Abstract
Schizophrenia (Sz), along with other neuropsychiatric disorders, is associated clinically with abnormalities in neocortical gamma frequency (30–80 Hz) oscillations. In Sz patients, these abnormalities include both increased and decreased gamma activity, and show a strong association with Sz symptoms. For several decades, administration of sub-anesthetic levels of ketamine has provided the most comprehensive experimental model of Sz-symptoms. While acute application of ketamine precipitates a psychotic-like state in a number of animal models, as well as humans, the underlying mechanisms behind this effect, including alteration of neuronal network properties, are incompletely understood, making an in vitro level analysis particularly important.
Previous in vitro studies have had difficulty inducing gamma oscillations in neocortical slices maintained in submerged-type recording chambers necessary for visually guided whole-cell recordings from identified neurons. Consequently, here, we validated a modified method to evoke gamma oscillations using brief, focal application of the glutamate receptor agonist kainate (KA), in slices prepared from mice expressing green fluorescent protein in GABAergic interneurons (GAD67-GFP knock-in mice). Using this method, gamma oscillations dependent on activation of AMPA and GABAA receptors were reliably elicited in slices containing mouse prelimbic cortex, the rodent analogue of the human dorsolateral prefrontal cortex.
Examining the effects of ketamine on this model, we found that bath application of ketamine significantly potentiated KA-elicited gamma power, an effect mimicked by selective NMDAR antagonists including a selective antagonist of NMDARs containing the NR2B subunit. Importantly, ketamine, unlike more specific NMDAR antagonists, also reduced the peak frequency of KA-elicited oscillatory activity. Our findings indicate that this effect is mediated not through NMDAR, but through slowing the decay kinetics of GABAA receptor mediated inhibitory postsynaptic currents in identified GABAergic interneurons. These in vitro findings may help explain the complexities of gamma findings in clinical studies of Sz and prove useful in developing new therapeutic strategies.
Keywords: Schizophrenia, Gamma Oscillations, Interneurons, Parvalbumin, GABA, NMDA
Abnormalities in neuronal oscillations, particularly those in the gamma frequency range (30–80 Hz), are a hallmark of a number of neuropsychiatric disorders (Herrmann and Demiralp, 2005). Such oscillations represent an essential mechanism for the temporal coordination of neural activity, and are critical for cognitive function (Buzsaki and Draguhn, 2004). Abnormalities in gamma oscillations have been consistently observed in clinical studies of Schizophrenia (Sz) (Kwon et al., 1999, Spencer et al., 2003, Spencer et al., 2004, Uhlhaas and Singer, 2010), leading to increased interest in defining the mechanisms behind these abnormalities, and determining how they play a role in the pathophysiology of this disease.
In general, failure of proper synchronization of gamma band oscillations is believed to underlie deficits in the integration of sensory input with information stored in memory, and is positively correlated with the severity of Sz related symptoms.(Spencer et al., 2004, Herrmann and Demiralp, 2005, Spencer et al., 2009). Clinical studies, including many from our group, however, have shown complexity in the amplitude of gamma response in Sz, some showing reduced gamma amplitude (Kwon et al., 1999, Spencer et al., 2003, Spencer et al., 2004, Uhlhaas and Singer, 2010), while others have shown more anatomically localized increases in gamma associated with positive symptoms of psychosis (Spencer et al., 2009). The variability of these clinical findings underlines the importance of establishing the mechanisms underlying control of gamma band synchronization and amplitude.
The NMDA receptor (NMDAR) antagonist, ketamine, a dissociative anesthetic and drug of abuse, has provided an important tool to gain insight into brain mechanisms underlying the symptoms of Sz (Krystal et al., 1994, Gunduz-Bruce, 2009). Early anecdotal observations found that recreational use of ketamine in healthy adults produced both cognitive dysfunction and psychosis (Krystal et al., 1994, Gunduz-Bruce, 2009). Subsequent clinical analysis under more controlled experimental conditions revealed subanesthetic doses of ketamine capable of modeling many of the symptoms typical of Sz, including positive, negative, and cognitive symptoms (Krystal et al., 1994, Gunduz-Bruce, 2009). Furthermore, behavioral effects reminiscent of Sz symptoms are observed following ketamine administration in animal models (Krystal et al., 1994, Bubenikova-Valesova et al., 2008, Gunduz-Bruce, 2009). Interestingly, acute ketamine administration has been found to increase the power of gamma oscillations in rodents (Pinault, 2008, Hakami et al., 2009), an effect reminiscent of what is clinically observed during psychosis. However, the brain regions affected and mechanisms behind this ketamine mediated effect remain unclear, making an in vitro level analysis essential. This is particularly important, as ketamine may target receptors other than NMDAR (Flood and Krasowski, 2000, Irifune et al., 2000, Kapur and Seeman, 2002).
In this study we explore the use of acute ketamine for modeling Sz, to determine this drugs effect on gamma oscillatory activity in mice. Experiments were performed in slices containing the mouse prelimbic cortex (PrL), the rodent analogue of the human dorsolateral prefrontal cortex (Vertes, 2004), as this region is heavily implicated in many of the cognitive impairments associated with Sz (Berman et al., 1986, Weinberger et al., 1986). Previous in vitro studies of gamma oscillations rely on constant perfusion of GABAergic and/or cholinergic agonists to stimulate such neuronal activity. However, generating gamma activity in neocortical preparations using this method has proven difficult (Hajos and Mody, 2009). Thus, here, we employed a modified method of inducing gamma oscillations in submerged neocortical slices: brief, focal application of kainate. In order to be able to study the effect of ketamine on identified GABAergic neurons controlling gamma oscillations, we used mice in which GABAergic neurons were labeled with green fluorescent protein (GFP); GAD67-GFP knock-in mice (Tamamaki et al., 2003, Brown et al., 2008, Chen et al., 2010).
2. Experimental Procedures
2.1 Animals
Wild-type, Swiss Webster mice (Charles River Laboratories) and heterozygous GAD67-GFP “knock-in” mice, which express GFP under control of the promoter for GAD67 (Tamamaki et al., 2003) of either sex, between the ages of P15-120 were utilized for this work. Evoked oscillatory activity was found to be consistent across this age range. Further, no significant genotype or sex differences in the gamma oscillations were observed. GAD67-GFP mice were used to identify GABAergic interneurons prior to whole-cell recordings (see below). Mice were housed at the VA Boston Healthcare System, Brockton campus under constant temperature (23°C) and a 12h:12h light–dark cycle with food and water available ad libitum. All experiments were carried out in accordance with the American Association for Accreditation of Laboratory Animal Care’s policy on care and use of laboratory animals and were approved by the local Institutional Animal Care and Use Committee.
2.2 Slice Preparation
Coronal slices containing the PrL were prepared as follows. Mice were deeply anesthetized using isoflurane, then quickly decapitated. The brain was removed and placed into ice cold modified artificial cerebrospinal fluid (ACSF) containing: (in mM) 252 Sucrose, 1.8 KCl, 1.2 KH2PO4, 2 MgSO4, 25.6 NaHCO3 and 10 glucose saturated with 95% O2/5% CO2. 450 µm (extracellular gamma recordings) or 300 µm (whole-cell recordings) slices were cut between +2.96 and +1.54 mm with respect to bregma (According to the Franklin/Paxinos atlas Franklin and Paxinos, 2008) using a Vibratome 3000 (Vibratome, Bannockburn, IL). Slices were transferred into a prechamber (BSC-PC; Warner Instruments) containing ACSF: (in mM) 124 NaCl, 1.8 KCl, 1.2 KH2PO4, 2 CaCl2, 1.3 MgSO4, 25.6 NaHCO3 and 10 glucose, continuously bubbled with 95% O2/5% CO2 (pH 7.4). Slices were allowed to recover for at least 1 hour before use. For recordings, slices were transferred to a submersion-style recording chamber (RC27L; Warner Instruments) and constantly perfused (5 mL/min) with warm ACSF (30°C).
2.3 Immunocytochemistry
Adult (6–12 months) GAD67-GFP mice were anesthetized with pentobarbital (50 mg/ml), and then perfused transcardially with 0.1% glutaraldehyde (Sigma, G5882) in 10% formalin (Sigma, HT5011). The brain was removed and post-fixed for one day in the same glutaraldehyde/formalin mixture, then further post-fixed for another day in 10% formalin. The brain was then placed in 30% sucrose for a final day. 40 µm-thick coronal slices containing the PrL were cut on a freezing microtome, and stored in phosphate-buffered saline (PBS) at 4°C.
Slices were washed three times in PBS for 10 min at room temperature, and then placed in a blocking solution of 0.3% Triton in PBS, containing 5% normal donkey serum, for one hour. Slices were incubated overnight on a shaking platform at room temperature in the primary rabbit polyclonal anti-GABA antibody (Sigma, A2052, 1:5000). On the following day, slices were washed three times in PBS, and incubated for 3 h in donkey anti-rabbit-AlexaFluor594 (Invitrogen, 1:100, Red). Slices were then washed twice in PBS, mounted onto subbed slides, and coverslipped using Vectashield Hardset Mounting Medium (Vector Laboratories, H1400).
Cellular localization and quantification of GFP and GABA positive neurons were performed using Neurolucida computer software (Version 7; Microbrightfield, Williston, VT, USA), and an Olympus BX51 fluorescent microscope. GFP positive cells were identified with excitation/emission at 488/509 nm wavelengths (green fluorescence). GABA AlexaFluor 594 labeled neurons were identified with excitation/emission at 590/617 nm (red fluorescence). Double labeled neurons were identified by the presence of both fluorescent signals.
2.4 Elicitation of Gamma Oscillations in Vitro
Extracellular field potential activity was recorded using glass micropipettes (2–5 MΩ) filled with ACSF and positioned ~50 µm deep in the PrL (Layer II/III). Similar to Gloveli et al. (2005), oscillatory activity was elicited by a brief (80 ms @ 30 psi) focal application of KA (1 mM) onto the PrL slice in close apposition to the location of the field potential electrode using a picospritzer (General Valve Corp.; Figure 1A). Field potentials evoked by kainate application (Figure 1B) were amplified using the 100X gain DC-coupled current-clamp mode of a Multiclamp700B amplifier (Axon Instruments). Signals were digitized at 10 kHz using a Digidata 1322A 16-bit data acquisition system (Axon Instruments), then filtered between 1 kHz and .1 Hz using pClamp 9.2 (Axon Instruments) and stored on a PC hard drive.
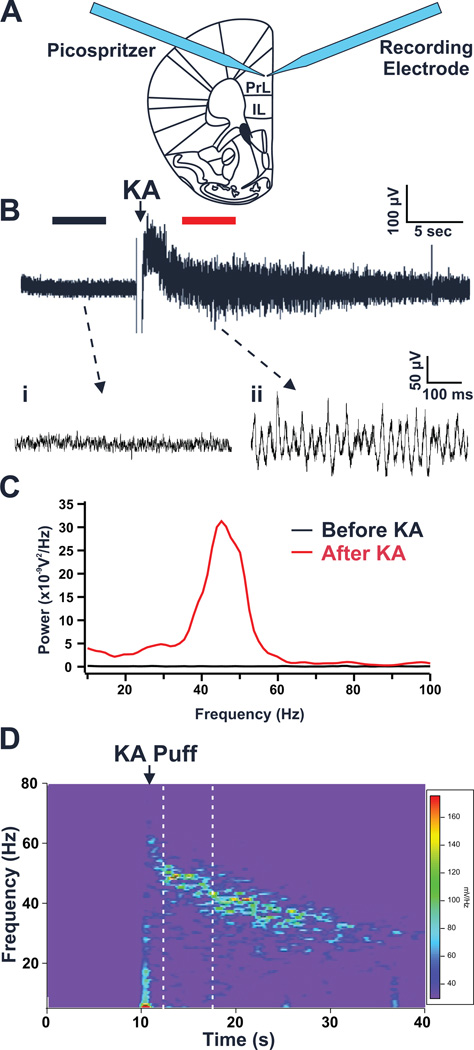
Figure 1. Focal kainate application elicits gamma oscillatory activity in prelimbic cortex (PrL) slices.
(A) In vitro oscillations were elicited by a brief focal application of 1 mM kainate (KA) in close proximity to an extracellular field potential recording electrode. (Abbreviation: PrL, prelimbic cortex IL, infralimbic cortex) (B) Representative field potential record from the PrL cortex showing local network activity before and after application of KA: (i) Under control conditions very little neuronal activity is observed; (ii) However, after kainate application, oscillatory activity is readily evident. (C) Power spectral density (PSD) profiles generated from 5 second segments of the field potential trace in B, before and after focal application of KA (segments used for PSD calculation denoted by black and red bars above trace). PSD profiles show that little to no activity is present prior to KA application. However, following KA application a large increase in oscillatory activity centered in the gamma frequency range is observed. (D) A spectrogram of the representative field potential recording in B shows a transient increase in power in the gamma frequency range (30–80Hz) following KA application. Warmer colors indicate higher power. The area between the dashed lines represents the 5 second segment of the field potential trace used for PSD analysis of elicited oscillations.
To analyze the characteristics of the oscillations, power spectral density (PSD) profiles (Figure 1C) illustrating the power of the oscillations at different frequencies were generated by Fourier transforms of the field potential records (Igor Pro, Wavemetrics). PSDs were calculated from a 5 s epoch of the field potential trace starting 2.5 s following application of KA, when the transient DC shift produced by kainate application had subsided (Fig. 1B, D). Under baseline conditions kainate was applied three times at 5 minute intervals. Only slices in which the three consecutive field potential recordings gave consistent PSD profiles across all trials (< 10 % difference in peak power, frequency) were used for analysis. These three PSD were then averaged to give the baseline PSD for that slice. Three measures were used to characterize the oscillations: peak power, peak frequency and total gamma power. Peak power was defined as the highest amplitude in the averaged PSD (> 10 Hz, bins of 1.2 Hz). The frequency at which the peak power was observed was defined as the peak frequency. Total gamma power was determined by integration of averaged PSD between 30 and 80 Hz. For determining the acute effects of drugs on oscillations, after analysis of oscillations under baseline conditions, drugs were applied to the slice for 15 minutes via bath perfusion; this was then followed by repetition of the above analysis paradigm.
2.5 Whole-cell Recordings
To determine if changes in the peak frequency of gamma oscillations produced by ketamine (see results) were due to changes in inhibitory synaptic transmission, whole cell recordings of miniature inhibitory postsynaptic currents (mIPSC) were performed using 3–5 MΩ glass micropipettes filled with pipette solution containing: (in mM) 135 CsCl, 10 HEPES, 2 MgCl2, 0.1 EGTA, 2 Na2ATP, 4, MgATP, 2.5 NaGTP, 1 spermine (pH 7.25 with CsOH). Individual neurons in GAD67-GFP mouse PrL slices were chosen based on their overall appearance and the presence (interneurons) or absence (pyramidal neuron) of GFP. Previous studies have shown that GFP selectively labels essentially all GABAergic neurons in these mice (Tamamaki et al., 2003, Brown et al., 2008). Patched neurons were voltage-clamped at −60 mV. Access resistance was maintained between 15–25 MΩ; recordings were discarded if this changed by more than 20 % during the experiment. Slices were constantly perfused with a cocktail of TTX (500 nM), DNQX (30 µM) and AP-5 (50 µM) to block action potentials, AMPA and NMDA receptors respectively. mIPSCs were recorded for 5 minutes under control conditions and then again following perfusion with 100 µM ketamine. As above, recordings were made with a Multiclamp700B amplifier and digitized at 10 kHz using a Digidata 1322A 16-bit data acquisition system (Axon Instruments), then low-pass filtered at 1 kHz using pClamp 9.2 and stored on a PC. Recordings were subsequently analyzed using Igor Pro (Wavemetrics) to determine amplitude and decay time constant (tau) of individual mIPSCs.
2.6 Statistical Analysis
Student’s t-test (2-tailed) was used for statistical comparison of results (SPSS 10, SPSS Inc.). Differences were considered to be significant at p < 0.05. Averaged values reported in this manuscript are expressed as mean ± S.E.M. for all data generated from analysis of oscillations and mIPSC.
2.7 Drugs
Acutely administered drugs used in this study were obtained from Sigma-Aldrich (Ketamine-HCl, PEAQX (also refered to as NVP-AAM077)), Ascent Scientific (MK-801, AP-5, Ro25-6981), and Tocris Bioscience (PPPA, Quinpirole, α-methyl-5-hydroxytryptamine malate). Concentrations used were determined by conducting a literature search for both known receptor binding specificity values and previous experimental usage of drugs in in vitro slice preparations (Wu and Johnson, 1996, Fischer et al., 1997, Auberson et al., 2002, Liu et al., 2003, Massey et al., 2004, Feng et al., 2005, Wang et al., 2008, Kline et al., 2009, Williams and Undieh, 2009, Ahmed et al., 2011).
3. Results
3.1 Generation of gamma frequency oscillations in acute PrL slices
Gamma frequency oscillations were generated in neocortical PrL slices via a brief focal application of the glutamate receptor agonist kainate (KA) using a picospritzer. We found this method reliably elicited transient local bursts of oscillatory activity in the slice. As shown in figure 1, PrL slices exhibited little or no spontaneous local field potential activity under control conditions (ACSF perfusion). However, application of KA (1 mM) onto the slice elicited a response characterized by a brief DC offset (~1–2 s), followed by 20–30 s of robust oscillatory activity (Figure 1A, B). A time-frequency analysis of this activity (Figure 1D) revealed that once the DC offset had subsided, the activity was localized in the gamma (30–80 Hz) frequency domain. These oscillations appeared to decline in frequency over time. Thus, we restricted analysis of this activity to a 5 second period following the DC offset, when the oscillations were consistently in the gamma range.
Analysis of KA-elicited oscillations, pooled across a large number of PrL slices from both wild-type and GAD67-GFP mice of either sex between the ages of p15-120 (51 brain slices obtained from 39 different mice; referred to henceforward as: n=51/39), revealed that this rhythmic activity possessed a single predominant peak with a mean frequency of 44.5 ± 0.9 Hz. Repeated applications of KA on individual slices at 5 min intervals reliably evoked very consistent levels of oscillatory activity (peak power, peak frequency, and total gamma power). Between slices, the magnitude of the evoked activity varied considerably, while the peak frequency of the oscillations remained quite consistent. Overall, the mean peak oscillatory power was 13.3 ± 1.8 nV2/Hz and the mean total gamma power was 172.4 ± 16.6 nV2.Hz.
To ensure this oscillatory activity was not generated by a mechanical artifact resulting simply from pressure ejection of solution onto the slice, we focally applied a non-neuroactive substance (ACSF) to the slice. ACSF application generated no oscillatory activity, and produced only a small mechanical artifact which subsided in about 1 sec (n = 3/1). KA on the other hand, in the same slices tested with ASCF, induced robust gamma frequency oscillations.
GAD67-GFP knock-in mice were used for the majority of our studies to allow us to examine the effects of ketamine on identified GABAergic neurons (see below). Comparison of the characteristics of KA-elicited oscillations in PrL slices prepared from wild-type (WT) mice (n = 10/9) with those prepared from GAD67-GFP mice (n = 23/17) revealed no significant differences in the peak power (WT: 9.8 ± 2.9 nV2/Hz, GAD67-GFP:12.8 ± 2.9 nV2/Hz) peak frequency (WT:45.4 ± 2.2 Hz, GAD67-GFP:44.5 ± 1.0 Hz) or total gamma power (WT:131.0 ± 36.8 nV2.Hz, GAD67-GFP:147.4 ± 23.2 nV2.Hz). Additionally, a comparison of oscillations from PrL slices obtained from male mice (n = 11/8) or female mice (n = 12/9) found no significant differences in the peak power (Male:16.8 ± 3.5 nV2/Hz, Female:16.8 ± 4.1 nV2/Hz), peak frequency (Male:42.7 ± 1.5 Hz, Female:44.6 ± 1.2 Hz), or total gamma power (Male:190.0 ± 34.1 nV2.Hz, Female:162.1 ± 25.7 nV2.Hz). Together these findings indicate there was no effect of gender or genotype on the KA- elicited oscillations.
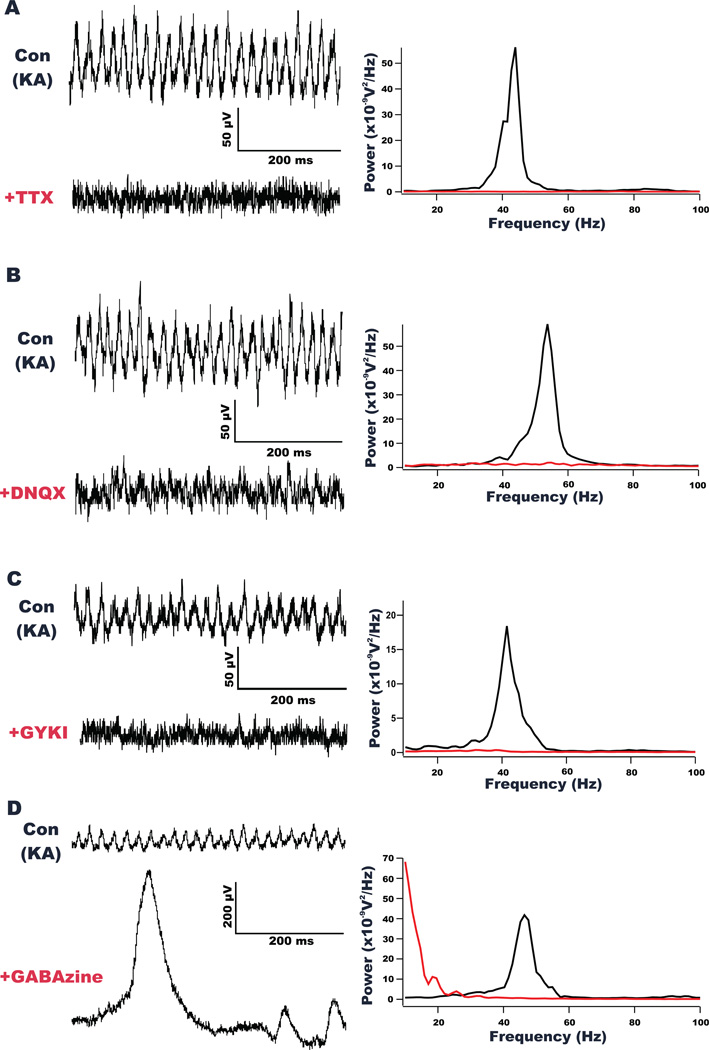
To ensure that the gamma oscillations elicited in our PrL preparation were similar in nature to those observed in other in vitro preparations, we employed a number of pharmacological agents to define the receptor systems involved in this activity. First, the oscillations were completely abolished following perfusion with TTX (500 nM), indicating that action potentials were required for their generation (n=5/5; peak oscillatory power reduced by 97.5 ± 1.1% and overall gamma power reduced by 95.4 ± 2%; p<0.05 for both; Figure 2A). PrL gamma oscillations were also dependent on AMPA and GABAA receptor activation. The oscillations were blocked by the AMPA/kainate receptor antagonist DNQX (5 µM; Figure 2B) in all slices tested (n = 5/5), with slices exhibiting a significant reduction (p<0.05) in total gamma power (85.2 ± 3.3%) and a reduction in peak oscillatory power (90.4 ± 2.9%). Additionally, the selective AMPA receptor antagonist, GYKI 52466 (50 µM; Figure 2C) resulted in a significant (p<0.05) reduction in both total gamma power (70.8 ± 13%) and peak oscillatory power (85.5 ± 10.3%) in all slices tested (n=7/7). Application of the GABAA receptor antagonist, GABAzine (100 µM), completely abolished the generation of KA-induced gamma oscillations in all slices tested (n=8/8). Concurrent with the blockage of gamma activity, large epileptiform-like spiking was revealed in 7 of the 8 slices recorded. While the block of gamma oscillatory was readily apparent with visual inspection of the traces (see figure 2D), the appearance of the large amplitude epileptiform spikes prevented us from quantifying the extent of the observed change in gamma oscillatory activity.
Figure 2. Gamma oscillations are blocked by tetrodotoxin (TTX), an AMPA/kainate receptor antagonist (DNQX), an AMPA receptor antagonist (GYKI) and a GABAA receptor antagonist (GABAzine).
(A) Field potential recordings from a representative PrL slice shows oscillatory activity elicited by KA under control conditions and after application of TTX (500 nM). Traces show a large decrease in oscillations in the presence of TTX. The power spectral density (PSD) analysis of a 5 second epoch of field potential activity after KA application under both conditions (right), shows TTX significantly blocked elicited gamma activity. (B) Representative recordings of oscillatory activity elicited by KA before and after application of DNQX (5 µM). As shown by the PSD analysis (right), the kainate and AMPA receptor antagonist significantly blocked elicited gamma activity. (C) Representative recordings of oscillatory activity elicited by KA before and after application of GYKI 52466 (50 µM). As shown by the PSD analysis (right), the AMPA receptor antagonist significantly blocked elicited gamma activity. (D) Representative recordings of oscillatory activity elicited by KA before and after application of GABAzine. As shown by the PSD analysis (right), the GABAA receptor antagonist blocked gamma oscillations. However, large amplitude epileptiform spiking activity was uncovered.
3.2 Effects of acute NMDAR antagonists on PrL gamma oscillations
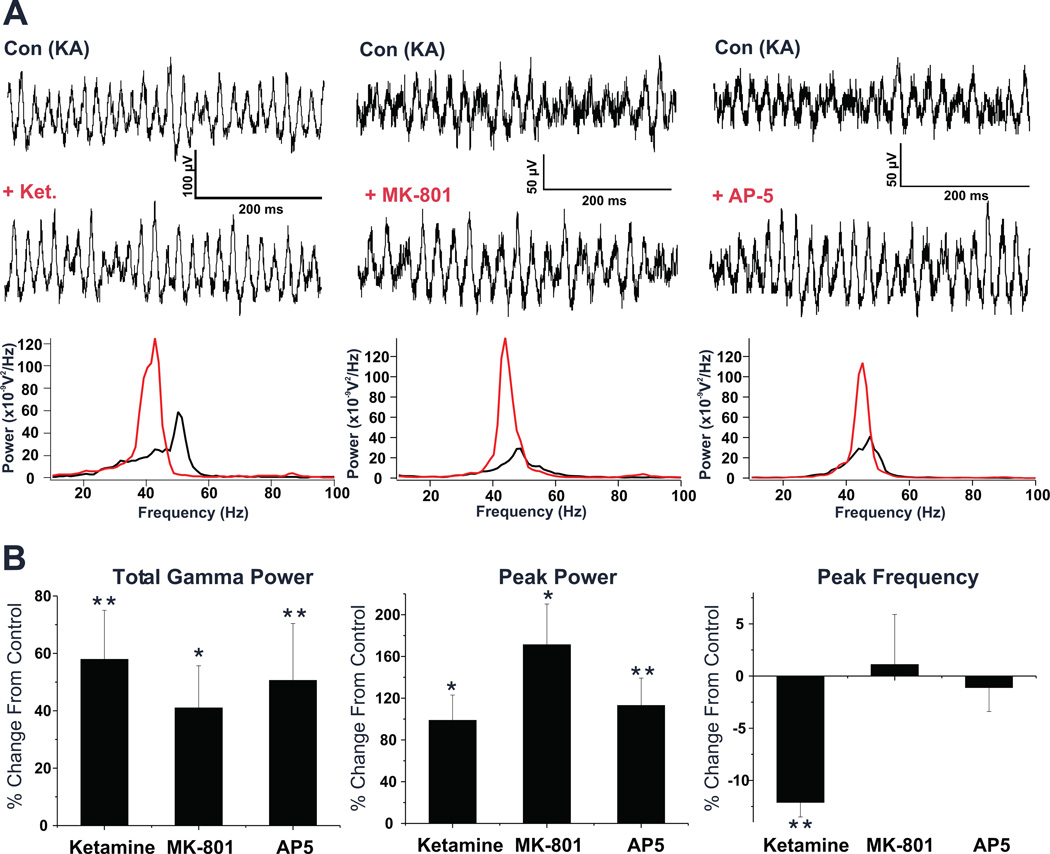
In order to characterize the acute effect of ketamine and other NMDA receptor antagonists, KA-elicited gamma oscillations were characterized in individual slices from mice aged p15-91, first under control conditions, and then again following 15 min of bath perfusion with an NMDAR antagonist. Ketamine (100 µM) significantly increased both the peak power of KA-elicited oscillations by 98.8 ± 24.2%, and total gamma power by 57.9 ± 17.1% (p<0.05; n=11 slices/9 mice; Fig. 3). Ketamine also significantly reduced the peak frequency of these oscillations (p<0.05; −12.1 ± 1.4%; Figure 3). Lower concentrations of ketamine (20 µM, n=3/2; 50 µM, n=4/2) did not significantly alter the power or peak frequency of the gamma oscillations. A slight increase in peak power was observed at 50 µM (29.4% ± 21.7), but this effect did not reach statistical levels of significance (p=0.59).
Figure 3. Acute administration of ketamine and selective NMDAR antagonists potentiates kainate induced gamma oscillations in the PrL cortex.
(A) Left: Field potential records from a representative PrL slice show KA induced gamma oscillations both before and after application of Ketamine (100 µM). Comparing the power spectra of a 5 second epoch of field potential activity recorded after KA application under both conditions, shows a large potentiation of the elicited oscillations and a reduction in the peak frequency. Middle: Field potential records of a representative PrL slice showing KA induced oscillations before and after MK-801 (20 µM) application. Comparing the power spectra of KA elicited activity under each condition shows a large potentiation with MK-801. Right: Field potential records of a representative PrL slice showing KA induced oscillations before and after AP-5 (50 µM) application. Comparing the power spectra of KA elicited activity under each condition shows a large potentiation with AP-5. (B) Bar graphs showing the overall effects of above NMDA antagonists on elicited oscillation across all slices tested. While all agents used significantly potentiated both total gamma power and peak power, only ketamine was observed to have a significant effect on the peak frequency of elicited oscillations. (* p < 0.05; ** p < 0.01)
Ketamine is not a particularly specific agent for the NMDAR, and has been suggested to interact with several other receptor subtypes. Therefore, we tested two additional antagonists with higher specificity for NMDAR; the non-competitive antagonist MK-801 and the competitive antagonist, AP-5 (Figure 3). MK-801 (20 µM) increased peak power by 171.2 ± 39.0%, and total gamma power by 41.0 ± 14.7% across all slices tested (p < 0.05; n=13/11). AP-5 (50 µM) also significantly increased peak power (113.0 ± 26.3%), and total gamma power (50.6 ± 19.8%) of the oscillations (p<0.05; n=17/15). In contrast to ketamine, no significant change was observed in the peak frequency of KA-elicited oscillations with either MK-801 or AP-5 (Figure 3B and Table 1).
Table 1.
Acute pharmacological modulation of KA elicited gamma oscillations.
| n | Total Gamma Power | Peak Power | Peak Frequency | |
|---|---|---|---|---|
| Ketamine (100 µM) | 11 | 57.9 ± 17.1** | 98.9 ± 24.2* | −12.1 ± 1.4** |
| AP-5 (50 µM) | 17 | 50.6 ± 19.8** | 113 ± 26.3** | −1.1 ± 2.3 |
| MK-801 (10 µM) | 13 | 41.0 ± 14.7* | 171.2 ± 39.0* | 1.1 ± 4.8 |
| Ro 25-6981 (1 µM) | 5 | 46.8 ± 11.0* | 114.3 ± 36.2* | 2.1 ± 4.5 |
| PEAQX (500 nM) | 6 | −4.0 ± 17.7 | 21.3 ± 28.1 | 6.3 ± 3.9 |
| PPPA (500 nM) | 5 | −9.4 ± 12.8 | −5.3 ± 25.4 | −1.0 ± 2.7 |
| Quinipirole (30 µM) | 6 | −13.0 ± 16.1 | −8.1 ± 25.6 | −1.6 ± 3.2 |
| α-methyl-5-HT (25 µM) | 6 | −6.4 ± 22.8 | 5.3 ± 26.9 | 3.0 ± 3.1 |
| Ketamine (100 µM) + Risperidone (1 µM) | 5 | 58.0 ± 36.3* | 99.3 ± 42.2 | −12.4 ± 0.8** |
Data is the mean ± SEM.
value is significantly (p < 0.05) different from control.
value is significantly (p < 0.01) different from control
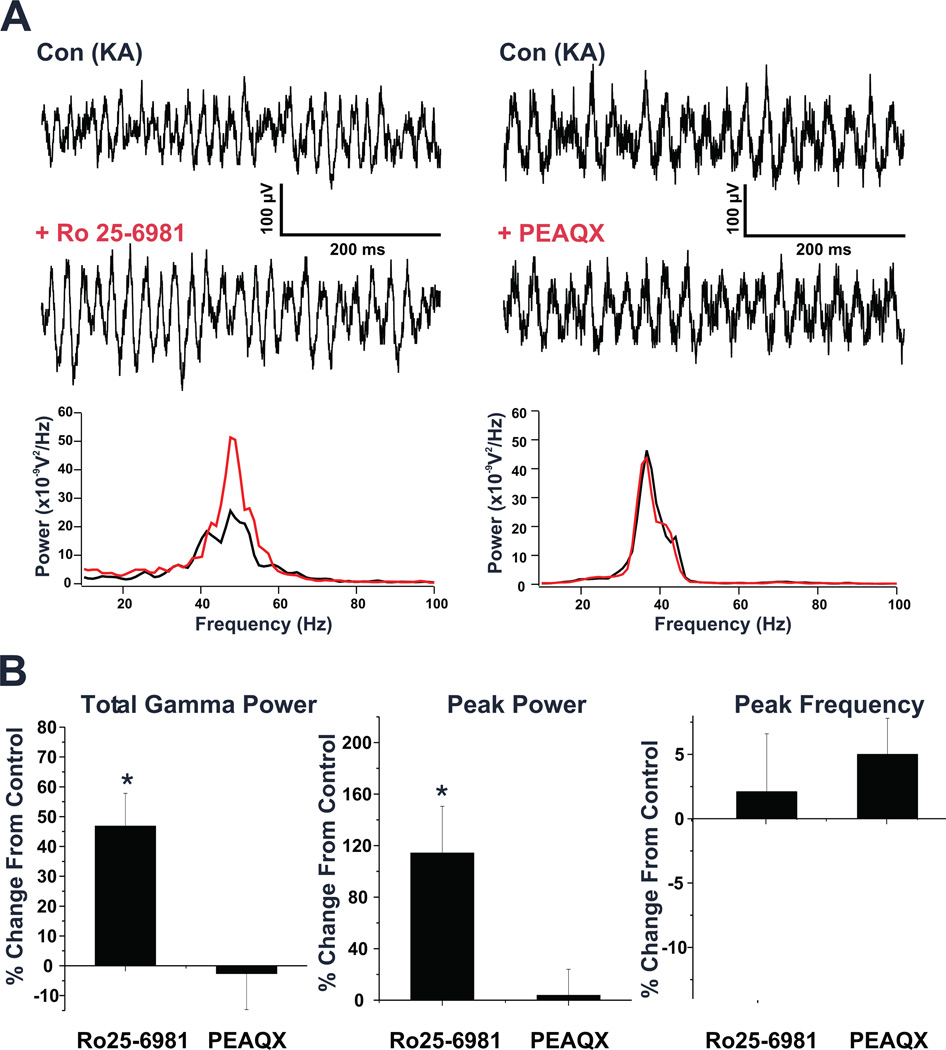
To determine the subtype of NMDAR responsible for this potentiation of gamma power we tested the effects of antagonists selective for NMDARs containing either NR2A or NR2B subunits. As shown in figure 4 and table 1, the NR2B selective antagonist, Ro 25-6981 (1 µM; >5,000× more potent at cloned NR2B/NR1 over NR2A/NR1 receptors; (Fischer et al., 1997)) significantly increased both the peak power (114.3 ± 36.2%) and total gamma power (46.8 ± 11.0%) of KA-elicited gamma oscillations (p<0.05; n=5/4), while the NR2A selective antagonist, PEAQX (500 nM; n=7/6; >10 fold selectivity for NR2A over NR2B (Auberson et al., 2002, Feng et al., 2005)) had no significant effect. Use of PEAQX at higher concentrations was also observed to not alter oscillatory activity (1 µM, n=4/4; 5 µM, n=1/1). Additionally, oscillations were unaffected by a less selective NR2A antagonist, PPPA (500 nM; n=5/4; >3 fold selectivity for NR2A over other NR2 subunits (Feng et al., 2005); Table 1). Thus, potentiation of gamma power was produced by antagonism of NR2B but not NR2A containing NMDAR.
Figure 4. NMDAR antagonist potentiation of oscillations is mediated by NMDAR containing the NR2B subunit.
(A) Left: Field potential records from a representative PrL slice show KA induced gamma oscillations both before and after application of the NR2B antagonist, Ro 25-6981 (1 µM). Comparing the power spectra of a 5 second epoch of field potential activity recorded after KA application under both conditions, shows a large potentiation of the elicited oscillations. Right: Field potential records of a representative PrL slice showing KA induced oscillations before and after application of the NR2A antagonist, PEAQX (500 nM). Comparing the power spectra of KA elicited activity under each condition shows no difference. (B) Bar graphs showing the overall effects of both Ro 25-6981 and PEAQX on elicited oscillations across all slices tested. Ro 25-6981 was found to significantly potentiate both total gamma power and peak power of elicited oscillations, while PEAQX had no effect. Neither agent significantly altered the peak frequency of these oscillations. (* p < 0.05)
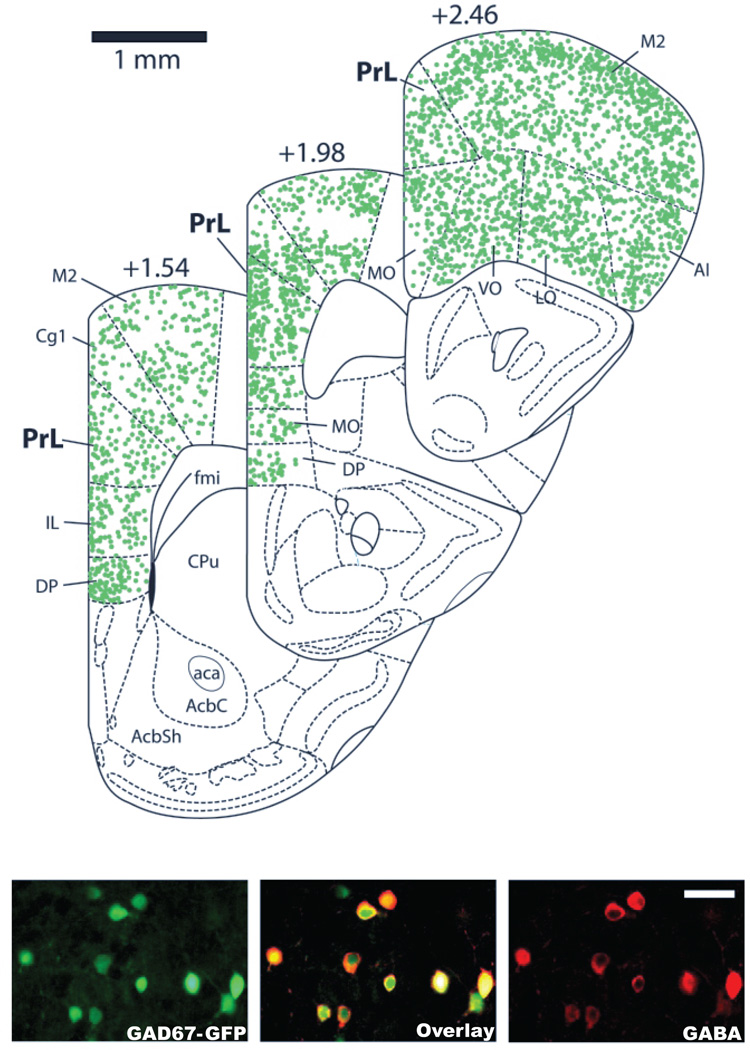
To address the reduction in peak oscillatory frequency observed specifically following ketamine treatment, we investigated potential effects ketamine might be having on GABAergic receptor function. GABAergic receptor decay kinetics play a direct role in determining the frequency of gamma oscillations in in vitro systems (Faulkner et al., 1998, Whittington et al., 2000b). While best known for its action as a NMDAR antagonist, ketamine has been suggested to influence GABAA receptor function (Flood and Krasowski, 2000, Irifune et al., 2000). Therefore, to determine if changes in the GABAergic receptor decay kinetics are responsible for the reduced oscillatory frequency observed with acute ketamine treatment, we analyzed the effect of ketamine (100 µM) on mIPSCs in PrL interneurons and pyramidal neurons. Use of slices from GAD67-GFP mice enabled us to readily distinguish interneurons in the slice based on their expression of GFP. To confirm the specificity of GFP expression in the PrL, cell counts were performed in slices fluorescently labeled for GABA (bilateral, three slices per animal; +1.54, 1.98, and 2.46 mm anterior to Bregma). Out of 5709 neurons analyzed in these slices, 96.7 ± 0.3% of GFP-positive cells were co-labeled with GABA, and 94.3 ± 0.8% of GABAergic neurons co-expressed GFP (Figure 5).
Figure 5. GAD67-GFP knock-in mice specifically express GFP in GABAergic Neurons in the PrL.
Representative fluorescence images of PrL slices from GAD67-GFP knock-in mice fluorescently labeled for GABA. Images show that both the GFP and GABA (A594) signals (40X Magnification) show strong colocalization. (Scale bar is equivalent to 50 µm)
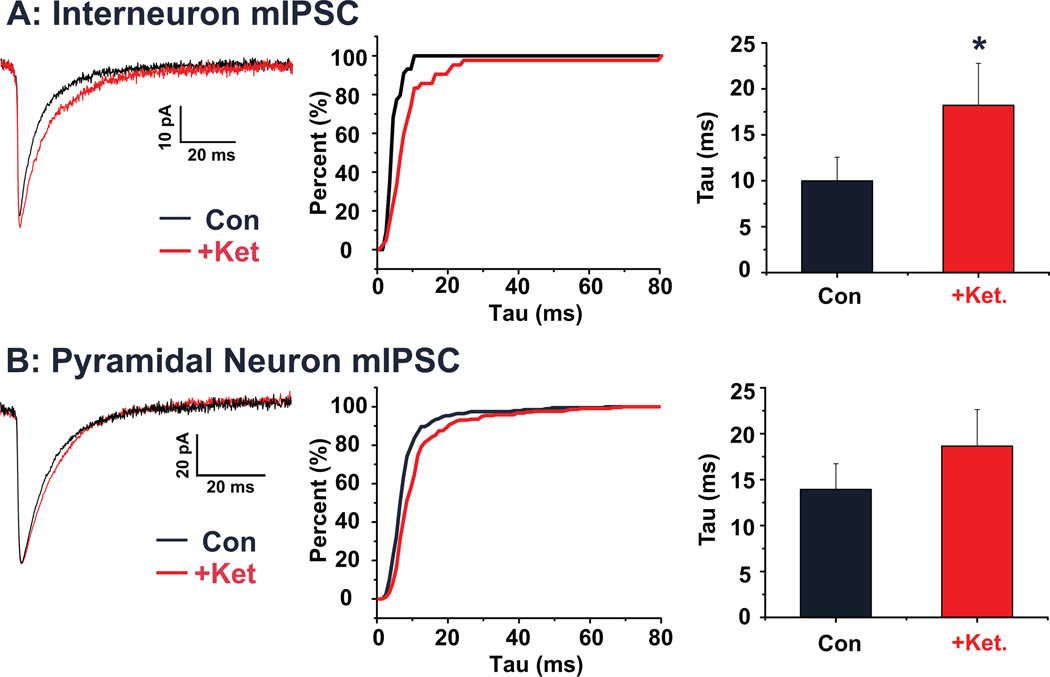
mIPSCs were isolated via bath perfusion with TTX, DNQX and AP-5, allowing us to focus exclusively on ketamine effects on GABA receptor mediated currents in the absence of ketamine effects on NMDAR. mIPSCs were first recorded from PrL interneurons, identified by their expression of GFP (n=5 cells; Figure 6A). Under control conditions, mIPSCs had a decay time constant (tau) of 10.0 ± 2.6 ms. Following application of ketamine, this value significantly increased to 18.2 ± 4.6 ms (p < 0.05). For PrL pyramidal neurons (n=7 cells; Figure 6B), under control conditions, mIPSCs had an average decay tau of 13.9 ± 2.8 ms. Following application of ketamine this value increased to 18.6 ± 4.0 ms. However, this increase was not found to be statistically significant (p = 0.31).
Figure 6. Acute ketamine increases mIPSC decay time in identified interneurons of the PrL.
(A) Average mIPSCs recorded from a representative PrL interneuron under control conditions (black) and following ketamine (red). The cumulative histogram plots the decay tau of interneuronal mIPSCs from the same representative cell under both control (black) and ketamine (red) treated conditions. Bar graph shows mean decay tau before and after ketamine application. Ketamine significantly (p < 0.05) increased the decay time. (B) Average mIPSCs recorded from a representative PrL pyramidal neuron under control conditions (black) and following ketamine (red). The cumulative histogram plots the decay tau of pyramidal mIPSCs from the same representative cell under both control (black) and ketamine (red) treated conditions. Bar graph shows mean decay tau before and after ketamine application. The increase in decay time in pyramidal neurons was not statistically significant. (* p < 0.05)
Previous studies have also suggested that ketamine is capable of acting as a partial agonist of both type-2 dopamine receptors (D2R) and type-2 serotonin receptors (5-HT2R)(Kapur and Seeman, 2002). Thus, to determine if either of these receptors plays a role in the observed ketamine mediated effects on KA-elicited oscillations, we examined the effects of both a D2R and 5-HT2R agonist on induced oscillations in the PrL. As shown in table 1, neither quinpirole (30 µM, n=6), a selective D2R agonist, or α-methyl-5-hydroxytryptamine (25 µM, n=6), a selective 5-HT2R agonist, had any significant effect on either the power or frequency of KA-elicited oscillations. Additionally, we utilized risperidone, an antipsychotic agent which acts as both a D2R and 5-HT2R antagonist, to see if blocking these two receptors would block the effect of ketamine on KA–elicited oscillation frequency. Co-application of ketamine (100 µM) and risperidone (1 µM) together (n=5) produced no significant change in the total gamma power (p = 0.99), peak power (p = 0.99), or peak frequency (p = 0.89) of KA-elicited oscillatory response in the PrL slice when compared to the effects of ketamine alone (Table 1). Taken together these results rule out the role of both D2R and 5-HT2R in the observed ketamine effects described above.
4. Discussion
In this report we investigated the effect of acute application of the psychomimetic drug, ketamine, on KA-elicited gamma oscillations in the mouse PrL cortex. Our results indicate that ketamine significantly alters PrL gamma oscillatory synchronization in a manner similar to clinically observed Sz-related abnormalities in two important ways.
First, acute ketamine administration produced a strong potentiation of the amplitude of KA-elicited gamma oscillations; increased gamma power has been found to be associated with positive Sz symptoms such as psychosis (Spencer et al., 2004, Herrmann and Demiralp, 2005).
Second, application of ketamine also significantly lowered the frequency of KA-elicited gamma oscillations. Decreased oscillatory frequency has been clinically associated with cognitive Sz symptoms (Spencer et al., 2003, Spencer et al., 2004, Herrmann and Demiralp, 2005).
4.1 Advantages of focal kainate application to elicit oscillations
While no model system is capable of accurately capturing the full complexity of Sz, we believe that our approach provides a useful means to investigate mechanisms underlying gamma oscillatory abnormalities, which represent a particularly important dimension of this, as well as other neuropsychiatric disorders. Previous in vitro slice studies of gamma oscillations, mainly in the hippocampal formation or entorhinal cortex, employed continuous bath application of KA and/or the cholinergic agonist carbachol to elicit oscillatory activity (Fisahn, 2005). However, in neocortical slice preparations, use of this method to stimulate oscillations, with the exception of two recent papers from one group (Anver et al., 2010, Oke et al., 2010), generally elicited lower frequency, alpha or beta oscillations (Kilb and Luhmann, 2003, Dupont et al., 2006, Roopun et al., 2008, van Aerde et al., 2008) and did not consistently induce oscillations in the gamma band (>30 Hz). In addition, most in vitro slice studies of oscillations use interface-type recording chambers which do not allow water-immersion objectives necessary for visually guided patch-clamp recordings (Hajos and Mody, 2009). Therefore, in order to study gamma oscillations in neocortical slices maintained in a submerged-type recording chamber, enabling the investigation of neurons identified by expression of fluorescent markers, we adapted the technique used by Gloveli and colleagues (2005) in the hippocampus. Gamma oscillations induced by pressure ejection of kainate onto the slice in close proximity to the recording electrode were reproducible in frequency and power. Similar to gamma oscillations studied in other brain regions, PrL gamma oscillations required neuronal firing and were dependent on glutamatergic AMPA receptors and on GABAA receptors, implicating recurrent circuitry involving glutamatergic neurons and GABAergic interneurons in their generation.
4.2 Increase in gamma power with acute ketamine application
Studies in healthy humans have demonstrated increased metabolism in the prefrontal cortex associated with the psychosis-like state produced by acute administration of ketamine (Breier et al., 1997, Holcomb et al., 2001, Honey et al., 2004, Honey et al., 2005, Corlett et al., 2006, Deakin et al., 2008), suggesting that acutely administered ketamine increases neuronal activity. Consistent with this idea and similar to previous in vivo studies of the effect of acute systemic ketamine on cortical gamma oscillatory activity in rats (Pinault, 2008, Hakami et al., 2009), we observed a large potentiation of induced gamma oscillations in the PrL slice with acute, bath application of subanesthetic concentrations of ketamine. Thus, our results suggest that this in vivo effect of systemic ketamine on prefrontal gamma oscillations is, at least partially, due to a direct effect on the PrL. Our results are also consistent with recent in vitro findings in the rat visual cortex which described two distinct (high and low frequency) local gamma oscillators, which are potentiated and entrained at higher concentrations of ketamine or other NMDAR antagonists (Anver et al., 2010, Oke et al., 2010), suggesting a mechanism by which NMDA hypofunction could generate psychosis (Anver et al., 2010).
The increase in the power of KA-elicited gamma oscillations induced by acute ketamine in the PrL appears to be directly mediated by its interaction with NMDAR, as this potentiation was also induced by other, more selective NMDAR antagonists. While the concentration (100 µM) of ketamine which we found to potentiate gamma power is relatively high, it is consistent with previous estimates of the ability of ketamine to block the effect of NMDAR in rat brain slices (Wu and Johnson, 1996). Ketamine has also been observed to an increased dopamine release in both humans and rodents when applied systemically (Aalto et al., 2005, Kamiyama et al., 2011). However, this effect is unlikely to account for our results, since the cell bodies of dopamine neurons are not present in the PrL slice and a previous study in rat prefrontal cortical slices found no effect of ketamine on dopamine release at ketamine concentrations up to 300 µM (Rodvelt et al., 2008). Additionally, ketamine has been suggested to act as partial agonist of both D2R and 5-HT2R (Kapur and Seeman, 2002). However, we found no evidence that either of these receptors were involved in the modulation of KA-elicited gamma oscillation by acute ketamine.
The potentiation of gamma power by ketamine and other NMDAR antagonists most likely involves blockade of NMDAR on local inhibitory GABAergic interneurons, since these neurons are around tenfold more sensitive to NMDAR antagonists than pyramidal cells (Grunze et al., 1996). Recent genetic manipulation studies have revealed that ablation of NMDAR specifically on fast-spiking interneurons containing parvalbumin increases the power of spontaneous gamma oscillations in the hippocampus and neocortex (Korotkova et al., 2010, Carlen et al., 2011). As described by Homayoun and Moghaddam (2007), acute application of NMDAR antagonists in the prefrontal cortex leads to disinhibition, and increased firing of pyramidal neurons, allowing these neurons to become more easily entrained in oscillatory activity.
Based on the effects of subunit-specific NMDAR antagonists, the potentiation of gamma oscillations observed in our PrL preparation was mediated specifically by NMDARs containing the NR2B subunit. This subunit was also found to mediate NMDAR antagonist effects on gamma oscillations observed in the visual cortex (Anver et al., 2010). While NR2B containing NMDARs show lower expression levels than NR2A containing receptors in the adult cortex, these receptors have longer lasting currents and conduct more calcium per unit of current compared to those with NR2A subunits (Yashiro and Philpot, 2008). Thus, these receptors are potentially better suited to alter cortical circuit function. Recently, meta analysis findings have implicated NR2B (GRIN2B) as a risk gene for Sz (Allen et al., 2008), and postmortem studies suggest trafficking of this NMDAR subunit is altered in Sz (Kristiansen et al., 2010a, Kristiansen et al., 2010b).
4.3 Decrease in oscillation frequency with acute ketamine application
All NMDAR antagonists tested in our system potentiated the power of KA-elicited gamma oscillations, allowing us to surmise that this increase is mediated specifically by NMDAR. However, only with ketamine did we observe a significant effect on oscillatory frequency. Such a finding is not completely unexpected, as ketamine has been found to produce multiple effects unrelated to its action as NMDAR antagonist (Rotaru et al., 2011 and see above). Previous studies have suggested that the decay time course of GABAA receptors is primarily responsible for determining the frequency of gamma oscillations (Faulkner et al., 1998, Whittington et al., 2000a, Vierling-Claassen et al., 2008). We thus investigated if ketamine affected mIPSCs in the PrL. Consistent with its effect on peak gamma frequency, ketamine significantly increased the decay time of mIPSCs recorded from identified interneurons. It is important to note that these recordings were performed in the presence of AP-5, a more selective NMDAR antagonist, indicating that the observed effect was not mediated by NMDAR. Several prior studies have suggested that ketamine can influence GABAA receptor function (Flood and Krasowski, 2000, Irifune et al., 2000). Additionally, recent work in the cerebellum has found that ketamine, but not other NMDAR antagonists (PCP), can positively modulate activity of particular GABAA receptor subtypes (Hevers et al., 2008). Thus, the observed decrease in the peak frequency of KA-induced oscillations in the PrL following ketamine is likely due to an alteration of the kinetics of GABAA receptors, particularly in PrL interneurons.
The decrease in the peak oscillatory frequency of KA-elicited oscillations observed in response to ketamine is quite intriguing, as it is reminiscent of clinical Sz studies which also observed reductions in the frequency (gamma to high beta) of neuronal oscillations elicited by complex sensory stimuli (Spencer et al., 2004, Hirano et al., 2008). Moreover, one of the most highly replicated findings in clinical studies is the inability of schizophrenics to generate oscillations in phase with a 40 Hz steady-state auditory input, although they respond normally to input in the beta frequency range. (Kwon et al., 1999, Brenner et al., 2003, Light et al., 2006). Such impairments in neural synchrony are associated with specific Sz-related cognitive impairments (Uhlhaas and Singer, 2006). Recording and modeling studies suggest that lower frequency oscillations coordinate neuronal activity across longer distances than those at higher frequencies (Kopell et al., 2000, Uhlhaas et al., 2008). Therefore, it is tempting to speculate that the decrease in oscillatory frequency observed following ketamine treatment could result in inappropriate synchronization over a larger cortical region, which could contribute to the psychosis and impaired cognition observed in clinical studies, and Sz-like behaviors in animal studies.
4.4 Conclusion
In conclusion, acute ketamine not only closely mimics the behavioral and cortical circuitry changes typical of Sz but is also capable of reproducing some of the gamma oscillation abnormalities observed in this disease. Our in vitro findings about the receptor complexity of the ketamine Sz model may help to explain some of the divergence of gamma findings in clinical studies of Sz, and prove useful in developing new therapeutic strategies to address the gamma oscillation abnormalities and associated clinical symptoms of Sz.
Highlights.
In vitro slice model of EEG gamma oscillation abnormalities seen in schizophrenia
Acute application of the psychomimetic ketamine strongly potentiated gamma power
Gamma power increases were mediated by NMDARs containing the NR2B subunit
Acute Ketamine also decreased gamma frequency by acting on GABA-A receptors
Acknowledgements
We would like to thank Dr. Kevin Spencer for his comments and input on this manuscript. This study was supported by the VA and by an ARRA supplement to National Institute of Mental Health (NIMH) RO1 grant MH040799 “Neurophysiological studies of Schizophrenia” to R.W. McCarley, NIMH R21 grant MH094803 “Modeling schizophrenia gamma deficits using cell-specific RNAi knockdown of GAD67” to R.E. Brown, and Grants-in Aids for Scientific Research from MEXT, Japan and Takeda Science Foundation to Y. Yanagawa.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- GABA
gamma-aminobutyric acid
- GAD67
Glutamic acid decarboxylase (67 kD)
- GFP
Green fluorescent protein
- KA
kainate
- mIPSC
miniature inhibitory postsynaptic current
- NMDAR
N-Methyl-D-aspartic acid receptor
- PrL
prelimbic cortex
- PSD
power spectral density
- Sz
schizophrenia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
James M. McNally, Email: james_mcnally@hms.harvard.edu.
Robert W. McCarley, Email: robert_mccarley@hms.harvard.edu.
James T. McKenna, Email: james_mckenna@hms.harvard.edu.
Yuchio Yanagawa, Email: yanagawa@med.gunma-u.ac.jp.
Ritchie E. Brown, Email: ritchie_brown@hms.harvard.edu.
References
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, Nagren K, Vilkman H, Gustafsson LL, Syvalahti E, Hietala J. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology (Berl) 2005;182:375–383. doi: 10.1007/s00213-005-0092-6. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Sabanov V, D'Hooge R, Balschun D. An N-methyl-d-aspartate-receptor dependent late-phase long-term depression in middle-aged mice identifies no GluN2-subunit bias. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.04.006. in press. [DOI] [PubMed] [Google Scholar]
- Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- Anver H, Ward PD, Magony A, Vreugdenhil M. NMDA Receptor Hypofunction Phase Couples Independent gamma-Oscillations in the Rat Visual Cortex. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Berman KF, Zec RF, Weinberger DR. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. II. Role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry. 1986;43:126–135. doi: 10.1001/archpsyc.1986.01800020032005. [DOI] [PubMed] [Google Scholar]
- Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. Am J Psychiatry. 1997;154:805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Sporns O, Lysaker PH, O'Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. doi: 10.1176/appi.ajp.160.12.2238. [DOI] [PubMed] [Google Scholar]
- Brown RE, McKenna JT, Winston S, Basheer R, Yanagawa Y, Thakkar MM, McCarley RW. Characterization of GABAergic neurons in rapid-eye-movement sleep controlling regions of the brainstem reticular formation in GAD67-green fluorescent protein knock-in mice. Eur J Neurosci. 2008;27:352–363. doi: 10.1111/j.1460-9568.2008.06024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenikova-Valesova V, Horacek J, Vrajova M, Hoschl C. Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci Biobehav Rev. 2008;32:1014–1023. doi: 10.1016/j.neubiorev.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, McKenna JT, Bolortuya Y, Winston S, Thakkar MM, Basheer R, Brown RE, McCarley RW. Knockdown of orexin type 1 receptor in rat locus coeruleus increases REM sleep during the dark period. Eur J Neurosci. 2010;32:1528–1536. doi: 10.1111/j.1460-9568.2010.07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlett PR, Honey GD, Aitken MR, Dickinson A, Shanks DR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, McKenna PJ, Robbins TW, Bullmore ET, Fletcher PC. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch Gen Psychiatry. 2006;63:611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Dupont E, Hanganu IL, Kilb W, Hirsch S, Luhmann HJ. Rapid developmental switch in the mechanisms driving early cortical columnar networks. Nature. 2006;439:79–83. doi: 10.1038/nature04264. [DOI] [PubMed] [Google Scholar]
- Faulkner HJ, Traub RD, Whittington MA. Disruption of synchronous gamma oscillations in the rat hippocampal slice: a common mechanism of anaesthetic drug action. Br J Pharmacol. 1998;125:483–492. doi: 10.1038/sj.bjp.0702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Morley RM, Jane DE, Monaghan DT. The effect of competitive antagonist chain length on NMDA receptor subunit selectivity. Neuropharmacology. 2005;48:354–359. doi: 10.1016/j.neuropharm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Fisahn A. Kainate receptors and rhythmic activity in neuronal networks: hippocampal gamma oscillations as a tool. J Physiol. 2005;562:65–72. doi: 10.1113/jphysiol.2004.077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E, Heitz MP, Kemp JA. Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther. 1997;283:1285–1292. [PubMed] [Google Scholar]
- Flood P, Krasowski MD. Intravenous anesthetics differentially modulate ligand-gated ion channels. Anesthesiology. 2000;92:1418–1425. doi: 10.1097/00000542-200005000-00033. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. New York: Elsevier Press; 2008. [Google Scholar]
- Gloveli T, Dugladze T, Saha S, Monyer H, Heinemann U, Traub RD, Whittington MA, Buhl EH. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz-Bruce H. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev. 2009;60:279–286. doi: 10.1016/j.brainresrev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Hajos N, Mody I. Establishing a physiological environment for visualized in vitro brain slice recordings by increasing oxygen supply and modifying aCSF content. J Neurosci Methods. 2009;183:107–113. doi: 10.1016/j.jneumeth.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, O'Brien TJ, Pinault D. NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hevers W, Hadley SH, Luddens H, Amin J. Ketamine, but not phencyclidine, selectively modulates cerebellar GABA(A) receptors containing alpha6 and delta subunits. J Neurosci. 2008;28:5383–5393. doi: 10.1523/JNEUROSCI.5443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Hirano Y, Maekawa T, Obayashi C, Oribe N, Kuroki T, Kanba S, Onitsuka T. Abnormal neural oscillatory activity to speech sounds in schizophrenia: a magnetoencephalography study. J Neurosci. 2008;28:4897–4903. doi: 10.1523/JNEUROSCI.5031-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Weiler M, Tamminga CA. Sequential regional cerebral blood flow brain scans using PET with H2(15)O demonstrate ketamine actions in CNS dynamically. Neuropsychopharmacology. 2001;25:165–172. doi: 10.1016/S0893-133X(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Honey RA, O'Loughlin C, Sharar SR, Kumaran D, Suckling J, Menon DK, Sleator C, Bullmore ET, Fletcher PC. Ketamine disrupts frontal and hippocampal contribution to encoding and retrieval of episodic memory: an fMRI study. Cereb Cortex. 2005;15:749–759. doi: 10.1093/cercor/bhh176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RA, Honey GD, O'Loughlin C, Sharar SR, Kumaran D, Bullmore ET, Menon DK, Donovan T, Lupson VC, Bisbrown-Chippendale R, Fletcher PC. Acute ketamine administration alters the brain responses to executive demands in a verbal working memory task: an FMRI study. Neuropsychopharmacology. 2004;29:1203–1214. doi: 10.1038/sj.npp.1300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irifune M, Sato T, Kamata Y, Nishikawa T, Dohi T, Kawahara M. Evidence for GABA(A) receptor agonistic properties of ketamine: convulsive and anesthetic behavioral models in mice. Anesth Analg. 2000;91:230–236. doi: 10.1097/00000539-200007000-00043. [DOI] [PubMed] [Google Scholar]
- Kamiyama H, Matsumoto M, Otani S, Kimura SI, Shimamura KI, Ishikawa S, Yanagawa Y, Togashi H. Mechanisms underlying ketamine-induced synaptic depression in rat hippocampus-medial prefrontal cortex pathway. Neuroscience. 2011;177:159–169. doi: 10.1016/j.neuroscience.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Mol Psychiatry. 2002;7:837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- Kilb W, Luhmann HJ. Carbachol-induced network oscillations in the intact cerebral cortex of the newborn rat. Cereb Cortex. 2003;13:409–421. doi: 10.1093/cercor/13.4.409. [DOI] [PubMed] [Google Scholar]
- Kline DD, Hendricks G, Hermann G, Rogers RC, Kunze DL. Dopamine inhibits N-type channels in visceral afferents to reduce synaptic transmitter release under normoxic and chronic intermittent hypoxic conditions. J Neurophysiol. 2009;101:2270–2278. doi: 10.1152/jn.91304.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68:557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Bakir B, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophr Res. 2010a;119:198–209. doi: 10.1016/j.schres.2010.02.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Patel SA, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor subunit and its Tbr-1/CINAP regulatory proteins in postmortem brain suggest altered receptor processing in schizophrenia. Synapse. 2010b;64:495–502. doi: 10.1002/syn.20754. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL. Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry. 2006;60:1231–1240. doi: 10.1016/j.biopsych.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Liu Z, Bunney EB, Appel SB, Brodie MS. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C. J Neurophysiol. 2003;90:3201–3212. doi: 10.1152/jn.00281.2003. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oke OO, Magony A, Anver H, Ward PD, Jiruska P, Jefferys JG, Vreugdenhil M. High-frequency gamma oscillations coexist with low-frequency gamma oscillations in the rat visual cortex in vitro. Eur J Neurosci. 2010;31:1435–1445. doi: 10.1111/j.1460-9568.2010.07171.x. [DOI] [PubMed] [Google Scholar]
- Pinault D. N-methyl d-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Rodvelt KR, Schachtman TR, Kracke GR, Miller DK. NMDA receptor blockade augmented nicotine-evoked dopamine release from rat prefrontal cortex slices. Neurosci Lett. 2008;440:319–322. doi: 10.1016/j.neulet.2008.05.106. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci. 2009;10:85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- van Aerde KI, Heistek TS, Mansvelder HD. Prelimbic and infralimbic prefrontal cortex interact during fast network oscillations. PLoS ONE. 2008;3:e2725. doi: 10.1371/journal.pone.0002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vierling-Claassen D, Siekmeier P, Stufflebeam S, Kopell N. Modeling GABA alterations in schizophrenia: a link between impaired inhibition and altered gamma and beta range auditory entrainment. J Neurophysiol. 2008;99:2656–2671. doi: 10.1152/jn.00870.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res. 2008;236:42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Faulkner HJ, Doheny HC, Traub RD. Neuronal fast oscillations as a target site for psychoactive drugs. Pharmacol Ther. 2000a;86:171–190. doi: 10.1016/s0163-7258(00)00038-3. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000b;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Williams SN, Undieh AS. Dopamine D1-like receptor activation induces brain-derived neurotrophic factor protein expression. Neuroreport. 2009;20:606–610. doi: 10.1097/WNR.0b013e32832a0a98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YN, Johnson SW. Pharmacological characterization of inward current evoked by N-methyl-D-aspartate in dopamine neurons in the rat brain slice. J Pharmacol Exp Ther. 1996;279:457–463. [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]