Abstract
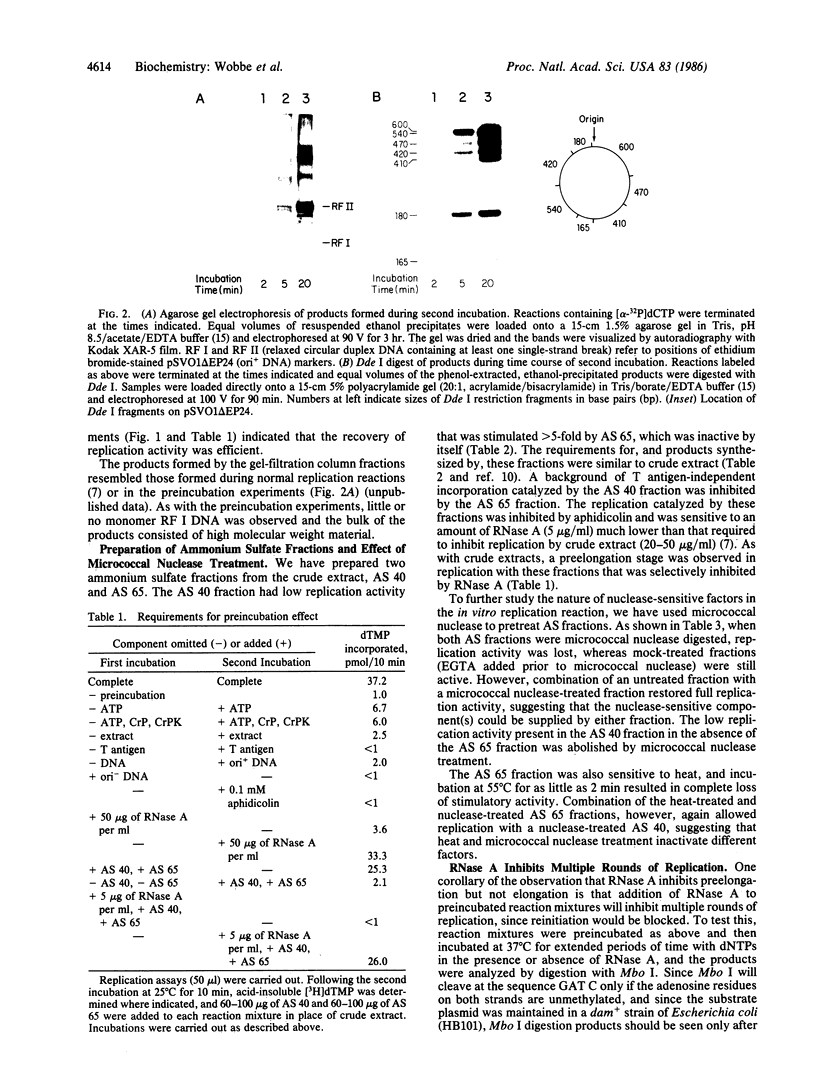
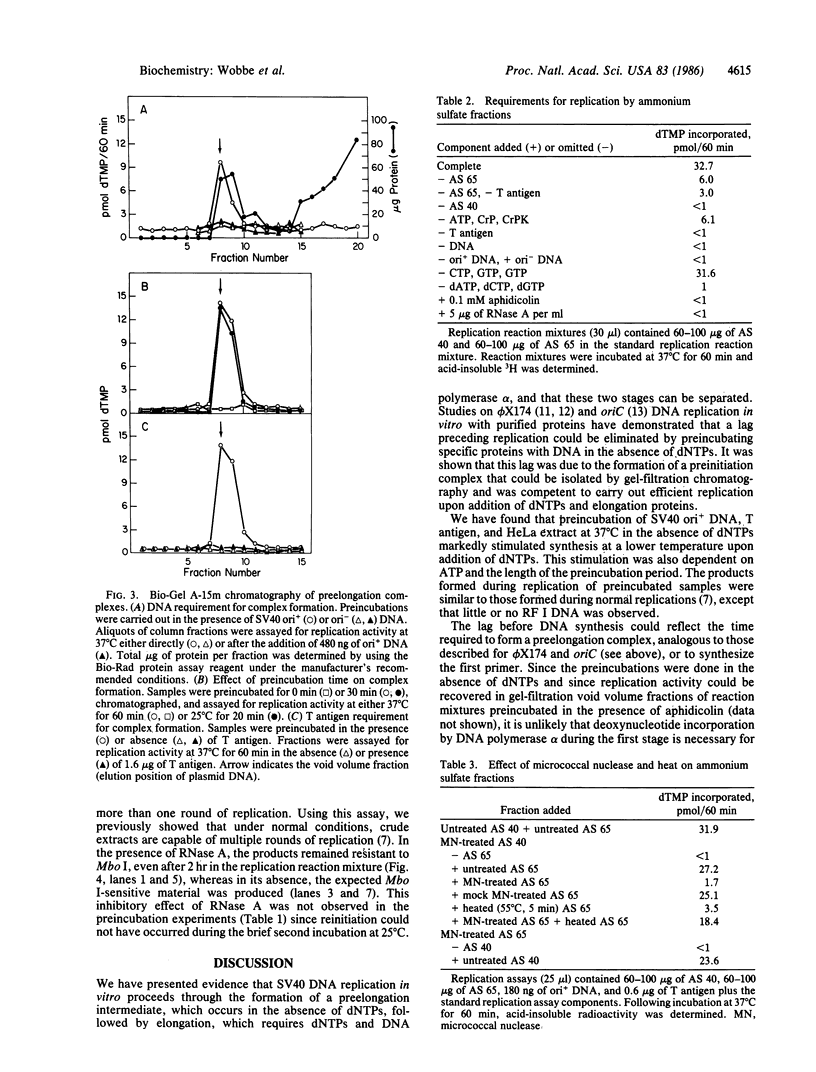
We have evidence for the formation of a stable preelongation complex during the replication of simian virus 40 (SV40) origin containing DNA (ori+ DNA) in vitro. Preincubation of ori+ DNA with HeLa cytosolic extracts and SV40-encoded large tumor antigen (T antigen) in the absence of deoxynucleoside triphosphates eliminates a lag that normally precedes replication. This effect requires ATP and is inhibited by RNase A; subsequent elongation is inhibited by aphidicolin but not by RNase A. A T antigen and SV40 origin-dependent complex can be isolated by gel-filtration chromatography of preincubation reaction mixtures. In both cases, the products formed by replication after complex formation resemble those formed during in vitro replication reactions described previously. HeLa cytosolic extract was separated into two ammonium sulfate fractions: a 0-40% fraction (AS 40) that shows low levels of DNA synthesis and a 40-65% fraction (AS 65) that is inactive by itself but stimulates synthesis when added to the AS 40 fraction. DNA synthesis by these combined fractions has the same requirements as crude extract, occurs in two stages as described above, and is sensitive to RNase A. Pretreatment of both fractions with micrococcal nuclease eliminated replication activity, whereas the combination of a pretreated fraction (either AS 40 or 65) with an untreated fraction was active. A heat-inactivated (55 degrees C, 5 min) AS 65 fraction restored replication activity to the combination of micrococcal nuclease-treated AS 40 and AS 65 fractions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ariga H., Sugano S. Initiation of simian virus 40 DNA replication in vitro. J Virol. 1983 Nov;48(2):481–491. doi: 10.1128/jvi.48.2.481-491.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., McKerlie M. L., Salzman N. P. Characterization of Simian virus 40 DNA component II during viral DNA replication. J Mol Biol. 1973 Feb 25;74(2):95–111. doi: 10.1016/0022-2836(73)90101-0. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Field J., Hurwitz J. Purification of a primase activity associated with DNA polymerase alpha from HeLa cells. J Biol Chem. 1984 Aug 10;259(15):9479–9486. [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marians K. J. Enzymology of DNA in replication in prokaryotes. CRC Crit Rev Biochem. 1984;17(2):153–215. doi: 10.3109/10409238409113604. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Wobbe C. R., Weissbach L., Dean F. B., Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1986 May;83(9):2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn U., Szyszko J., Coombs D., Krause M. 7S-K nuclear RNA from simian virus 40-transformed cells has sequence homology to the viral early promoter. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7090–7094. doi: 10.1073/pnas.80.23.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B., Gerard R. D., Guggenheimer R. A., Gluzman Y. T antigen and template requirements for SV40 DNA replication in vitro. EMBO J. 1985 Nov;4(11):2933–2939. doi: 10.1002/j.1460-2075.1985.tb04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T., Selzer G., Som T. Inhibition of ColE1 RNA primer formation by a plasmid-specified small RNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1421–1425. doi: 10.1073/pnas.78.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., McMacken R., Kornberg A. Isolation of an intermediate which precedes dnaG RNA polymerase participation in enzymatic replication of bacteriophage phi X174 DNA. Proc Natl Acad Sci U S A. 1976 Mar;73(3):752–756. doi: 10.1073/pnas.73.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobbe C. R., Dean F., Weissbach L., Hurwitz J. In vitro replication of duplex circular DNA containing the simian virus 40 DNA origin site. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5710–5714. doi: 10.1073/pnas.82.17.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende A., Baker T. A., Ogawa T., Kornberg A. Initiation of enzymatic replication at the origin of the Escherichia coli chromosome: primase as the sole priming enzyme. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3954–3958. doi: 10.1073/pnas.82.12.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]