Abstract
Objectives
Xeroderma pigmentosum group G (XPG) protein is essential for the nucleotide excision repair (NER) system, and genetic variations in XPG/ERCC5 that affect DNA repair capacity may contribute to the risk of tobacco-induced cancers, including squamous cell carcinoma of the head and neck (SCCHN). We investigated the association between XPG/ERCC5 polymorphisms and risk of squamous cell carcinoma of the head and neck (SCCHN).
Methods
We genotyped 12 tagging and potentially functional single nucleotide polymorphisms (SNPs) of XPG/ERCC5 in a case-control study of 1,059 non-Hispanic white patients with SCCHN and 1,066 cancer-free age-and sex matched controls and evaluated their associations with SCCHN risk.
Results
Multivariate logistic regression showed that only an intronic tagging SNP (rs4150351A/C) of XPG/ERCC5 was associated with a decreased risk of SCCHN (adjusted OR=0.76, 95% CI=0.62–0.92 for AC vs. AA; adjusted OR=0.81, 95% CI=0.67–0.98 for AC/CC vs. AA), but this association was nonsignificnant after corrections by the permutation test (empirical P=0.105). In the genotype-phenotype correlation analysis using peripheral lymphocytes from 44 SCCHN patients, we found that rs4150351 AC/CC was associated with a statistically significant increase in XPG/ERCC5 mRNA expression.
Conclusion
These findings suggest that genetic variation in XPG/ERCC5 may not affect the SCCHN risk, although rs4150351 C variant genotypes were associated with the increased expression of XPG/ERCC5 mRNA and nonsignificantly decreased risk of SCCHN. Larger population-based and additional functional studies are warranted to validate our findings.
Keywords: ERCC5, polymorphism, SCCHN, risk
Introduction
DNA repair plays a critical role in protecting the genome from insults of environmental carcinogens, such as tobacco smoke and ultraviolet (UV) radiation [1, 2]. To date, more than 150 genes are involved in at least five distinct DNA repair pathways in humans: nucleotide excision repair (NER), base excision repair (BER), mismatch repair (MMR), homologous recombination (HR) and non-homologous end joining (NHEJ) [3, 4]. Of those pathways, NER is the most versatile repair mechanism responsible for many different forms of DNA damage, including bulky adducts cross-links, oxidative DNA damage, thymidine dimers, and alkylating damage [5]. Studies have shown that there are inter-individual variations of DNA repair capacity (DRC) in the general population and that a suboptimal DRC has an effect on risk of smoking-related cancers, such as lung cancer and squamous cell carcinoma of the head and neck (SCCHN) [6, 7]. Furthermore, accumulated evidence has also shown that genetic variants in one or more NER genes contribute to phenotypic variation of DRC, thereby modifying the susceptibility to cancer [8-12].
Xeroderma pigmentosum group G (XPG), also known as ERCC5, is one of eight NER core genes (i.e., ERCC1, XPA, XPB/ERCC3, XPC, XPD/ERCC2, XPE/DDB1, XPF/ERCC4, and XPG/ERCC5) in humans, which mainly functions as a structure-specific endonuclease that cleaves the damaged DNA strand on the 3’ endside [13, 14]. In addition, XPG stimulates BER of oxidative DNA damage to facilitate efficient transcription by RNA polymerase II [15, 16]. As one of the key factors of the NER pathway, XPG/ERCC5 has been widely explored for its role in carcinogenesis with various tumor cell lines or tissues. For example, its expression levels were found to be correlated with risk and prognosis of several human cancers, including SCCHN [17-21]. Recent studies further suggested that XPG/ERCC5 expression levels were related to cellular NER activity and response to cisplatin and irofulven of therapeutic agents, potentially making it an attractive therapeutic target for human cancers [17, 21, 22].
Located on chromosome 13q32.3-q33.1, XPG/ERCC5 contains 15 exons, spanning 32 kb in length. At least 446 single nucleotide polymorphisms (SNPs) in the XPG/ERCC5 gene have been identified (http://www.ncbi.nlm.nih.gov/projects/SNP/); however, only few are potentially functional, which may affect gene expression, protein functions, or cellular DRC [23, 24]. Of those SNPs, the His1104Asp polymorphism (rs17655) located in the exon 15 has been largely investigated in genetic and epidemiologic studies of susceptibility to cancers of the breast [25], lung [26], stomach [27], bladder [28], colorectum [29], and head and neck [30-33]. Additionally, it has been reported that rs17655 of XPG/ERCC5 together with SNPs of several other NER genes jointly contributed to the variability of DRC [34]. These support the hypothesis that variants of XPG/ERCC5 may be associated with development of human cancers. However, to our knowledge, few studies have comprehensively investigated associations between SNPs of XPG/ERCC5 and SCCHN risk [30-33].
Using a comprehensive approach of selecting 12 SNPs of XPG/ERCC5 that tag all common (minor allele frequency [MAF] ≥0.05) of the gene from the NIEHS database (http://egp.gs.washington.edu/) and the HapMap database (http://www.hapmap.org/), we conducted a large case-control study of 1,059 non-Hispanic white patients with SCCHN and 1,066 cancer-free age-and-sex matched controls to investigate associations between these SNPs and SCCHN risk and to evaluate modification effects of both the tagging and potentially functional SNPs in XPG/ERCC5 on SCCHN risk.
Materials and Methods
Study population
Participant recruitment was described previously [33, 35, 36]. Briefly, all patients with histopathologically confirmed SCCHN were consecutively recruited from The University of Texas M.D. Anderson Cancer Center between October 1999 and October 2007. Among all patients initially contacted for participation, approximately 90% of eligible cases agreed to participate in the study. There was no age, sex, histology or stage restrictions, but patients with second SCCHN primary tumors, primary tumors of the nasopharynx or sinonasal tract, metastasized cancer from other organs, or any histopathologic diagnosis other than SCCHN, were excluded. Cancer-free controls, frequency-matched to cases on age (±5 years) and sex, were recruited from those visitors to outpatients at M.D. Anderson Cancer Center. These individuals were not related genetically to the enrolled cases or to each other. The overall response rate of controls was approximately 85%. The designed questionnaires were used to acquire subjects’ information on demographic data and environmental exposure history, such as age, sex, smoking and alcohol consumption. After the interview, approximately 30-ml venous blood sample was collected from each study participant. Among all cases, only 44 subjects had some left-over frozen PBMCs (blood mononuclear cells) available for culture, which had different genotypes for SNPs, and were used for evaluating mRNA expression levels. All subjects were non-Hispanic whites and a total of 1,059 cases and 1,066 controls that completed the interview and donated a one-time blood sample were included in the analysis. This study was approved by the institutional review board of M. D. Anderson. Informed consent was obtained from all study subjects.
SNPs selection and genotyping
Polymorphisms of the XPG/ERCC5 gene were selected by a comprehensive approach combining potentially functional or tagging SNPs. The NIEHS database (http://egp.gs.washington.edu/) and the HapMap database (http://www.hapmap.org/) were used to search for all common SNPs (MAF ≥ 0.05 in European populations) located in or within a 3-kb region centered around the XPG/ERCC5 gene, potentially functional significance of which was then predicted by the online software, Pupasuite 2 (http://pupasview.bioinfo.ochoa.fib.es/) and FuncPred (http://manticore.niehs.nih.gov/snpfunc.htm). In this study, potentially functional SNPs included all those related to amino acid changing, transcription factor binding sites (TFBS), exonic splicing enhancers (ESE), exonic splicing silencers (ESS) and miRNA binding sites. Furthermore, common tagging SNPs (Hardy-Weinberg equilibrium P ≥ 0.05 and call rate ≥85%) were identified by the Haploview software on the basis of pairwise linkage disequilibrium (LD) (r2 threshold: 0.8) and with a priority of forcing the potentially functional SNPs in the selection. As a result, 12 SNPs (Table 1 and Supplementary Fig. 1) were selected for genotyping by using the SNPlex assay in the DNA Core Facility at MD Anderson Cancer Center, according to the protocol of the manufacturer (Applied Biosystems, Foster City, CA). The output data from the SNPlex assays were analyzed using the GeneMapper software (Applied Biosystems) to determine the genotypes. In addition, the polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) method was also used to revaluate the genotypes of samples that failed in the SNPlex assays. Approximately 10% of the samples were randomly selected to perform the repeated genotyping assays, and the results were 100% concordant.
Table 1.
Primary information and genotyping results of selected SNPs in XPG/ERCC5
| Gene and locus | NCBI rs # | Location | Type | Base change | MAF in Hapmap database (European) | MAF in our cases | MAF in our controls | HWE | Genotyping rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| XPG/ERCC5 | rs2094258 | 5’ near gene | TFBS | C>T | 0.22 | 0.18 | 0.18 | 0.09 | 98.4 |
| 13q22 | rs2296147 | 5’UTR | TFBS | T>C | 0.38 | 0.49 | 0.47 | 0.44 | 99.8 |
| rs4771436 | intron_1 | tagging | T>G | 0.23 | 0.23 | 0.23 | 0.94 | 99.8 | |
| rs1047768 | exon_2 (H46H) | ESE or ESS | C>T | 0.45 | 0.41 | 0.41 | 0.67 | 99.9 | |
| rs2227869 | exon_8 (C529S) | non-synonymous | G>C | 0.05 | 0.04 | 0.04 | 0.95 | 100 | |
| rs4150351 | intron_12 | tagging | A>C | 0.18 | 0.17 | 0.19 | 0.11 | 99.9 | |
| rs4150355 | intron_12 | tagging | C>T | 0.32 | 0.38 | 0.36 | 0.91 | 99.5 | |
| rs4150383 | intron_14 | tagging | G>A | 0.17 | 0.18 | 0.17 | 0.97 | 99.9 | |
| rs4150386 | intron_14 | tagging | A>C | 0.12 | 0.12 | 0.12 | 0.72 | 100 | |
| rs17655 | exon_15 (D1104H) | non-synonymous | G>C | 0.27 | 0.22 | 0.22 | 0.10 | 100 | |
| rs873601 | 3’UTR | miRNA binding | A>G | 0.38 | 0.26 | 0.27 | 0.45 | 99.9 | |
| rs4150393 | 3’ near gene | tagging | A>G | 0.10 | 0.12 | 0.13 | 0.10 | 100 |
NCBI = National Center for Biotechnology Information; MAF= minor allele frequency; HWE= Hardy-Weinberg equilibrium; TFBS=transcription factor binding sites; ESE=exonic splicing enhancers; ESS=exonic splicing silencers
Quantitative measurement of ERCC5 mRNA expression
Quantitative real-time reverse transcription-PCR (qRT-PCR) was used to determine the expression level of XPG/ERCC5 in peripheral blood mononuclear cells (PBMCs). Total RNA was isolated from phytohemagglutinin-stimulated peripheral blood lymphocytes from 44 SCCHN patients by using the TRIzol reagent (Invitrogen™, USA). Expression levels of the target and reference genes were analyzed using an ABI7900 sequence detection system (Applied Biosystems, Foster City, CA) with a final volume of 5 μl containing 5 ng of the cDNA, 0.25 μl primers, and 2.5 μl Master mix. The thermal cycling conditions were: 95°C for 5 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. 18S RNA was measured as an endogenous control to normalize for differences in the amount of cDNA used in each reaction. XPG/ERCC5 and 18S mRNA levels were quantified in separate tubes in duplicates, and expression level of XPG/ERCC5 relative to that of 18S was calculated using the equation ratio = CtERCC5/Ct18S*100%.
Statistical analysis
Deviation of genotype frequencies for each SNP from the Hardy-Weinberg equilibrium was tested by a goodness-of-fit χ2 test. Distributions of demographic characteristics, selected variables, and frequencies of genotypes of XPG/ERCC5 between cases and controls were evaluated by using the χ2 test. The associations between XPG/ERCC5 genotypes and SCCHN risk were estimated by computing odds ratios (ORs) and 95% confidence intervals (CIs) in different genetic models from both univariate and multivariate Logistic regression with adjustment for age, sex, smoking status and alcohol use. We also corrected multiple testing on associations of all SNPs by using Stata 10.0 through 1000 permutations that randomly permutated the case/control status independent of genotypes. The SAS TTEST procedure was used to compare the expression levels of XPG/ERCC5 between cases with different genotypes. All of the statistical analyses were two-sided with a significance level of 0.05 and performed with Statistical Analysis System software (v.9.2 SAS Institute, Cary, NC).
Results
Characteristics of study subjects
The details of 1,059 cases and 1,066 controls enrolled in this study are shown in Table 2. The mean age was 57.0 years (±11.1) for cases and 56.6 years (±11.0) for controls, and the frequency-matching on age and sex between cases and controls was adequate (P=0.522 and 0.660, respectively). However, cases were more likely to be smokers (71.9% vs. 51.1%, P< 0.001) and drinkers (72.6% vs. 56.7%, P< 0.001) than controls. Among all SCCHN cases, 317 (29.9%) had primary tumors of the oral cavity, 538 (50.8%) of the oropharynx and 204 (19.3%) of the hypopharynx/larynx. In addition, 259 cases (24.5) were of I-II stages, and 800 cases (75.5%) were of III-IV stages.
Table 2.
Distributions of selected variables in SCCHN cases and cancer-free controls.
| Variables | Cases N (%) | Controls N (%) | Pa |
|---|---|---|---|
| All subjects | 1,059 (100) | 1,066 (100) | |
| Age, yr | 0.522 | ||
| ≤57(median) | 570 (53.8) | 559 (52.4) | |
| >57(median) | 489 (46.2) | 507 (47.6) | |
| Sex | 0.660 | ||
| Females | 262 (24.7) | 255 (23.9) | |
| Males | 797 (75.3) | 811 (76.1) | |
| Smoking status | <0.001 | ||
| No | 298 (28.1) | 521 (48.9) | |
| Yes | 761 (71.9) | 545 (51.1) | |
| Alcohol status | <0.001 | ||
| No | 290 (27.4) | 462 (43.3) | |
| Yes | 769 (72.6) | 604 (56.7) | |
| Tumor site | |||
| Oral cavity | 317 (29.9) | ||
| Oropharynx | 538 (50.8) | ||
| Hypopharynx/ Larynx | 204 (19.3) | ||
| Stage | |||
| I-II | 259 (24.5) | ||
| III-IV | 800 (75.5) |
Two-sided χ2 test
Associations between XPG/ERCC5 variants and SCCHN risk
The position, MAF and genotyping rate of the 12 selected SNPs are presented in Table 1. The observed genotype frequencies for these 12 SNPs were all in Hardy-Weinberg equilibrium in the controls (P>0.004, 0.05/12), and the SNP calling rates were all >98.0%. The genotype frequencies of XPG/ERCC5 SNPs in the cases and the controls are summarized in Table 3. In the single locus analyses, only the genotype frequencies of rs4150351 were significantly different between the cases and the controls (P=0.005). After adjustment for age, sex, smoking and alcohol status, multivariate Logistic regression analysis further revealed that variant genotypes of rs4150351A/C were significantly associated with a decreased risk of SCCHN (adjusted OR=0.76, 95% CI=0.62−0.92 for AC vs. AA and adjusted OR=0.81, 95% CI=0.67–0.98 for AC/CC vs. AA). To reduce the false discovery rate, we further used permutation to asses statistical significance of SNPs (1000 permutations) and found that the P value for rs4150351 was the smallest among of all 12 SNPs, but the p value changed to non-significant (Empirical P=0.105). No significant associations with SCCHN risk were identified for other 11 SNPs examined in this study (Table 3).
Table 3.
Logistic regression analysis for associations between XPG/ERCC5 polymorphisms and SCCHN risk.
| XPG/ERCC5 Locus | Genotype | Cases a N (%) | Controls a N (%) | Pb | Crude OR (95% CI) | Adjusted OR (95% CI) c | Pc |
|---|---|---|---|---|---|---|---|
| N=1,038 | N=1,053 | ||||||
| rs2094258 | CC | 706 (68.0) | 721 (68.5) | 0.808d | 1.00 | 1.00 | |
| CT | 295 (28.4) | 291 (27.6) | 1.04 (0.85-1.26) | 1.00 (0.82-1.22) | 0.991 | ||
| TT | 37 (3.6) | 41 (3.9) | 0.92 (0.58-1.46) | 0.93 (0.58-1.48) | 0.749 | ||
| CT/TT | 332 (32.0) | 332 (31.5) | 0.851e | 1.02 (0.85-1.23) | 0.99 (0.82-1.20) | 0.934 | |
|
| |||||||
| N=1,056 | N=1,065 | ||||||
| rs2296147 | TT | 280 (26.5) | 294 (27.6) | 0.619 d | 1.00 | 1.00 | |
| CT | 532 (50.4) | 543 (51.0) | 1.03 (0.84-1.26) | 1.02 (0.83-1.25) | 0.876 | ||
| CC | 244 (23.1) | 228 (21.4) | 1.12 (0.88-1.43) | 1.13 (0.88-1.45) | 0.346 | ||
| CT/CC | 776 (73.5) | 771 (72.4) | 0.591 e | 1.06 (0.87-1.28) | 1.06 (0.87-1.28) | 0.572 | |
|
| |||||||
| N=1,057 | N=1,064 | ||||||
| rs4771436 | TT | 630 (59.6) | 637 (59.9) | 0.971 d | 1.00 | 1.00 | |
| GT | 374 (35.4) | 372 (34.9) | 1.02 (0.85-1.22) | 1.01 (0.84-1.22) | 0.920 | ||
| GG | 53 (5.0) | 55 (5.2) | 0.97 (0.66-1.44) | 0.98 (0.65-1.46) | 0.908 | ||
| GT/TT | 427 (40.4) | 427 (40.1) | 0.929 e | 1.01 (0.85-1.20) | 1.01 (0.84-1.20) | 0.954 | |
|
| |||||||
| N=1,059 | N=1,065 | ||||||
| rs1047768 | CC | 369 (34.8) | 379 (35.6) | 0.911 d | 1.00 | 1.00 | |
| CT | 506 (47.8) | 507 (47.6) | 1.03 (0.85-1.24) | 0.98 (0.81-1.19) | 0.855 | ||
| TT | 184 (17.4) | 179 (16.8) | 1.06 (0.82-1.36) | 1.07 (0.82-1.38) | 0.630 | ||
| CT/TT | 690 (65.2) | 686 (64.4) | 0.720 e | 1.03 (0.87-1.23) | 1.00 (0.84-1.21) | 0.971 | |
|
| |||||||
| N = 1,059 | N = 1,066 | ||||||
| rs2227869 | GG | 987 (93.2) | 974 (91.4) | 0.278 d | 1.00 | 1.00 | |
| CG | 70 (6.6) | 90 (8.4) | 0.77 (0.56-1.06) | 0.73 (0.52-1.01) | 0.060 | ||
| CC | 2 (0.2) | 2 (0.2) | 0.99 (0.14-7.02) | 0.84 (0.12-6.16) | 0.864 | ||
| CG/CC | 72 (6.8) | 92 (8.6) | 0.123 e | 0.77 (0.56-1.06) | 0.73 (0.52-1.01) | 0.060 | |
|
| |||||||
| N = 1,057 | N = 1,065 | ||||||
| rs4150351 | AA | 740 (70.0) | 693 (65.1) | 0.005 d | 1.00 | 1.00 | |
| AC | 275 (26.0) | 342 (32.1) | 0.75 (0.62-0.91) | 0.76 (0.62-0.92) | 0.006 | ||
| CC | 42 (4.0) | 30 (2.8) | 1.31 (0.81-2.12) | 1.46 (0.89-2.39) | 0.138 | ||
| AC/CC | 317 (30.0) | 372 (34.9) | 0.016 e | 0.80 (0.67-0.96) | 0.81 (0.67-0.98) | 0.030 | |
|
| |||||||
| N = 1,054 | N = 1,061 | ||||||
| rs4150355 | CC | 408 (38.7) | 436 (41.1) | 0.323 d | 1.00 | 1.00 | |
| CT | 488 (46.3) | 487 (45.9) | 1.07 (0.89-1.29) | 1.06 (0.88-1.28) | 0.550 | ||
| TT | 158 (15.0) | 138 (13.0) | 1.22 (0.94-1.60) | 1.22 (0.93-1.61) | 0.155 | ||
| CT/TT | 646 (61.3) | 625 (58.9) | 0.267 e | 1.11 (0.93-1.32) | 1.10 (0.92-1.31) | 0.323 | |
|
| |||||||
| N = 1,059 | N = 1,063 | ||||||
| rs4150383 | GG | 720 (68.0) | 732 (68.9) | 0.906 d | 1.00 | 1.00 | |
| AG | 308 (29.1) | 300 (28.2) | 1.04 (0.86-1.26) | 1.02 (0.84-1.24) | 0.822 | ||
| AA | 31 (2.9) | 31 (2.9) | 1.02 (0.61-1.69) | 1.04 (0.61-1.76) | 0.886 | ||
| AG/AA | 339 (32.0) | 331 (31.1) | 0.674 e | 1.04 (0.87-1.25) | 1.02 (0.85-1.24) | 0.804 | |
|
| |||||||
| N = 1,059 | N = 1,066 | ||||||
| rs4150386 | AA | 823 (77.7) | 825 (77.4) | 0.974 d | 100 | 1.00 | |
| AC | 223 (21.1) | 227 (21.3) | 0.99 (0.80-1.21) | 0.98 (0.79-1.22) | 0.862 | ||
| CC | 13 (1.2) | 14 (1.3) | 0.93 (0.44-1.99) | 0.87 (0.40-1.89) | 0.716 | ||
| AC/CC | 236 (22.3) | 241 (22.6) | 0.876 e | 0.98 (0.80-1.20) | 0.97 (.79-1.20) | 0.806 | |
|
| |||||||
| N = 1,059 | N = 1,066 | ||||||
| rs17655 | GG | 648 (61.2) | 654 (61.4) | 0.608 d | 1.00 | 1.00 | |
| CG | 359 (33.9) | 350 (32.8) | 1.04 (0.86-1.24) | 1.02 (0.84-1.23) | 0.860 | ||
| CC | 52 (4.9) | 62 (5.8) | 0.85 (0.58-1.24) | 0.85 (0.57-1.27) | 0.434 | ||
| CG/CC | 411 (38.8) | 412 (38.6) | 0.965 e | 1.01 (0.85-1.20) | 0.99 (0.83-1.19) | 0.937 | |
|
| |||||||
| N = 1,058 | N = 1,066 | ||||||
| rs873601 | AA | 565 (53.4) | 572 (53.7) | 0.323 d | 1.00 | 1.00 | |
| AG | 427 (40.4) | 411 (38.5) | 1.05 (0.88-1.26) | 1.08 (0.90-1.30) | 0.397 | ||
| GG | 66 (6.2) | 83 (7.8) | 0.81 (0.57-1.14) | 0.82 (0.57-1.17) | 0.265 | ||
| AG/GG | 493 (46.6) | 494 (46.3) | 0.931 e | 1.01 (0.85-1.20) | 1.04 (0.87-1.24) | 0.678 | |
|
| |||||||
| N = 1,059 | N = 1,066 | ||||||
| rs4150393 | AA | 821 (77.5) | 814 (76.4) | 0.787 d | 1.00 | 1.00 | |
| AG | 217 (20.5) | 228 (21.4) | 0.94 (0.77-1.16) | 0.94 (0.76-1.17) | 0.574 | ||
| GG | 21 (2.0) | 24 (2.2) | 0.87 (0.48-1.57) | 0.87 (0.47-1.60) | 0.649 | ||
| AG/GG | 238 (22.5) | 252 (23.6) | 0.537 e | 0.94 (0.77-1.15) | 0.93 (0.76-1.15) | 0.514 | |
The numbers were not the same for each SNP because of their different calling rates due to few uncalled samples.
Chi square test for genotype distributions between cases and controls.
Adjusted by age, gender, smoking and alcohol status in Logistic regress models
for genotyping distribution
for dominant genetic models
We further evaluated the effect of rs4150351AC/CC genotypes on SCCHN risk stratified by selected variables including age, sex, smoking status, alcohol status, tumor site and stage (Table 4). Although the protective effect of rs4150351AC/CC genotypes were statistically significant in some groups, heterogeneity test showed that there was no significant heterogeneity (P>0.05) between every two strata, suggesting no risk effect modification by the variables under investigation.
Table 4.
Stratified analysis for XPG/ERCC5 rs4150351 genotypes and SCCHN risk
| Variables | rs4150351 (Cases/ Controls)
|
Crude OR (95% CI) | Adjusted OR (95% CI)a | P for Heterogeneity | |
|---|---|---|---|---|---|
| AA | AC/CC | ||||
| XPG/ERCC5 | 740/693 | 317/372 | 0.80 (0.67-0.96) | 0.81 (0.67-0.98) | |
| Age | |||||
| ≤57(median) | 401/365 | 167/194 | 0.78 (0.61-1.01) | 0.79 (0.61-1.02) | 0.653 |
| >57(median) | 339/328 | 150/178 | 0.82 (0.63-1.06) | 0.85 (0.65-1.12) | |
| Sex | |||||
| Females | 177/164 | 85/91 | 0.87 (0.61-1.25) | 0.92 (0.63-1.34) | 0.424 |
| Males | 563/529 | 232/281 | 0.78 (0.63-0.96) | 0.77 (0.62-0.96) | |
| Smoking status | |||||
| Never | 202/332 | 96/189 | 0.84 (0.62-1.13) | 0.84 (0.62-1.14) | 0.756 |
| Ever | 538/361 | 221/183 | 0.81 (0.64-1.03) | 0.79 (0.62-1.00) | |
| Alcohol status | |||||
| Never | 199/306 | 91/155 | 0.90 (0.66-1.24) | 0.90 (0.66-1.24) | 0.399 |
| Ever | 541/387 | 226/217 | 0.75 (0.59-0.94) | 0.76 (0.60-0.96) | |
| Tumor site | |||||
| Oral cavity | 220/693 | 97/372 | 0.82 (0.63-1.08) | 0.83 (0.63-1.10) | 0.953 |
| Oropharynx | 378/693 | 160/372 | 0.79 (0.63-0.99) | 0.78 (0.62-0.99) | |
| Hypopharynx/Larynx | 142/693 | 60/372 | 0.79 (0.57-1.09) | 0.80 (0.57-1.14) | |
| Stage | |||||
| I-II | 175/693 | 83/372 | 0.88 (0.66-1.18) | 0.89 (0.66-1.21) | 0.522 |
| III-IV | 565/693 | 234/372 | 0.77 (0.63-0.94) | 0.79 (0.64-0.96) | |
Adjusted for age, sex, smoking status and alcohol status in Logistic regression models
Because 10 of 12 SNPs (rs2094258, rs2296147, rs4771436, rs1047768, rs2227869, rs4150351, rs4150355, rs4150383, rs4150386 and rs17655) are in the same block and in incomplete LD (0<r2<0.8) (Supplementary Fig.1), we conducted the haplotype analysis for these 10 SNPs. A total of 53 haplotypes were derived from the observed genotypes, of which CCTCGATGAG was the most common haplotype in cases and controls with the frequencies of 26.3 and 24.3%, respectively. However, no significant associations were found between all other haplotypes and risk of SCCHN, compared with the common CCTCGATGAG haplotype (data not shown).
Correlation analysis for XPG/ERCC5 expression and XPG/ERCC5 genotypes
To further explore the functional relevance of XPG/ERCC5 polymorphisms, we conducted genotype-phenotype correlation analysis between variant genotypes of rs4150351 and levels of XPG/ERCC5 mRNA expression in lymphocytes from 44 SCCHN cases. The means±SD of relative levels were 241.6±19.2, 231.3±16.2 and 213.0±24.1 for subjects with genotypes AA (n=28), AC (n=14) and CC (n=2), respectively. The results showed that cases with rs4150351 AC/CC genotypes (n = 16) had significantly lower normalized Ct values (i.e., high initial concentration), indicating higher levels of ERCC5 expression, compared to cases with the AA genotypes (n = 28) (P = 0.037; Fig. 1).
Fig. 1. Boxplot for levels of XPG/ERCC5 mRNA expression in peripheral blood lymphocytes from 44 SCCHN cases with known XPG/ERCC5 variant genotypes (rs4150351).
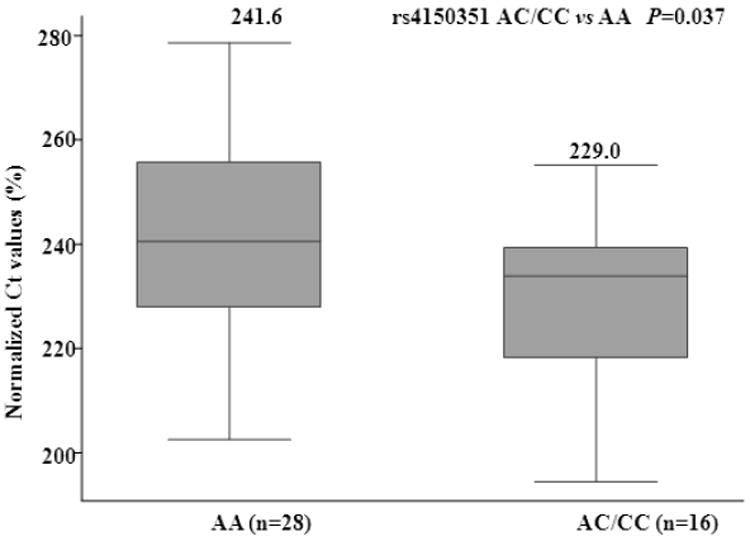
Data represented median and quartiles. The relative levels of XPG/ERCC5 were normalized to that of 18S using the equation ratio = CtERCC5 / Ct18S * 100%. A lower normalized Ct value represents higher expression. The means±SD of relative levels were 241.6±19.2 and 229.0±17.5 for subjects with AA and AC/CC genotypes, respectively.
Discussion
In the present study, we assessed the effect of genetic variation in XPG/ERCC5 on susceptibility to SCCHN by genotyping 12 tagging or potentially functional SNPs of the gene. After rigorous corrections for multiple tests, we found that none of SNPs in ERCC5/XPG was significantly associated with SCCHN risk, though variant genotypes of XPG/ERCC5 rs4150351 were associated with an increased expression of ERCC5/XPG mRNA and nondignificantly reduced SCCHN risk; the latter is likely due to limited statistical power of the present study.
XPG/ERCC5 acts as a structure-specific endonuclease that is critical to both NER subpathways of the transcription-coupled repair (TCR), which specifically targets and repairs DNA damage on the transcribed strand of actively expressed genes, and the global genomic repair (GGR), which removes DNA damage from the remaining genome [37]. In addition, patients with large truncations in the XPG protein frequently present features of combined XP and Cockayne syndrome (XP-CS). In animal experiments, complete inactivation of the XPG/ERCC5 gene leads to severe developmental defects in mice; these suggest that the XPG protein is involved in additional housekeeping functions besides the role in the NER pathway [38, 39]. It has been reported that some genetic mutations in XPG/ERCC5 affect the NER endonuclease activity [18, 41] and that decreased expression of XPG/ERCC5 in lymphocytes has been associated with increased risk of some cancers, including SCCHN [18, 40]. Our previous study also found that the combined effects of select functional SNPs in the core NER genes including ERCC5 were significantly associated with the DRC phenotype in nonmelanoma skin cancer cases and healthy controls [41].
In the present study, the results showed that XPG/ERCC5 rs4150351 was associated with ERCC5/XPG expression levels but with non-significantly reduced risk of SCCHN. Rs4150351 is an intronic SNP of XPG/ERCC5, and its functional significance has not been elucidated. Some studies have reported that an intronic SNP may alter mRNA levels of genes by affecting transcription, RNA elongation, splicing, or maturation [42-44]. Interestingly, we found that variant (AC/CC) genotypes of XPG/ERCC5 rs4150351, compared with the AA genotype, were indeed associated with increased mRNA levels of XPG/ERCC5 in PBMCs from SCCHN patients. These data suggest a potentially functional impact of this intronic SNP on the mRNA levels, but the underlying molecular mechanism needs further investigation.
Several studies have investigated the associations of SNPs in XPG/ERCC5 with risk of various cancers [11, 25-33]. Of these SNPs, the most frequently studied one was His1104Asp (rs17655, G/C) located in the XPG C-terminus, which is required for its interactions with other components of the NER pathway, such as XPB, XPD and TFIIH subunits [45]. The His1104Asp amino acid change may influence these protein-protein interactions; however, such a potentially functional relevance has not been tested in published reports, and studies on the association between His1104Asp and risk of human cancers have generated inconsistent results [25-33], possibly because of differences in study design, sample size, tumor sites or ethnicity. For example, Abbasi et al. found an increased risk of laryngeal cancer only for 1104Asp/His heterozygous carriers [31], whereas another two studies reported no associations between His1104Asp genotypes and risk of SCCHN [30, 32]. Our previous analysis with 829 SCCHN patients and 854 cancer-free controls [33] and the current analysis with a larger sample size did not find an association between His1104Asp and risk of SCCHN in non-Hispanic whites. The possible discrepancy of results between different studies may be caused by different genetic background by ethnicity. For example, the frequency of rs17655 C allele was 0.47 in the study by Wen et al (in Chinese populations) [32], but 0.22 in our study (non-Hispanic white populations). Furthermore, some studies also investigated the effect of other two XPG/ERCC5 SNPs (rs1047768 and rs4771436) on SCCHN risk [11, 31] but found no significant associations, consistent with the findings in the present study.
To more fully explore the SNPs of ERCC5, we also imputed genotypes by using these 12 SNPs within a ±3-kb region around the XPG/ERCC5 on chromosome 13q22. The imputation used IMPUTE 2 (http://mathgen.stats.ox.ac.uk/impute/impute_v2.html) and the CEU data from 1000 genomes (June 2010 release, pilot1) as a reference panel (http://www.1000genomes.org/). Finally, only 36 imputed SNPs (MAF>0.05 and an estimate of r2 between imputed and true genotypes above 0.3 as thresholds of quality) were evaluated for the association with SCCHN risk, but we did not any significant associations. These findings further suggested that it is likely that SNPs of ERCC5 may play a very limited role in the etiology of SCCHN, if any.
Our study has a number of strengths. This large SCCHN case-control study provided us sufficient statistical power to detect a moderate effect of ERCC5 SNPs on SCCHN risk. Furthermore, we comprehensively selected 12 tagging and potentially functional SNPs of XPG/ERCC5 that covered all common SNPs of the gene by using database searching and functional prediction in silico. However, the present study also has several potential limitations. Firstly, it was a hospital-based, and thus the genotype frequency in the control group may not represent the true frequency in the general population because of potential selection bias. However, the agreement with Hardy-Weinberg equilibrium for all 12 SNPs and similar allele frequencies of our controls to those reported in CEU populations from the HapMap database suggested the minimal selection bias, if any, in terms of genotype frequencies. Secondly, although our sample size was relatively large, the small sample size in subgroup analysis may have limited statistical power. Therefore, additional larger studies in different populations are needed to validate our findings. Finally, we may have missed some potentially functional SNPs that are not available in the existing databases we used for SNPs selection. For example, recent 1000 genome database has identified that another SNP (rs76871136) of ERCC5/XPG has a MAF of 14% for Europeans and may change a Gly to a stop codon; however, frequency information of this SNP is currently unavailable in the Hapmap database. Thus, additional studies need to focus on such SNPs and provide more comprehensive information for the association between SNPs of ERCC5 and SCCHN risk.
In conclusion, we comprehensively evaluated the effect of all available common genetic variants in XPG/ERCC5 on SCCHN risk but found a weak association, which disappeared after corrections for multiple testing, between XPG/ERCC5 rs4150351 variant genotypes (AC/CC) and SCCHN risk in this non-Hispanic white study population. Although this XPG/ERCC5 polymorphism may be functional, its role in susceptibility to SCCHN remains to be confirmed in larger epidemiological studies and in-depth mechanistic studies.
Supplementary Material
Acknowledgments
We thank Margaret Lung, Jessica Fiske, Shara Challa and Ana Neumann for their assistance in recruiting the subjects and gathering the questionnaire information, Kexin Chen, Zhibin Hu, Yujing Huang, Yawei Qiao, Jianzhong He, Kejing Xu and Min Zhao for laboratory assistance, and Dakai Zhu for his technical support. This work was supported by National Institute of Health grants R01 CA131274 and R01 ES011740 (Q. Wei) and P30 CA016672 (The University of Texas M. D. Anderson Cancer Center). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- OR
odds ratios
- CI
confidence interval
- ERCC5
excision repair cross complement group 5
- XPG
xeroderma pigmentosum group G
- SNP
single nucleotide polymorphism
- DRC
DNA repair capacity
- LD
linkage disequilibrium
- MAF
minor allele frequency
- SCCHN
squamous cell carcinoma of the head and neck
Footnotes
Conflict of interest The authors have declared no conflicts of interest.
References
- 1.Phillips DH. Smoking-related DNA and protein adducts in human tissues. Carcinogenesis. 2002;23:1979–2004. doi: 10.1093/carcin/23.12.1979. [DOI] [PubMed] [Google Scholar]
- 2.Stokes MP, Comb MJ. A wide-ranging cellular response to UV damage of DNA. Cell Cycle. 2008;7:2097–2099. doi: 10.4161/cc.7.14.6326. [DOI] [PubMed] [Google Scholar]
- 3.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002;511:145–178. doi: 10.1016/s1383-5742(02)00009-1. [DOI] [PubMed] [Google Scholar]
- 5.Wood RD. DNA damage recognition during nucleotide excision repair in mammalian cells. Biochimie. 1999;81:39–44. doi: 10.1016/s0300-9084(99)80036-4. [DOI] [PubMed] [Google Scholar]
- 6.Wei Q, Cheng L, Hong WK, Spitz MR. Reduced DNA repair capacity in lung cancer patients. Cancer Res. 1996;56:4103–4107. [PubMed] [Google Scholar]
- 7.Cheng L, Eicher SA, Guo Z, Hong WK, Spitz MR, Wei Q. Reduced DNA repair capacity in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7:465–468. [PubMed] [Google Scholar]
- 8.Berwick M, Vineis P. Markers of DNA repair and susceptibility to cancer in humans: an epidemiologic review. J Natl Cancer Inst. 2000;92:874–897. doi: 10.1093/jnci/92.11.874. [DOI] [PubMed] [Google Scholar]
- 9.Goode EL, Ulrich CM, Potter JD. Polymorphisms in DNA repair genes and associations with cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1513–1530. [PubMed] [Google Scholar]
- 10.Wang LE, Hu Z, Sturgis EM, Spitz MR, Strom SS, Amos CI, Guo Z, Qiao Y, Gillenwater AM, Myers JN, Clayman GL, Weber RS, El-Naggar AK, Mao L, Lippman SM, Hong WK, Wei Q. Reduced DNA repair capacity for removing tobacco carcinogen-induced DNA adducts contributes to risk of head and neck cancer but not tumor characteristics. Clin Cancer Res. 16:764–774. doi: 10.1158/1078-0432.CCR-09-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michiels S, Danoy P, Dessen P, Bera A, Boulet T, Bouchardy C, Lathrop M, Sarasin A, Benhamou S. Polymorphism discovery in 62 DNA repair genes and haplotype associations with risks for lung and head and neck cancers. Carcinogenesis. 2007;28:1731–1739. doi: 10.1093/carcin/bgm111. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Wang LE, Wei Q. DNA repair phenotype and cancer susceptibility--a mini review. Int J Cancer. 2009;124:999–1007. doi: 10.1002/ijc.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakasugi M, Reardon JT, Sancar A. The non-catalytic function of XPG protein during dual incision in human nucleotide excision repair. J Biol Chem. 1997;272:16030–16034. doi: 10.1074/jbc.272.25.16030. [DOI] [PubMed] [Google Scholar]
- 14.O’Donovan A, Davies AA, Moggs JG, West SC, Wood RD. XPG endonuclease makes the 3’ incision in human DNA nucleotide excision repair. Nature. 1994;371:432–435. doi: 10.1038/371432a0. [DOI] [PubMed] [Google Scholar]
- 15.Bessho T. Nucleotide excision repair 3’ endonuclease XPG stimulates the activity of base excision repairenzyme thymine glycol DNA glycosylase. Nucleic Acids Res. 1999;27:979–983. doi: 10.1093/nar/27.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klungland A, Hoss M, Gunz D, Constantinou A, Clarkson SG, Doetsch PW, Bolton PH, Wood RD, Lindahl T. Base excision repair of oxidative DNA damage activated by XPG protein. Mol Cell. 1999;3:33–42. doi: 10.1016/s1097-2765(00)80172-0. [DOI] [PubMed] [Google Scholar]
- 17.Koeppel F, Poindessous V, Lazar V, Raymond E, Sarasin A, Larsen AK. Irofulven cytotoxicity depends on transcription-coupled nucleotide excision repair and is correlated with XPG expression in solid tumor cells. Clin Cancer Res. 2004;10:5604–5613. doi: 10.1158/1078-0432.CCR-04-0442. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L, Sturgis EM, Eicher SA, Spitz MR, Wei Q. Expression of nucleotide excision repair genes and the risk for squamous cell carcinoma of the head and neck. Cancer. 2002;94:393–397. doi: 10.1002/cncr.10231. [DOI] [PubMed] [Google Scholar]
- 19.Cheng L, Spitz MR, Hong WK, Wei Q. Reduced expression levels of nucleotide excision repair genes in lung cancer: a case-control analysis. Carcinogenesis. 2000;21:1527–1530. [PubMed] [Google Scholar]
- 20.Bartolucci R, Wei J, Sanchez JJ, Perez-Roca L, Chaib I, Puma F, Farabi R, Mendez P, Roila F, Okamoto T, Taron M, Rosell R. XPG mRNA expression levels modulate prognosis in resected non-small-cell lung cancer in conjunction with BRCA1 and ERCC1 expression. Clin Lung Cancer. 2009;10:47–52. doi: 10.3816/CLC.2009.n.007. [DOI] [PubMed] [Google Scholar]
- 21.Walsh CS, Ogawa S, Karahashi H, Scoles DR, Pavelka JC, Tran H, Miller CW, Kawamata N, Ginther C, Dering J, Sanada M, Nannya Y, Slamon DJ, Koeffler HP, Karlan BY. ERCC5 is a novel biomarker of ovarian cancer prognosis. J Clin Oncol. 2008;26:2952–2958. doi: 10.1200/JCO.2007.13.5806. [DOI] [PubMed] [Google Scholar]
- 22.Furuta T, Ueda T, Aune G, Sarasin A, Kraemer KH, Pommier Y. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 2002;62:4899–4902. [PubMed] [Google Scholar]
- 23.Nouspikel T, Clarkson SG. Mutations that disable the DNA repair gene XPG in a xeroderma pigmentosum group G patient. Hum Mol Genet. 1994;3:963–967. doi: 10.1093/hmg/3.6.963. [DOI] [PubMed] [Google Scholar]
- 24.Emmert S, Schneider TD, Khan SG, Kraemer KH. The human XPG gene: gene architecture, alternative splicing and single nucleotide polymorphisms. Nucleic Acids Res. 2001;29:1443–1452. doi: 10.1093/nar/29.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgensen TJ, Visvanathan K, Ruczinski I, Thuita L, Hoffman S, Helzlsouer KJ. Breast cancer risk is not associated with polymorphic forms of xeroderma pigmentosum genes in a cohort of women from Washington County, Maryland. Breast Cancer Res Treat. 2007;101:65–71. doi: 10.1007/s10549-006-9263-3. [DOI] [PubMed] [Google Scholar]
- 26.Kiyohara C, Yoshimasu K. Genetic polymorphisms in the nucleotide excision repair pathway and lung cancer risk: a meta-analysis. Int J Med Sci. 2007;4:59–71. doi: 10.7150/ijms.4.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain SK, Mu LN, Cai L, Chang SC, Park SL, Oh SS, Wang Y, Goldstein BY, Ding BG, Jiang Q, Rao J, You NC, Yu SZ, Papp JC, Zhao JK, Wang H, Zhang ZF. Genetic variation in immune regulation and DNA repair pathways and stomach cancer in China. Cancer Epidemiol Biomarkers Prev. 2009;18:2304–2309. doi: 10.1158/1055-9965.EPI-09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Closas M, Malats N, Real FX, Welch R, Kogevinas M, Chatterjee N, Pfeiffer R, Silverman D, Dosemeci M, Tardon A, Serra C, Carrato A, Garcia-Closas R, Castano-Vinyals G, Chanock S, Yeager M, Rothman N. Genetic variation in the nucleotide excision repair pathway and bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2006;15:536–542. doi: 10.1158/1055-9965.EPI-05-0749. [DOI] [PubMed] [Google Scholar]
- 29.Mort R, Mo L, McEwan C, Melton DW. Lack of involvement of nucleotide excision repair gene polymorphisms in colorectal cancer. Br J Cancer. 2003;89:333–337. doi: 10.1038/sj.bjc.6601061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao J, Cao W, Cozen W, Mack TM, Zhang ZF. Polymorphism of Xeroderma Pigmentosum group G and the risk of lung cancer and squamous cell carcinomas of the oropharynx, larynx and esophagus. Int J Cancer. 2006;118:714–720. doi: 10.1002/ijc.21413. [DOI] [PubMed] [Google Scholar]
- 31.Abbasi R, Ramroth H, Becher H, Dietz A, Schmezer P, Popanda O. Laryngeal cancer risk associated with smoking and alcohol consumption is modified by genetic polymorphisms in ERCC5, ERCC6 and RAD23B but not by polymorphisms in five other nucleotide excision repair genes. Int J Cancer. 2009;125:1431–1439. doi: 10.1002/ijc.24442. [DOI] [PubMed] [Google Scholar]
- 32.Wen SX, Tang PZ, Zhang XM, Zhao D, Guo YL, Tan W, Lin DX. Association between genetic polymorphism in xeroderma pigmentosum G gene and risks of laryngeal and hypopharyngeal carcinomas. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:703–706. [PubMed] [Google Scholar]
- 33.An J, Liu Z, Hu Z, Li G, Wang LE, Sturgis EM, El-Naggar AK, Spitz MR, Wei Q. Potentially functional single nucleotide polymorphisms in the core nucleotide excision repair genes and risk of squamous cell carcinoma of the head and neck. Cancer Epidemiol Biomarkers Prev. 2007;16:1633–1638. doi: 10.1158/1055-9965.EPI-07-0252. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Desai M, Agrawal M, Kennedy DO, Senie RT, Santella RM, Terry MB. Polymorphisms in nucleotide excision repair genes and DNA repair capacity phenotype in sisters discordant for breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1614–1619. doi: 10.1158/1055-9965.EPI-06-0218. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Calderon JI, Zhang Z, Sturgis EM, Spitz MR, Wei Q. Polymorphisms of vitamin D receptor gene protect against the risk of head and neck cancer. Pharmacogenet Genomics. 2005;15:159–165. doi: 10.1097/01213011-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Huang YJ, Niu J, Liu Z, Wang LE, Sturgis EM, Wei Q. The functional IGFBP7 promoter -418G>A polymorphism and risk of head and neck cancer. Mutat Res. 2010;702:32–39. doi: 10.1016/j.mrgentox.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanawalt PC. Controlling the efficiency of excision repair. Mutat Res. 2001;485:3–13. doi: 10.1016/s0921-8777(00)00071-9. [DOI] [PubMed] [Google Scholar]
- 38.Harada YN, Shiomi N, Koike M, Ikawa M, Okabe M, Hirota S, Kitamura Y, Kitagawa M, Matsunaga T, Nikaido O, Shiomi T. Postnatal growth failure, short life span, and early onset of cellular senescence and subsequent immortalization in mice lacking the xeroderma pigmentosum group G gene. Mol Cell Biol. 1999;19:2366–2372. doi: 10.1128/mcb.19.3.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emmert S, Slor H, Busch DB, Batko S, Albert RB, Coleman D, Khan SG, Abu-Libdeh B, DiGiovanna JJ, Cunningham BB, Lee MM, Crollick J, Inui H, Ueda T, Hedayati M, Grossman L, Shahlavi T, Cleaver JE, Kraemer KH. Relationship of neurologic degeneration to genotype in three xeroderma pigmentosum group G patients. J Invest Dermatol. 2002;118:972–982. doi: 10.1046/j.1523-1747.2002.01782.x. [DOI] [PubMed] [Google Scholar]
- 40.Lalle P, Nouspikel T, Constantinou A, Thorel F, Clarkson SG. The founding members of xeroderma pigmentosum group G produce XPG protein with severely impaired endonuclease activity. J Invest Dermatol. 2002;118:344–351. doi: 10.1046/j.0022-202x.2001.01673.x. [DOI] [PubMed] [Google Scholar]
- 41.Wang LE, Li C, Strom SS, Goldberg LH, Brewster A, Guo Z, Qiao Y, Clayman GL, Lee JJ, El-Naggar AK, Prieto VG, Duvic M, Lippman SM, Weber RS, Kripke ML, Wei Q. Repair capacity for UV light induced DNA damage associated with risk of nonmelanoma skin cancer and tumor progression. Clin Cancer Res. 2007;13:6532–6539. doi: 10.1158/1078-0432.CCR-07-0969. [DOI] [PubMed] [Google Scholar]
- 42.Baralle M, Pastor T, Bussani E, Pagani F. Influence of Friedreich ataxia GAA noncoding repeat expansions on pre-mRNA processing. Am J Hum Genet. 2008;83:77–88. doi: 10.1016/j.ajhg.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buratti E, Brindisi A, Pagani F, Baralle FE. Nuclear factor TDP-43 binds to the polymorphic TG repeats in CFTR intron 8 and causes skipping of exon 9: a functional link with disease penetrance. Am J Hum Genet. 2004;74:1322–1325. doi: 10.1086/420978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giacopelli F, Rosatto N, Divizia MT, Cusano R, Caridi G, Ravazzolo R. The first intron of the human osteopontin gene contains a C/EBP-beta-responsive enhancer. Gene Expr. 2003;11:95–104. doi: 10.3727/000000003108748991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakasugi M, Sancar A. Order of assembly of human DNA repair excision nuclease. J Biol Chem. 1999;274:18759–18768. doi: 10.1074/jbc.274.26.18759. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


