Abstract
Murine embryonic stem cell (mESC)-derived cardiomyocytes represent a promising source of cells for use in the development of models for studying early cardiac development as well as cell-based therapies in postnatal pathologies. Here, we report a highly efficient cardiac differentiation system in which high density embyoid body (EB) cultures leads to a marked increase of cardiomyocytes production from multiple mESC lines without the addition of any cardiogenic growth factors. Our results show that high density EB cultures significantly increase the yield of functional cardiomyocytes, which express typical cardiac markers, exhibit normal rhythmic Ca2+ transients, and respond to both β-adrenergic and electric stimulations. During the differentiation period, the inhibition of bone morphogenetic protein (BMP) signaling significantly attenuates the increase of cardiac differentiation as well as the increased expression of cardiac-specific genes, NK2 transcription factor related 5 (Nkx2.5) and myosin light chain 2v (Mlc2v) by high density EB cultures. Therefore, we believe that we offer a novel and efficient means of cardiomyocyte production for practical use of mESCs in cardiac regenerative medicine.
Keywords: Embryonic stem cells, cardiac differentiation, high density, cardiomyocytes, cardiac troponin T
1. Introduction
Embryonic stem cells (ESCs) are derived from the inner cell mass of blastocyst stage embryos [1]. The remarkable property of ESCs to differentiate into any cell lineage of the three embryonic layers - ectoderm, endoderm and mesoderm - has received a great deal of interest for potential in therapeutic applications [2; 3]. Accordingly, there have been many attempts to increase the efficiency of ESC differentiation toward specific cell lineages [4; 5; 6]. The cardiogenic capacity of ESCs has been thoroughly investigated; however, only a very small portion of ESCs spontaneously differentiate into cardiomyocytes in conventional culture strategies which induce differentiation through embyoid body (EB) formation [7].
In order to capitalize on the potential of ESCs for cardiac repair and regenerative medicine, a highly efficient and easily reproducible differentiation system must be established. Investigations into the dynamic cardiac differentiation/development process have provided critical guidance for improvement of the production of cardiomyocyte from ESCs. Previous studies demonstrated that various signaling pathways, such as bone morphogenetic protein (BMP) [8; 9] and Wnt/β-catenin [7; 10] signaling, regulate the differentiation of cardiomyocytes in ESCs. Although modulating cardiogenic factors can increase the efficiency of cardiac differentiation, their widespread implementation is likely to be complex due to considerations like the maintenance of growth factor activity and the logistics involved in the timing of cardiogenic factor administration. In certain cases, the reproducibility of cardiac differentiation could be a challenging issue due to differences in the systems and cell lines utilized [11]. Despite the fact that many established strategies produce cardiomyocytes in sufficient quantities for small-scale experimental approaches, the number of cardiomyocytes required for clinical application modeling is higher. Furthermore, even if the requisite number of cardiomyocytes could be produced using the currently available strategies, the cost of cardiogenic growth factors and equipment required for such production would likely prove to be a significant obstacle.
We made the serendipitous observation during cardiac differentiation that EBs with higher density in a local area showed stronger and wider contracting regions after differentiation compared to those sparsely situated. Additionally, the spontaneous contraction of each EB was synchronized when they contacted each other. These findings led us to hypothesize that culturing EBs at high density can increase the efficiency of cardiac differentiation.
In this study, we established a novel strategy using high density cultures of EBs that significantly improves cardiac differentiation of mESCs. Because the majority of progress made to date in the field of degenerative heart disease is dependent on animal studies, significant emphasis is placed on knowledge gained from mouse developmental models and stem cell research performed in mouse models. Consequently, the development of new approaches allowing efficient, direct differentiation of mESCs into cardiomyocytes is of growing interest. Our present study provides a highly efficient, simple and practical approach for studying cardiac differentiation, therapeutics and regeneration.
4. Materials and Methods
2.1. Primary generation of mESCs
In this study, two lines of primary mouse ESCs were used. Timed matings of C57BL/6 mouse were performed and on day 3.5 of pregnancy, the females were sacrificed and the blastocysts were flushed from the uterine horns using M2 medium (Sigma-Aldrich, MO). After washing with M2 medium, the zona pellucid was removed with acidic Tyrode’s Solution (Sigma-Aldrich, MO). After washing, the blastocysts were plated onto mouse embryonic feeder (MEF) cells for 7–10 days.
2.2. mESC culture
In this study, primary and commercially available mESCs (E14G) were cultured on MEF feeder cells for 3 days in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) supplemented with 1% penicillin and streptomycin (Invitrogen), 2 mM L-glutamine (Invitrogen), 0.1 mM nonessential amino acid (MEM-NEAA; Invitrogen), 1mM sodium pyruvate (Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma), LIF-conditioned media (1:500 dilution), and 15% Knock-out serum replacement (KO-SR; Invitrogen). To deplete the feeder cells, 70% confluent mESCs cultures were dissociated into single cells with 0.25% trypsin-EDTA (Invitrogen) and were cultured for two days on gelatin-coated 60 mm tissue culture dishes in Iscove’s modified Dulbecco’s medium supplemented with 2mM L-glutamine, 1% penicillin and streptomycin, 0.1 mM 1-thioglycerol (Sigma), LIF-conditioned medium, and 15% KO-SR.
2.3. mESC differentiation
mESC differentiation was induced by transferring approximately 540 cells in 15 µl differentiation medium to the lid of a 100 mm petri dish and cultured for two days as a hanging drop in order to form embroid bodies (EBs). Each dish contained around 80 EBs that were cultured in differentiation medium containing IMDM supplemented with 2mM L-glutamine, 0.1 mM 1-thioglycerol, and 15 % fetal bovine serum (Gemini bio-products or Hyclone) [16; 34]. The day of hanging drop preparation was defined as EB day 0. At EB Day 2, EBs were transferred to a 60 mm tissue culture dish for adhesion culture in the presence of 5 ml differentiation medium before harvesting.
2.4. Fluorescence-activated cell sorting (FACS) analysis
After 10 days of EB culture in differentiation medium, EBs were dissociated into single cell suspensions using a 1:1 solution of collagenase A and collagenase B (Roche) (6.67 mg/ml) for 30 min with periodic pipetting followed by digestion with Accutase® (Invitrogen) for 5 min. Cells were fixed with 2% paraformaldehyde in PBS. Cells were blocked and permeabilized with 10% normal goat serum (NGS; Invitrogen). Cells were then incubated for 60 min with primary antibody and were incubated with FITC-conjugated secondary antibody for 60 min. Samples were analyzed using FACSCalibur and Cell Quest software (BD Pharmingen).
2.5. Immunocytochemistry
Cells were fixed with 4% paraformaldehyde in PBS and permeabilized for 10 min with PBST. Cells were pre-incubated with 10% normal goat serum (NGS; Invitrogen) in PBST for 45 min, and then incubated for 60 min with primary antibodies and were incubated for 60 min with FITC-conjugated secondary antibody. Samples were visualized with a fluorescence microscope (Nikon) with a 100× objective.
2.6. Ca2+ transient measurement
For Ca2+ imaging, beating EBs were manually dissected and then dissociated with Collagenase A and Collagenase B (6.67 mg/ml, Roche) and plated onto gelatin-coated cover slips. After 2–3 days in culture, cells were loaded with 25 µM cell permeant Ca2+ indicator dye fluo-4 AM (Invitrogen) together with 0.1% Pluronic F-127 (Invitrogen) in Tyrode’s solution containing 2.5 mM Ca2+ at 37 °C for 30 min. The coverslips were mounted and observed using an inverted fluorescence microscope (Nikon Diaphot-TDM). 30 µM blebbistatin (Sigma) was used to arrest contraction of the cardiomyocytes. Data was captured with a NeuroCCD camera (RedShirtImaging), acquired at 125 frames per second, and analyzed with Neuroplex Software (RedShirtImaging). Fluorescence was measured by manually defining each region of interest using the analysis software and quantified in relation to baseline fluorescence (F/F0). The samples were electrically stimulated by field pacing (10v, 10 ms bipolar pulses at 1 - 4Hz). The samples were chemically stimulated with the β-adrenoceptor agonist isoproterenol (Sigma), which was added to the chamber and mixed for a final concentration of 500 nM.
2.7. Statistical analysis
The results were reported as the mean ± standard error (S.E.M.), and experiments were analyzed by Student’s t-test. Statistical significance was defined as P values < 0.05.
3. Results
3. 1. High density EB cultures promotes cardiac differentiation
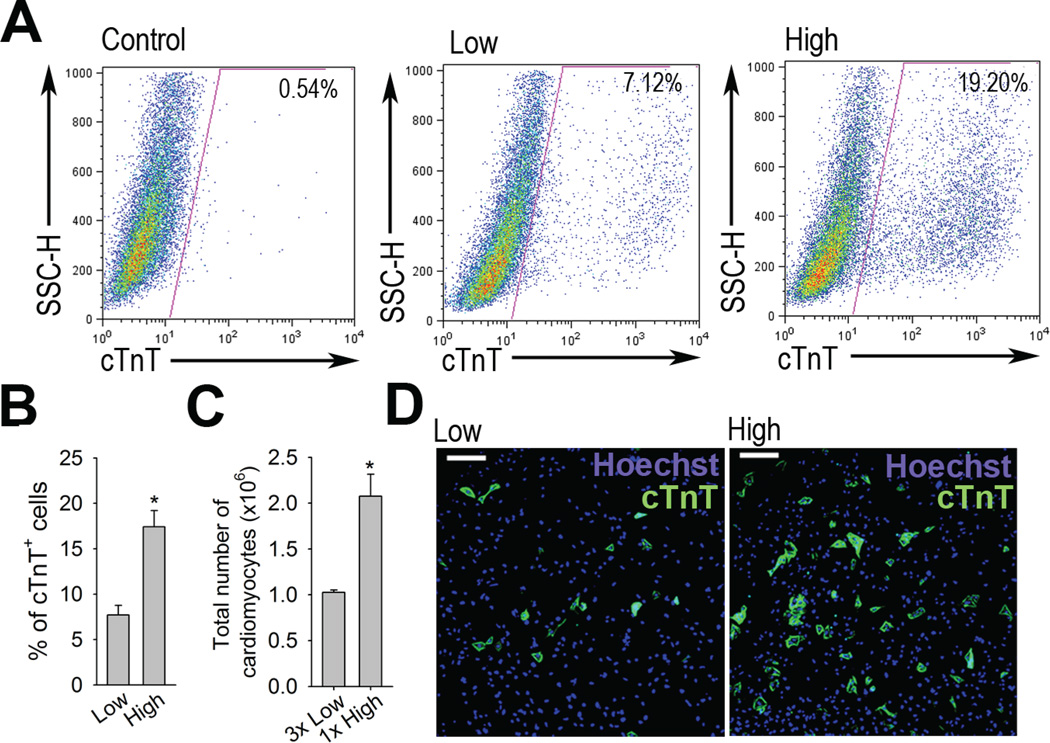
To compare the different culture densities of EBs during cardiac differentiation, we cultured EBs from either one (low density) or three dishes (high density) of 80 EBs in each 60 mm tissue culture dish (Details see Material and Methods). In order to quantify cardiac differentiation, we used flow-cytometry analysis to determine the percentage of cardiomyocytes based on the presence of cardiac specific marker, cardiac troponin T (cTnT) [12] on EB day 10. As shown in Figure 1A and 1B, the percentage of cTnT+ cells was 7.7 ± 1.1 % in low density cultures and 17.4 ± 1.8 % in high density cultures. Cell counting revealed that the total number of cardiomyocytes from three dishes of low density EB cultures (80 EBs/60mm dish) is 1.03 ± 0.02 × 106, while the total number of cardiomyocytes from one dish of high density EB culture (240 EBs/60mm dish) is 2.08 ± 0.24 × 106 (Figure 1B and 1C). The effect of high density cultures to promote cardiac differentiation was reproducibly observed in different type of primary mESC line and E14G mESC line (Figure S1A and S1B). These results suggest that cardiomyocyte differentiation is enhanced in high density EB cultures compared to low density EB cultures. Enhanced cardiomyocyte differentiation was also confirmed by cTnT staining. After enzymatic dissociation of EBs cultured in low and high density, we replated 106 cells from each group in 12-well plate. As shown in Figure 1C, more cTnT+ cardiomyocytes were observed in wells plated with cells from high density cultures. Larger areas of cTnT+ cells and contracting cells were also observed in EBs cultured in high density (Supplemental Figure 1C, Movie S1 and S2). Taken together, cardiomyocyte differentiation is significantly enhanced by high density EB cultures in mESCs.
Fig 1.
High density EB cultures promotes cardiac differentiation. (A) Flow cytometry profile of differentiated mESCs on EB day 10. Differentiated EBs were dissociated into single cells and analyzed for cTnT expression as described in Materials and Methods. Numbers represent the percentage of cTnT+ cells within the indicated region and the figure is representative of six independent experiments using primary mESC line. (B) Graph depicts the percentage of cTnT+ cells cultured in low density and high density culture condition. (C) Graph shows that total number of cardiomyocytes from 3 dishes of low density EB cultures (85 EBs/60mm dish) and 1 dish of high density EB culture (255 EBs/60mm dish). Data are expressed as mean ± S.E.M. of six independent experiments. (D) Immunostaining showing the cTnT+ cells (green fluorescence) of enzymatically dissociated EBs from low and high density EB cultures. Scale bar, 100 µM.
3.2. Cardiomyocytes from low and high density cultures show comparable functional properties
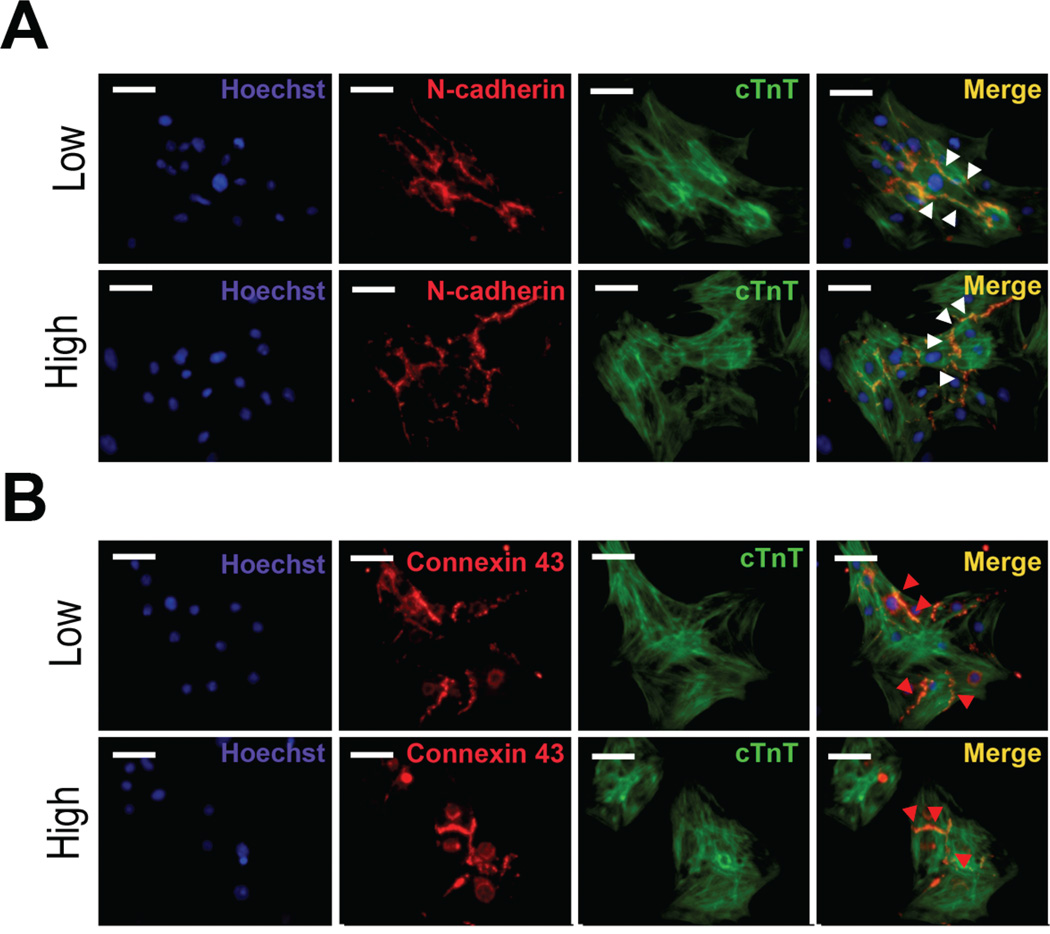
In order to determine the syncytial function of cardiomyocytes, we examined the expression of cardiac junctional proteins. After enzymatic dissociation of beating EBs cultured in low and high density, we replated cells. Immunofluorescence showed that there were no significant differences in the expression of N-cadherin, a major isoform of cadherins in the adherens junction or connexin 43 (Cx43), an important connexin isoform in gap junctions of cardiomyocytes [13] (Figure. 2A and 2B).
Fig 2.
Cardiomyocytes derived from low and high EB cultures shows comparable N-cadherin and connexin43 (Cx43) protein expression. Immunocytochemistry showed that N-cadherin (A) and Cx43 (B) are expressed in dissociated cTnT+ cardiomyocytes derived from low and high density EB cultures. Insets represent magnified views of the white boxes and show that N-cadherin (white arrow heads) and Cx43 (red arrow heads) are expressed at cell-cell interface. Scale bar, 50 µm.
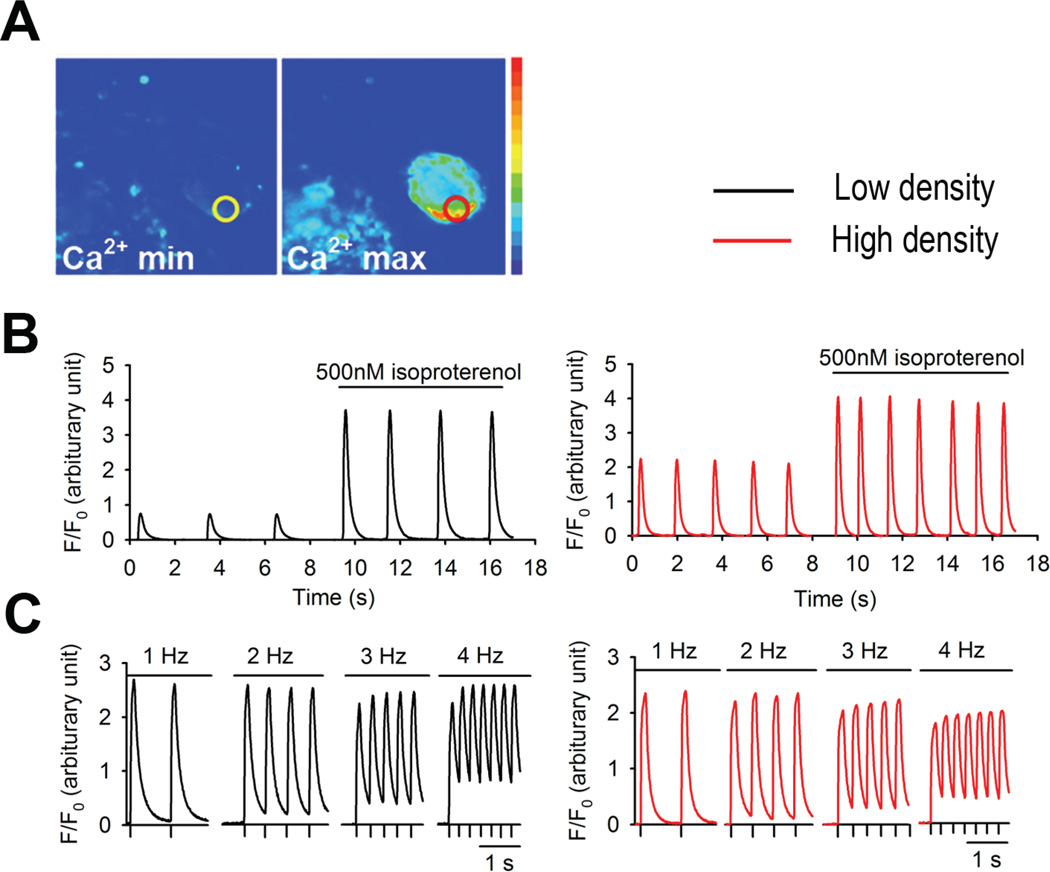
In order to compare the contractile properties of cardiomyocytes derived from low and high density EB cultures real-time intracellular Ca2+ transients were measured. We assessed the transient changes in intracellular Ca2+ concentration of cardiomyocyte clusters by measuring minimum (Ca2+min) and maximum (Ca2+max) [Ca2+]i using fluorescent microscopic methods (Figure 3A). We observed periodic Ca2+ oscillations in cardiomyocyte clusters derived from both low and high density EB cultures. The duration and amplitude of the Ca2+ transients between low and high density cultures-derived cardiomyocyte clusters showed modest differences, possibly due to compositional heterogeneity in the cluster. More importantly, however, we found that both Ca2+ transient amplitude and frequency in low and high-density cultures-derived cardiomyocyte clusters were increased by isoproterenol (500 nM) treatment (Figure 3B), suggesting the existence of functional β-adrenergic signaling pathways. Additionally, [Ca2+]i oscillations responded to increasing frequencies of external electrical field stimulation (Figure 3C), indicating that cardiac excitation-contraction coupling, a key factor for the ultimate cardiac contractility, is present in cardiomyocytes differentiated using either low or high density EB cultures.
Fig 3.
Cardiomyocytes derived from low and high EB cultures shows comparable real time [Ca2+]i transients. Cardiomyocyte clusters derived from beating EBs were loaded with the cell permeant Ca2+ indicator dye fluo-4 AM. Fluorescence was measured by manually defining a region of interest centered on spontaneously beating clusters and comparing that measurement to baseline fluorescence (F/F0). (A) Representative fluorescence and pseudo-colored images of spontaneous cardiomyocyte clusters showing minimal (Ca2+min) and maximal (Ca2+max) fluo-4 fluorescence intensity. Circles indicate the area used to measure Ca2+. (B) Increase in [Ca2+]i transient amplitude and frequency in cardiomyocyte clusters derived from low and high density EB cultures during isoproterenol stimulation (500 nM). (C) Ca2+ transients of cardiomyocyte clusters from low and high density EB cultures in response to electrical pacing at various frequencies (1–4 Hz).
3.3. Inhibition of BMP signaling attenuates cardiogenesis associated with high density EB cultures
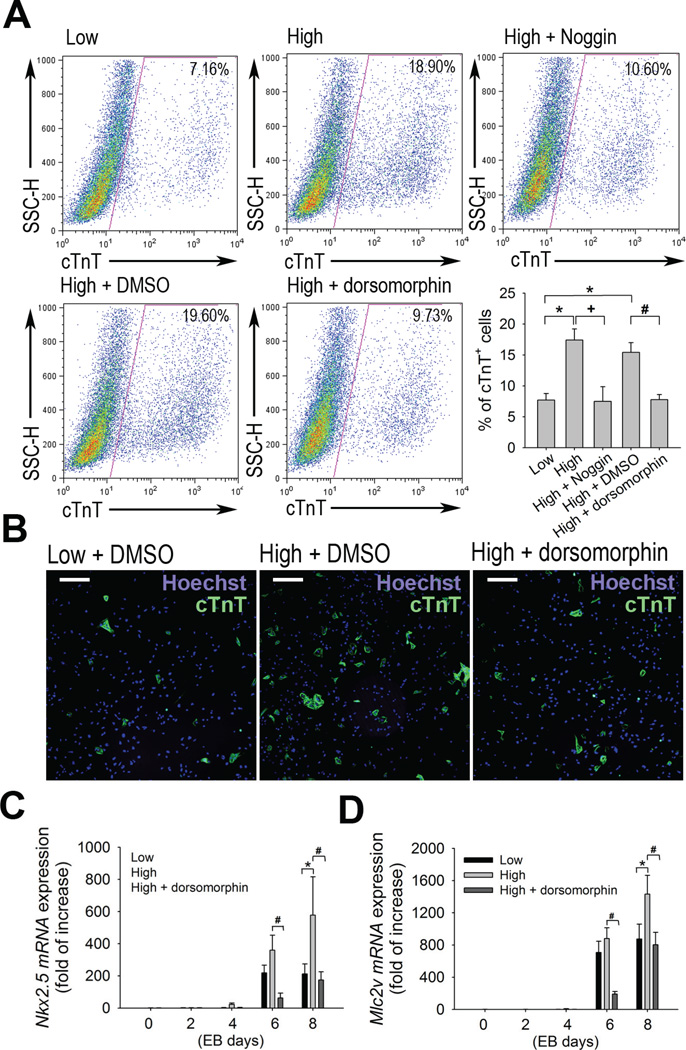
It has been well-known that the BMP signaling positively regulates cardiogenesis [14] and are expressed predominantly during ESC differentiation into cardiomyocytes [10]. In order to test whether BMP signaling is required for the enhancement of cardiogenesis in high density cultures, we performed flow cytometry on the basis of cTnT expression. EBs from high density cultures were treated at the end of EB day 4 with dorsomorphin, a small molecule which specifically blocks BMP-induced Smad activation [15] or Noggin, a BMP antagonist which binds and inactivates BMP. Cardiomyocyte differentiation induced by high density cultures was significantly attenuated by dorsomorphin or noggin treatment (Figure 4A). Consistent with flow cytometry analysis, immunoflurescence staining of cTnT showed that the increased number of cTnT+ cells was reduced in the group treated with dorsomorphine (Figure 4B).
Fig 4.
Inhibition of BMP signaling attenuates increase of cardiac differentiation in high density EB cultures. (A) Representative FACS profile of differentiated mESCs on EB day 10. EBs were cultured in low density or high density. DMSO vehicle control, small molecule BMP antagonist dorsomorphin (2 µM) or peptide antagonist Noggin (100 ng/ml) were added to the culture from EB day 5 to day 10. Differentiated EBs were dissociated into single cells and analyzed for cTnT expression. Data are expressed as mean ± S.E.M of three independent experiments. *P<0.05 vs. low density cultures; +P<0.05 vs. high density cultures; #P<0.05 vs. high density cultures+DMSO. Data are expressed as mean ± S.E.M. (B) Immunostaining showing the cTnT+ cells (green fluorescence) of enzymatically dissociated EBs cultured in low, high density or high density with dorsomorphin. 106 cells from each condition were replated onto gelatinized culture plate after dissociation into single cells. Scale bar, 100 µM. (C-D) Quantitative RT-PCR analysis showing Nkx 2.5 and Mlc2v mRNA expression. EBs were cultured in low, high density, or high density with dorsomorphin and were harvested for RNA isolation on the indicated EB days. Values were normalized to GAPDH and EB day 0 values were arbitrarily set to 1.0. *P<0.05 vs. low density cultures, #P<0.05 vs. high density cultures. Data are expressed as mean ± S.E.M.
In order to compare gene expression in low and high density EB differentiation, we examined cardiac specific transcription factor, Nkx2.5, and myosin light chain 2v (Mlc2v) which is a cardiac contractile protein gene [16] as key markers of cardiac differentiation. Figure 4C and 4D showed that the expressions of these cardiac muscle transcription factor genes were higher in high density experiments than low density and that their expressions were significantly decreased after inhibition of BMP signaling. Our findings suggest that BMP signaling is required for enhanced cardiac differentiation of high density EB cultures.
4. Discussion
In this study, we evaluated the effects of high density EB cultures in multiple mESC lines on cardiac differentiation. Our results demonstrate that EBs in high density cultures markedly promotes cardiogenesis and generate greater quantities of functional cardiomyocytes, which expressed typical cardiac markers, exhibited normal rhythmic Ca2+ transients, and responded to both β-adrenergic and electrical stimulation.
Strategies that facilitate efficient generation of cardiomyocytes from ESCs are critical for developing cell-based cardiac therapy [17; 18] because large amounts of cardiomyocytes are necessary for such interventions. A number of groups have shown that cardiomyocyte differentiation is regulated by diverse signaling pathways such as Wnt [19; 20], BMPs [9; 21];, Hedgehog [22] and Notch [23]. Although cardiomyocyte differentiation methods have been designed based on these regulatory factors they are not efficient and are difficult to reproduce [24]. In this study, we showed a marked increase in the yield of cardiomyocytes by increasing EB culture density without adding ectopic cardiogenic factors. Although previous studies have shown that EB-based differentiation of serum-stimulated ESCs generates cardiomyocytes, the efficiency of this process is low (typically 1–3% from mESCs) [18]. In the present study, however, we showed that the yield of cardiomyocytes is greatly improved solely by increasing EB culture density. Therefore, this study provides a simple and reproducible tool for generation of cardiomyocytes from ESCs. Notably, the baseline Ca2+ transient duration and amplitude (Fig. 3B) between low and high density EB-derived cardiomyocyte clusters showed modest differences possibly due to the existence of non-cardiomyocytes within each beating cluster and/or the difference in differentiation state of cardiomyocytes in the cluster. Future efforts will be needed to develop strategies to derive highly purified, phenotypically mature cardiomyocytes from mESCs.
BMPs are reported to be strong cardiogenic factors [25; 26]. In this study, we have shown that BMP signaling is required in high density EB cultures-enhanced cardiac differentiation (Fig. 4). During development, BMPs secreted from adjacent tissues direct the lateral plate mesoderm toward cardiac lineages by activating several important transcription factors [27]. We hypothesized that BMPs secreted by cells within EBs in high density cultures contribute to the significant increase observed in cardiomyocyte differentiation. Indeed, inhibition of BMP signaling pathway significantly attenuated cardiomyocyte differentiation and cardiac gene expression. Although it is highly unlikely that BMPs are the only factors necessary for the improved cardiac differentiation in high density cultures, it is clear that BMP signaling plays an important role in the increases in cardiac differentiation of cardiovascular progenitor cells. It is possible that BMPs synergistically regulate cardiomyocyte differentiation with other factors such as Wnt-11 [28], Chibby [29], retinoic acid [30], ascorbic acid [31], nitric oxide [32].
There are significant advantages associated with high density EB culture such as simplification of the differentiation step of ESC cardiac differentiation. Previously established protocols suggest that ESC cardiac differentiation is comprised of 3 steps: formation of EBs, collection and further cultivation of EBs in suspension, and plating/induction of outgrowth of EBs in tissue culture plates [11; 33]. In this study, we directly transferred EBs to adhesion culture and induced mESC differentiation. This protocol also maximizes the production of differentiated cardiomyocytes from mESCs while minimizing cost. In this study, we significantly improved cardiac differentiation by using FBS-based differentiation medium instead of expensive growth factors. Therefore, we believe that we offer an efficient means of cardiomyocyte production for practical use of mESCs in cardiac regenerative medicine.
Highlights.
High density embryoid body cultures increase the yield of functional cardiomyocytes. These cardiomyocytes exhibit cardiac markers and electrophysiological activities. The cardiogenic effect of high density cultures requires BMP signaling.
Supplementary Material
Acknowledgements
This work was supported by the Yale startup fund, NIH 1K02HL101990-01, UL1 RR024139 and AHA 09SDG2080420 (YQ), NIH 5T32 HL007950 (PJ), and NIH DK57751 and DK061747 (BEE). We thank Dr. Michael Simons for sharing his lab equipment.
The abbreviations used are:
- BMP
bone morphogenetic protein
- cTnT
cardiac troponin T
- Cx43
connexin 43
- EB
embryoid body
- ESC
embryonic stem cell
- MEF
mouse embryonic fibroblast
- MI
myocardial infarction
- MLC2v
myosin light chain 2v
- Nkx2.5
NK2 transcription factor related 5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Liew CG, Moore H, Ruban L, Shah N, Cosgrove K, Dunne M, Andrews P. Human embryonic stem cells: possibilities for human cell transplantation. Ann Med. 2005;37:521–532. doi: 10.1080/07853890500379463. [DOI] [PubMed] [Google Scholar]
- 3.Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto H, Quinn G, Asari A, Yamanokuchi H, Teratani T, Terada M, Ochiya T. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37:983–993. doi: 10.1053/jhep.2003.50202. [DOI] [PubMed] [Google Scholar]
- 5.Zhang WJ, Park C, Arentson E, Choi K. Modulation of hematopoietic and endothelial cell differentiation from mouse embryonic stem cells by different culture conditions. Blood. 2005;105:111–114. doi: 10.1182/blood-2004-04-1306. [DOI] [PubMed] [Google Scholar]
- 6.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 7.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Wijk B, Moorman AF, van den Hoff MJ. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc Res. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–611. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 10.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- 12.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noorman M, van der Heyden MA, van Veen TA, Cox MG, Hauer RN, de Bakker JM, van Rijen HV. Cardiac cell-cell junctions in health and disease: Electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47:23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Monzen K, Shiojima I, Hiroi Y, Kudoh S, Oka T, Takimoto E, Hayashi D, Hosoda T, Habara-Ohkubo A, Nakaoka T, Fujita T, Yazaki Y, Komuro I. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol Cell Biol. 1999;19:7096–7105. doi: 10.1128/mcb.19.10.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzmenkin A, Liang H, Xu G, Pfannkuche K, Eichhorn H, Fatima A, Luo H, Saric T, Wernig M, Jaenisch R, Hescheler J. Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB J. 2009;23:4168–4180. doi: 10.1096/fj.08-128546. [DOI] [PubMed] [Google Scholar]
- 17.Chien KR, Domian IJ, Parker KK. Cardiogenesis and the complex biology of regenerative cardiovascular medicine. Science. 2008;322:1494–1497. doi: 10.1126/science.1163267. [DOI] [PubMed] [Google Scholar]
- 18.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Arnold SJ, Stappert J, Bauer A, Kispert A, Herrmann BG, Kemler R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev. 2000;91:249–258. doi: 10.1016/s0925-4773(99)00309-3. [DOI] [PubMed] [Google Scholar]
- 20.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 22.Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134:1593–1604. doi: 10.1242/dev.02824. [DOI] [PubMed] [Google Scholar]
- 23.Grego-Bessa J, Luna-Zurita L, del Monte G, Bolos V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama YS, Chen H, Shou W, Ballestar E, Esteller M, Rojas A, Perez-Pomares JM, de la Pompa JL. Notch signaling is essential for ventricular chamber development. Dev Cell. 2007;12:415–429. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoji K, Yuasa S, Onizuka T, Hattori F, Tanaka T, Hara M, Ohno Y, Chen H, Egasgira T, Seki T, Yae K, Koshimizu U, Ogawa S, Fukuda K. G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell. 2010;6:227–237. doi: 10.1016/j.stem.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 26.Varga AC, Wrana JL. The disparate role of BMP in stem cell biology. Oncogene. 2005;24:5713–5721. doi: 10.1038/sj.onc.1208919. [DOI] [PubMed] [Google Scholar]
- 27.Alsan BH, Schultheiss TM. Regulation of avian cardiogenesis by Fgf8 signaling. Development. 2002;129:1935–1943. doi: 10.1242/dev.129.8.1935. [DOI] [PubMed] [Google Scholar]
- 28.Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun. 2004;325:968–975. doi: 10.1016/j.bbrc.2004.10.103. [DOI] [PubMed] [Google Scholar]
- 29.Singh AM, Li FQ, Hamazaki T, Kasahara H, Takemaru K, Terada N. Chibby, an antagonist of the Wnt/beta-catenin pathway, facilitates cardiomyocyte differentiation of murine embryonic stem cells. Circulation. 2007;115:617–626. doi: 10.1161/CIRCULATIONAHA.106.642298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wobus AM, Kaomei G, Shan J, Wellner MC, Rohwedel J, Ji G, Fleischmann B, Katus HA, Hescheler J, Franz WM. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol. 1997;29:1525–1539. doi: 10.1006/jmcc.1997.0433. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T, Lord B, Schulze PC, Fryer RM, Sarang SS, Gullans SR, Lee RT. Ascorbic acid enhances differentiation of embryonic stem cells into cardiac myocytes. Circulation. 2003;107:1912–1926. doi: 10.1161/01.CIR.0000064899.53876.A3. [DOI] [PubMed] [Google Scholar]
- 32.Kanno S, Kim PK, Sallam K, Lei J, Billiar TR, Shears LL., 2nd Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12277–12281. doi: 10.1073/pnas.0401557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachinidis A, Fleischmann BK, Kolossov E, Wartenberg M, Sauer H, Hescheler J. Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc Res. 2003;58:278–291. doi: 10.1016/s0008-6363(03)00248-7. [DOI] [PubMed] [Google Scholar]
- 34.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.