and cPLA2 phosphorylation (Fig. 5 B,C,E,F). In primary astrocytes, inhibition of NADPH oxidase and ROS production did not suppress Aβ42-induced ERK1/2 and cPLA2 phosphorylation (Fig.5 A,C,D,F). Apparently, results here are not in agreement with our previous studies showing Aβ42-induced ERK1/2 and cPLA2 phosphorylation in astrocytes through NADPH oxidase (Zhu et al., 2006, Zhu et al., 2009). One possible explanation is differences in use of NADPH oxidase inhibitors. In the study by Zhu et al., apocynin was used as NADPH oxidase inhibitor. Several studies indicate that apocynin can inhibit NADPH oxidase only in blood derived cells (leukocytes, microglia, etc) (Heumuller et al., 2008), and in other cell types, this compound can act as an antioxidant (Heumuller et al., 2008). In fact, in other cases, apocynin can even exert as an oxidant (Riganti et al., 2006). These results suggest that effects of apocynin may different depending on experimental conditions, and thus should be interpreted with caution. In our study, we used gp91ds-tat which is a specific NADPH oxidase inhibitor. Differences in effects of NADPH oxidase inhibition on ERK1/2 and cPLA2 phosphorylation could also result from the preparation of astrocytes and the purity of the cell culture. Therefore, more investigations are required to unravel the mechanism(s) underlying the Aβ-RAGE interaction resulting in activations of ERK1/2 and cPLA2 in astrocytes.
In summary, this study demonstrates the important role of Aβ42-RAGE interaction for NADPH oxidase complex assembling, resulting in subsequent ROS generation, activation of MAPK/ERK pathway, and the phosphorylation of cPLA2 in CECs and primary astrocytes. Our results also revealed two possibly different RAGE-dependent signal transducing pathways in CECs and astrocytes. In CEC, it is possible to demonstrate requirement for NADPH oxidase-mediated generation of ROS to activate ERK1/2 and cPLA2, whereas, astrocytes may activate ERK1/2 and cPLA2 independent of NADPH oxidase. Understanding the precise molecular mechanisms underlying Aβ-mediated oxidative stress should prove to provide new insights into the development of preventive and treatment strategies for AD.
*Highlights
-
>
Aβ binding to RAGE to activate NADPH oxidase in endothelial cells and astrocytes.
-
>
Aβ binding to RAGE to activate cPLA2 in endothelial cells and astrocytes.
-
>
NADPH oxidase activation is not needed for Aβ to activate cPLA2 in astrocytes.
Acknowledgements
This work was supported by Alzheimer Association Grant NIRG-06-24448; NIH Grant 1P01 AG18357, R21NS052385 and R21AG032579.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Duchen MR. The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:2309–2314. doi: 10.1098/rstb.2005.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue L-F, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS. RAGE potentiates A[beta]-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. NADPH Oxidase: An Update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Bailey TL, Rivara CB, Rocher AB, Hof PR. The nature and effects of cortical microvascular pathology in aging and Alzheimer's disease. Neurological Research. 2004;26:573–578. doi: 10.1179/016164104225016272. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Behl C, Holsboer F. Oxidative stress in the pathogenesis of Alzheimer's disease and antioxidant neuroprotection. Fortschr Neurol Psychiatr. 1998;66:113–121. doi: 10.1055/s-2007-995246. [DOI] [PubMed] [Google Scholar]
- Bell R, Zlokovic B. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathologica. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzin TM, Zipser BD, Rafii MS, Kuo--Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, Stopa EG. Agrin and microvascular damage in Alzheimer's disease. Neurobiology of Aging. 2000;21:349–355. doi: 10.1016/s0197-4580(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Borroni B, Akkawi N, Martini G, Colciaghi F, Prometti P, Rozzini L, Di Luca M, Lenzi GL, Romanelli G, Caimi L, Padovani A. Microvascular damage and platelet abnormalities in early Alzheimer's disease. Journal of the Neurological Sciences. 2002;203–204:189–193. doi: 10.1016/s0022-510x(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends in Pharmacological Sciences. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- Callaghan D, Bai J, Huang A, Vukic V, Xiong H, Jones A, Walker D, Leu LF, Beach TG, Sue L, Zhang W. P4–182: Inhibition of ABCG2 transport function by amyloid-beta peptide augments cellular oxidative stress and inflammatory gene expression in cells. Alzheimer's and Dementia. 2008;4:T724–T724. [Google Scholar]
- Chaney MO, Stine WB, Kokjohn TA, Kuo Y-M, Esh C, Rahman A, Luehrs DC, Schmidt AM, Stern D, Yan SD, Roher AE. RAGE and amyloid beta interactions: Atomic force microscopy and molecular modeling. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2005;1741:199–205. doi: 10.1016/j.bbadis.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Cherny VV, Henderson LM, Xu W, Thomas LL, DeCoursey TE. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. The Journal of Physiology. 2001;535:783–794. doi: 10.1111/j.1469-7793.2001.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiol Aging. 1995;16:53–57. doi: 10.1016/0197-4580(95)80007-e. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. How do heart disease and stroke become risk factors for Alzheimer's disease? Neurological Research. 2006;28:637–644. doi: 10.1179/016164106X130362. [DOI] [PubMed] [Google Scholar]
- Deane R, Zlokovic BV. Role of the blood-brain barrier in the pathogenesis of Alzheimer's disease. Curr Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Westerman M, Bui D, Bell K, Ashe KH, Sweatt JD. {beta}-Amyloid Activates the Mitogen-Activated Protein Kinase Cascade via Hippocampal {alpha}7 Nicotinic Acetylcholine Receptors: In Vitro and In Vivo Mechanisms Related to Alzheimer's Disease. J Neurosci. 2001;21:4125–4133. doi: 10.1523/JNEUROSCI.21-12-04125.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuelle MB, Michal T, Robert JM, Bernhard H. Amyloid β-Peptide Induces Cell Monolayer Albumin Permeability, Impairs Glucose Transport, and Induces Apoptosis in Vascular Endothelial Cells. Journal of Neurochemistry. 1997;68:1870–1881. doi: 10.1046/j.1471-4159.1997.68051870.x. [DOI] [PubMed] [Google Scholar]
- Ervin JF, Pannell C, Szymanski M, Welsh-Bohmer K, Schmechel DE, Hulette CM. Vascular Smooth Muscle Actin Is Reduced in Alzheimer Disease Brain: A Quantitative Analysis. Journal of Neuropathology & Experimental Neurology. 2004;63:735–741. doi: 10.1093/jnen/63.7.735. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PGM. Cerebral microvascular pathology in aging and Alzheimer's disease. Progress in Neurobiology. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VK. Beta-amyloid-induced migration of monocytes across human brain endothelial cells involves RAGE and PECAM-1. Am J Physiol Cell Physiol. 2000;279:C1772–1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O'Banion MK. Inflammatory processes in Alzheimer's disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schroder K, Brandes RP. Apocynin Is Not an Inhibitor of Vascular NADPH Oxidases but an Antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Hicks JB, Lai Y, Sheng W, Yang X, Zhu D, Sun GY, Lee JC. Amyloid-beta peptide induces temporal membrane biphasic changes in astrocytes through cytosolic phospholipase A2. Biochim Biophys Acta. 2008;1778:2512–2519. doi: 10.1016/j.bbamem.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman A, Ott A, Breteler MMB, Bots ML, Slooter AJC, van Harskamp F, van Duijn CN, Van Broeckhoven C, Grobbee DE. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. The Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Hsu M-J, Hsu CY, Chen B-C, Chen M-C, Ou G, Lin C-H. Apoptosis Signal-Regulating Kinase 1 in Amyloid -beta- Peptide-Induced Cerebral Endothelial Cell Apoptosis. J Neurosci. 2007;27:5719–5729. doi: 10.1523/JNEUROSCI.1874-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Cerebrovascular effects of amyloid-beta peptides: mechanisms and implications for Alzheimer's dementia. Cell Mol Neurobiol. 2003;23:681–689. doi: 10.1023/a:1025092617651. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Pax AB. Increased collagen content of cerebral microvessels in Alzheimer's disease. Brain Research. 1995;705:349–352. doi: 10.1016/0006-8993(95)01250-8. [DOI] [PubMed] [Google Scholar]
- Levy R. The role of cytosolic phospholipase A2-alfa in regulation of phagocytic functions. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2006;1761:1323–1334. doi: 10.1016/j.bbalip.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Luc B, Patrick RHOF, Andre D. Brain Microvascular Changes in Alzheimer's Disease and Other Dementias. Annals of the New York Academy of Sciences. 1997;826:7–24. doi: 10.1111/j.1749-6632.1997.tb48457.x. [DOI] [PubMed] [Google Scholar]
- Lue L-F, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, Stern DM, Yan SD. Involvement of Microglial Receptor for Advanced Glycation Endproducts (RAGE) in Alzheimer's Disease: Identification of a Cellular Activation Mechanism. Experimental Neurology. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- McDonald DR, Bamberger ME, Combs CK, Landreth GE. beta -Amyloid Fibrils Activate Parallel Mitogen-Activated Protein Kinase Pathways in Microglia and THP1 Monocytes. J Neurosci. 1998;18:4451–4460. doi: 10.1523/JNEUROSCI.18-12-04451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Moses GS, Jensen MD, Lue LF, Walker DG, Sun AY, Simonyi A, Sun GY. Secretory PLA2-IIA: a new inflammatory factor for Alzheimer's disease. J Neuroinflammation. 2006;3:28. doi: 10.1186/1742-2094-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Kudo I. Phospholipase A2. J Biochem. 2002;131:285–292. doi: 10.1093/oxfordjournals.jbchem.a003101. [DOI] [PubMed] [Google Scholar]
- Origlia N, Arancio O, Domenici L, Yan SS. MAPK, OI-amyloid and synaptic dysfunction: the role of RAGE. Expert Review of Neurotherapeutics. 2009;9:1635–1645. doi: 10.1586/ern.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. NADPH Oxidase-Derived Reactive Oxygen Species Mediate the Cerebrovascular Dysfunction Induced by the Amyloid -beta- Peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proceedings of the National Academy of Sciences. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing L, Jiu-Hong K, Rong-Liang Z. NADPH oxidase produces reactive oxygen species and maintains survival of rat astrocytes. Cell Biochemistry and Function. 2005;23:93–100. doi: 10.1002/cbf.1171. [DOI] [PubMed] [Google Scholar]
- Riganti C, Costamagna C, Bosia A, Ghigo D. The NADPH oxidase inhibitor apocynin (acetovanillone) induces oxidative stress. Toxicology and Applied Pharmacology. 2006;212:179–187. doi: 10.1016/j.taap.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Ruitenberg A, den Heijer T, Bakker SL, van Swieten JC, Koudstaal PJ, Hofman A, Breteler MM. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Toki S, Chowei H, Saito T, Nakano N, Hayashi Y, Takeuchi M, Makita Z. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer's disease. Brain Research. 2001;888:256–262. doi: 10.1016/s0006-8993(00)03075-4. [DOI] [PubMed] [Google Scholar]
- Shelat P, B., Chalimoniuk M, Wang J-H, Strosznajder J, B., Lee J, C., Sun A, Y., Simonyi A, Sun G, Y. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A<sub>2</sub> in cortical neurons. Journal of Neurochemistry. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson D, Rash K, Smalstig B, Roberts E, Johnstone E, Sharp J, Panetta J, Little S, Kramer R, Clemens J. Cytosolic phospholipase A2 is induced in reactive glia following different forms of neurodegeneration. Glia. 1999;27:110–128. doi: 10.1002/(sici)1098-1136(199908)27:2<110::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Stephenson DT, Lemere CA, Selkoe DJ, Clemens JA. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer's disease brain. Neurobiol Dis. 1996;3:51–63. doi: 10.1006/nbdi.1996.0005. [DOI] [PubMed] [Google Scholar]
- Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A<sub>2</sub> in oxidative and inflammatory responses in neurodegenerative diseases. Journal of Neurochemistry. 2007;103:1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- Suo Z, Tan J, Placzek A, Crawford F, Fang C, Mullan M. Alzheimer's [beta]-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Research. 1998;807:110–117. doi: 10.1016/s0006-8993(98)00780-x. [DOI] [PubMed] [Google Scholar]
- Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, Sosunov A, McKhann G, Funatsu Y, Nakamichi N, Nagai T, Mizoguchi H, Ibi D, Hori O, Ogawa S, Stern DM, Yamada K, Yan SS. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-OI and neuronal dysfunction. Proceedings of the National Academy of Sciences. 2009;106:20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Town T, Suo Z, Wu Y, Song S, Kundtz A, Kroeger J, Humphrey J, Crawford F, Mullan M. Induction of CD40 on human endothelial cells by Alzheimer's [beta]-amyloid peptides. Brain Research Bulletin. 1999;50:143–148. doi: 10.1016/s0361-9230(99)00122-7. [DOI] [PubMed] [Google Scholar]
- Vukic V, Callaghan D, Walker D, Lue L-F, Liu QY, Couraud P-O, Romero IA, Weksler B, Stanimirovic DB, Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer's brain is mediated by the JNK-AP1 signaling pathway. Neurobiology of Disease. 2009;34:95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wautier M-P, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier J-L. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- Xu J, Chen S, Ku G, Ahmed SH, Xu J, Chen H, Hsu CY. Amyloid beta Peptide-Induced Cerebral Endothelial Cell Death Involves Mitochondrial Dysfunction and Caspase Activation. J Cereb Blood Flow Metab. 2001;21:702–710. doi: 10.1097/00004647-200106000-00008. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-[beta] peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Lee JM, Chen SD, Xu J, Hsu CY. Amyloid-beta Induces Smac Release via AP-1/Bim Activation in Cerebral Endothelial Cells. J Neurosci. 2002;22:9764–9770. doi: 10.1523/JNEUROSCI.22-22-09764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KF, Pasternak SH, Rylett RJ. Oligomeric aggregates of amyloid [beta] peptide 1–42 activate ERK/MAPK in SH-SY5Y cells via the [alpha]7 nicotinic receptor. Neurochemistry International. 2009;55:796–801. doi: 10.1016/j.neuint.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Zanetti M, d'Uscio LV, Peterson TE, Katusic ZS, O'Brien T. Hypertension. 2005. Analysis of Superoxide Anion Production in Tissue; pp. 65–72. [DOI] [PubMed] [Google Scholar]
- Zhu D, Hu C, Sheng W, Tan KS, Haidekker MA, Sun AY, Sun GY, Lee JC-M. NAD(P)H oxidase-mediated reactive oxygen species production alters astrocyte membrane molecular order via phospholipase A2. Biochem J. 2009;421:201–210. doi: 10.1042/BJ20090356. [DOI] [PubMed] [Google Scholar]
- Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JCM. Phospholipases A2 Mediate Amyloid-beta Peptide-Induced Mitochondrial Dysfunction. J Neurosci. 2006;26:11111–11119. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. New therapeutic targets in the neurovascular pathway in Alzheimer's disease. Neurotherapeutics. 2008;5:409–414. doi: 10.1016/j.nurt.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]