Abstract
Purpose
To determine the value of a topical carbonic anhydrase inhibitor on the macular thickness and function in choroideremia patients with cystoid macular edema (CME).
Methods
Two choroideremia patients with CME, observed by spectral-domain optical coherence tomography (SD-OCT), were treated with a topical form of carbonic anhydrase inhibitor. Examinations performed before and during treatment included: best corrected visual acuity (BCVA) by using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts, contrast sensitivity measured with briefly presented grating targets (grating CS) and the Pelli-Robson chart (P-R CS), microperimetry (MP), and SD-OCT.
Results
The two choroideremia patients treated with dorzolamide 2% formulation had a noticeable reduction in macular thickness by SD-OCT. This reduction was found in both eyes after 2 months of treatment. After an additional 3 months of the same treatment regimen, a more noticeable reduction in macular thickness was observed. The two study patients had improvement of their visual acuity, in at least one eye, on ETDRS charts, but no clinically significant changes for the other measures of visual function.
Conclusion
The present study shows the potential efficacy of topical dorzolamide for treating choroideremia patients with CME.
Keywords: Cystoid macular edema, Choroideremia, Carbonic anhydrase inhibitor, Spectral-domain OCT
Introduction
Choroideremia (CHM) is a rare X-linked recessive degenerative chorioretinal disorder. The gene underlying CHM encodes the Rab Escort Protein 1 (REP1). The REP1 gene is ubiquitously expressed and acts as an escort protein of native regulators of intracellular trafficking.1,2 Progressive atrophy of the retinal photoreceptors, retinal pigment epithelium (RPE), and choroid are typical findings in CHM. Affected males develop night blindness in their teenage years, followed by loss of peripheral vision and legal blindness by middle age.3
Genead and Fishman reported the presence of cystic macular changes of variable degrees using spectral-domain optical coherence tomography (SD-OCT) with an overall prevalence of 63% in patients with choroideremia.4 Cystoid macular edema (CME) has also been previously reported in different hereditary retinal diseases such retinitis pigmentosa,5 Usher syndrome,6 X-linked retinoschisis,7 enhanced S-cone syndrome,8,9 and gyrate atrophy.10
Prior reports showed that the use of a topical carbonic anhydrase inhibitor (CAI) is effective for the improvement of CME with a modest increase in the visual acuity (in some of the subjects) in patients with retinal dystrophies such as retinitis pigmentosa,11,12 X-linked retinoschisis,7,13 and enhanced S-cone syndrome.9,14
SD-OCT performs noninvasive, real-time imaging of the retina and anterior segment of the eye that provides high-resolution, high-speed measurements and cross-sectional imaging of the intraretinal architectural morphology, especially the photoreceptor layer.15,16 The improvement in resolution provided by SD-OCT allows the junction between photoreceptor inner and outer segment (IS/OS) to be clearly seen. The presence of the IS/OS indicates normal alignment of the photoreceptors, and this alignment is essential for normal visual function.17
Microperimetry has gained increasing clinical interest as a tool for the evaluation of visual function in chorioretinal diseases.18 This test facilitates allows a real-time functional evaluation, directly correlated to fundus characteristics, with continuous fixation monitoring. In this way, microscotoma of the central visual field are detected under direct visualization, a task not possible with conventional perimetry.19,20
Although contrast sensitivity is not routinely measured in the clinic, evaluating contrast sensitivity can provide a sensitive index of visual function in patients undergoing treatment with CAIs.9 Additionally, contrast sensitivity is a good predictor of performance for many everyday activities.21 The measurement of foveal contrast sensitivity has been recommended as an outcome measure in clinical trials of therapeutic interventions22 and the usefulness of contrast sensitivity measurement was recently highlighted in an NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium.23
The aim of the current study was to determine the efficacy of a topical therapy with dorzolamide 2% ophthalmic solution on the retinal thickness and macular visual function in two choroideremia patients with CME evident on SD-OCT testing. Retinal thickness and macular visual function measurements were performed before the treatment was initiated and at two and five months post-treatment. Measurements of macular visual function included: best-corrected visual acuity, microperimetry sensitivity, Pelli-Robson letter contrast sensitivity (P-R CS), and contrast sensitivity for briefly presented grating targets (grating CS).
Patients and Methods
Patients
Two choroideremia patients with CME evident on SD-OCT testing were informed about the objectives of the study and volunteered to participate. Informed consent was obtained according to the tenets of the Declaration of Helsinki. The study was approved by an institutional review board at the University of Illinois at Chicago.
Clinical Examinations
Ophthalmic examinations included best-corrected visual acuity (BCVA) using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement (Goldmann applanation tonometry), and dilated funduscopy using both direct and indirect ophthalmoscopy.
Retinal Thickness Mapping
SD-OCT was performed using a spectral OCT/SLO system (OPKO instrumentations, Miami, FL) to obtain both OCT and SLO images with an axial resolution of <10 microns, and a transverse resolution of 20 microns (in tissue). The system uses an SLO fundus image for alignment, orientation, and registration of the OCT image topographic maps. Both the Line scan (B-scan) and the 3D Retinal Topography scan protocols were used for image acquisition. The Line scan mode allows the capture of cross-sectional B-scan OCT images of the vitreoretinal, retinal, and chorioretinal structures. We used the “Max Frame Count” of 32 frames. The “Max Frame Count” is the maximum sequentially captured frames of OCT and SLO images, which are captured and displayed as individual frames. The 3D Retinal Topography mode covers an area of 9.0 × 9.0 mm with a 2.0 mm depth. The retinal thickness map is displayed as 9 ETDRS-like subfields including central, parafoveal and perifoveal superior, temporal, inferior, and nasal subfields. The central subfield included the circle centered on the fovea with a radius of 0.5 mm. The parafoveal subfields included the concentric ring of retina around the central subfield with an inner radius of 0.5 mm and an outer radius of 1.5mm from the fovea. The perifoveal subfields included the outer ring of retina beyond the parafoveal subfields concentric with the fovea and with an inner radius of 1.5 mm and an outer radius of 3 mm. The macular thickness data were compared with normative data provided by the manufacturer, comprised of 225 eyes of 119 normative control subjects (mean age of 47.8 ± 16.3 years).
Macular Microperimetry (MP) Testing
MP was performed using the OPKO Spectral OCT/SLO system. All tests were performed after dilation of the pupil with 1% tropicamide and 2.5% phenylephrine. The non-tested eye was patched during testing. The subjects underwent a practice test and were instructed to fixate on a red square and to depress a button as soon as they saw the stimulus light. The Polar 3 testing grid was used for all subjects. Polar 3 is a standardized grid composed of 28 points arranged in three concentric circles (2.3°, 6.6°, and 11° in diameter) within the central macula. The inner circle is composed of four points, whereas the middle and outer circles are each composed of 12 points. Parameters included a Goldmann III size stimulus (area of 4 mm2, diameter of 26 min of arc or 0.4 degrees), a 200 ms duration of stimulus presentation, and a 4-2 test strategy. The system automatically tracks fundus location according to retinal vessel alignment to ensure accurate stimuli placement. MP sensitivity data were compared with normative sensitivity values obtained for 34 normal subjects with the same instrument.24
Contrast Sensitivity (CS) Testing
Contrast sensitivity for large letters was measured using the Pelli-Robson contrast sensitivity chart (P-R CS). The test distance was 1 m, as recommended by the manufacturer. P-R CS measurements were obtained from each eye of both patients. Additionally, contrast sensitivity was measured using a 2 cycle-per-degree grating target that was briefly presented (100 ms) on a video display (grating CS). For the grating CS measurement, the patients’ task was to judge the grating orientation (horizontal or vertical), and the contrast of the grating was adjusted on each trial to determine contrast sensitivity. For patient 1, grating CS was measured binocularly because fixation was unstable for monocular grating CS measurements. For patient 2, grating CS measurements were only performed in the right eye. For the left eye of this patient, measurements could not be obtained at the maximum contrast that could be produced by the display.
Results
Patient 1
Patient 1 was a 45-year-old Caucasian man of Swedish, Irish, and French ancestry. He had a history of night blindness for as long as he could remember. Additionally, there was a history for a progressive decrease in his peripheral and central vision of each eye. He complained of photoaversion and difficulty with color discrimination. A general review of systems indicated a history of anxiety. A review of the family pedigree indicated two maternal uncles as well as his brother who had night blindness and peripheral field restrictions.
BCVA at baseline was correctable to 0.50 log MAR (20/50−2 on a Snellen acuity chart) OD and to 0.48 log MAR (20/60−2) OS. The patient’s manifest refraction was −3.25+0.75×170° OD and −3.50+0.75×170° OS. Ocular pressures were 12 mmHg OD and 13 mmHg OS. Color vision screening with Ishihara color plates showed only the ability to identify the test plates in each eye. The corneas, anterior chambers, and lenses were clear. The vitreous was fibrillar. Fundus exam showed atrophic changes in the macula with relative sparing of the foveal zone in each eye. The optic discs were normal with peripapillary pigment atrophy and the retinal vessels were attenuated. There were extensive and diffuse RPE and choroidal peripheral retinal pigmentary degenerative changes in both eyes.
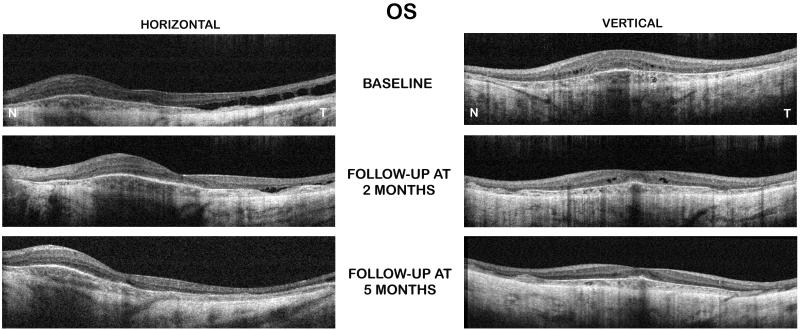
A SD-OCT exam at baseline showed the presence of foveal and extrafoveal microcysic changes in each eye with diffuse CME temporal to fovea in the left eye (Figure 1). SD-CT also showed a marked disorganization of the retinal laminar structures with marked attenuation of the outer retinal structures especially the outer segment/inner segment (OS/IS) junction of the photoreceptors and RPE/choroidal complex. Also, a central macular thickening was observed in each eye (left>right). Of interest, there was a noticeable thickening of the retinal nerve layer nasal to the fovea in each eye.
Figure 1.
Spectral-OCT scans of patient 1 (left eye) showed a noticeable reduction of cystoid macular edema and retinal thickness after two and five months of treatment with topical dorzolamide 2% three times a day (TID).
Microperimetry testing of 28 individual points within a 6 degree radius from the center of the foveola (12 degree circle, Polar 3 pattern) showed areas of dense scotomas and markedly subnormal individual point sensitivities that were below the 99% confidence limit of normal sensitivity. The overall average test score was 2.3 dB OD and 3.0 dB OS, which was reduced below the lower limit of normal (mean-2SD) at 14.1 dB.24 The patient showed surprisingly stable foveal fixation in each eye (92% of eye movements were within 2 degrees around the projected fixation target OD and 88% OS).
At the baseline visit, P-R CS was substantially reduced in both eyes, as compared to normal.25 The reduction in the left eye (0.65 log units) was greater than that in the right eye (0.50 log units). The binocular measurement of grating CS showed an even larger CS deficit (approximately 1.2 log units, as compared to normal). These large reductions in contrast sensitivity measured at baseline are consistent with the marked losses in microperimetric sensitivity.
Treatment with topical dorzolamide 2% three times a day in each eye was initiated. At 2 months after treatment began, the patient reported a mild subjective improvement of his central vision. No changes in his peripheral, night, or color vision were subjectively observed.
BCVA was correctable to 0.36 log MAR (20/50+1) OD and to 0.28 log MAR (20/50+1) OS. The patient’s manifest refraction was similar to the baseline visit. Ocular pressures were 10 mmHg OD and 11 mmHg OS. Color vision screening with the Ishihara color plates mildly improved to 3/17 plates in each eye. The anterior segment and dilated fundus examinations were essentially as noted at baseline.
SD-OCT examination of the right eye was showed a mild decrease in the retinal thickness and macular cysts size and number. However, the left eye showed a marked decrease in the macular thickness when compared to the baseline scans (Figure 1). The central 1-mm foveal subfield thickness decreased from 310 μm and 302 μm (at the baseline visit) to 249 μm and 254 μm, in the right and left eyes, respectively, (at the 2 months follow-up visit) (Table).
Table.
The retinal thickness and visual function data pre and post-treatment with topical dorzolamide 2% formulation in the study cohort
| Retinal thickness (μm) |
ETDRS log MAR visual acuity (Snellen acuity) |
MP sensitivity (dB) |
Log P-R CS | Log grating CS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OD | OS | OD | OS | OD | OS | OD | OS | OD | OS | ||
| Patient 1 | Baseline | 310 | 302 | 0.50 (20/50−2) | 0.48 (20/60−2) | 2.3 | 3.0 | 1.20 | 1.05 | 0.37 (binocular) | |
|
Two-month
follow-up |
249 | 254 | 0.36 (20/50+1) | 0.28 (20/50+1) | 2.6 | 3.4 | 1.20 | 1.05 | 0.81 (binocular) | ||
|
Five-month
follow-up |
236 | 211 | 0.24 (20/40+1) | 0.16 (20/30) | 3.1 | 3.3 | 1.20 | 1.20 | 0.64 (binocular) | ||
| Patient 2 | Baseline | 390 | 395 | 0.46((20/50−2) | 0.70 (20/200) | 3.0 | 1.6 | 1.05 | 0.90 | 0.64 | † |
|
Two-month
follow-up |
276 | 261 | 0.40 (20/50−1) | 0.60 (20/80+1) | 3.3 | 1.5 | 1.05 | 0.90 | 0.89 | ‡ | |
|
Five-month
follow-up |
248 | 253 | 0.44 (20/50−2) | 0.60 (20/80−1) | ** | 1.1 | 1.05 | 0.75 | 0.68 | ‡ | |
ETDRS= Early Treatment Diabetic Retinopathy Study; MP= Microperimetry; P-R CS= Pelli-Robson Letter Contrast Sensitivity; Grating CS= 2.0 cpd Grating Contrast Sensitivity.
The patient could not complete the test due to eye fatigue
Sensitivity was below measurable limits
Sensitivity not measured
The overall average test score on MP testing was similar to baseline line in each eye (2.6 dB OD and 3.4 dB OS), which was still reduced below the lower limit of normal at 14.1 dB. The patient showed an improvement in his ocular fixation as 95% of eye movements were within 2 degrees around the projected fixation target OD and 95% OS.
P-R CS was unchanged following two months of treatment. However, grating CS improved by approximately 0.4 log units. Although there was an improvement in grating CS, the grating CS value following two months of treatment was still below normal (approximately 0.8 log units below normal).
After 5 months of treatment, BCVA further improved to 0.24 log MAR (20/40+1) OD and to 0.16 log MAR (20/30) OS. SD-OCT exam showed additional reduction of the cystoid macular edema (OS>OD) with almost entire resolution of the temporal diffuse macular edema in the left eye (Figure 1). Of note, in each eye, the SD-OCT scans now showed the foveal depression, which was absent on most of the baseline scans. The central 1-mm foveal subfield thickness further decreased to 236 μm OD and 211 μm OS (Table).
No changes in the overall average MP sensitivity values were observed (3.1dB OD and 3.3 dB OS). However, the patient showed a small improvement in his ocular fixation as 100% of eye movements were within 2 degrees around the projected fixation target OD and 100 % OS.
Following 5 months of treatment, P-R CS remained unchanged in the right eye, whereas an improvement of 0.15 log units was found in the left eye. However, P-R CS was still reduced by approximately 0.5 log units, in both eyes, as compared to normal. In contrast to the slight improvement in P-R CS, there was a small reduction in grating CS (approximately 0.17 log units).
Patient 2
Patient 2 was a 63-year-old Caucasian man of German ancestry. He had a history of night blindness and peripheral vision impairment that began in the third decade. Additionally, there was a history for a progressive decrease in central vision of each eye. He complained of infrequent floaters and difficulty with color discrimination. A general review of systems was unremarkable and a review of the family pedigree showed no known family members affected with hereditary retinal diseases.
BCVA at baseline was correctable to 0.46 log MAR (20/50−2) OD and to 0.70 log MAR (20/200) OS. The patient’s manifest refraction was −2.00+1.50×140° OD and −0.75+1.75×40° OS. Ocular pressures were 12 mmHg in each eye. Color vision screening with Ishihara color plates showed 2/17 plates identified correctly OD and only the test plate OS. Anterior segment examination was unremarkable except for 1+ nuclear sclerosis in each eye of no clinical significance.
Dilated fundus exam showed normal optic discs with peripapillary pigment atrophy and mild attenuation of the retinal vessels in each eye. There was moderately extensive RPE and choroidal atrophy throughout the mid-peripheral retina and the posterior pole with relative sparing of the foveola with isolated areas of pigment clumping in the mid-peripheral retina of each eye.
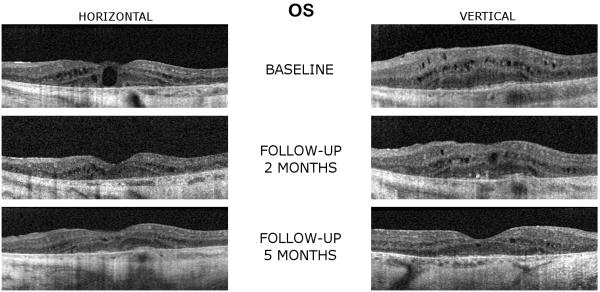
A SD-OCT exam at the baseline showed bilateral microcystic and macrocystic macular changes in each eye (OS>OD) with central macular thickening in both eyes. There were marked RPE and choroidal architecture disorganization with loss of OS/IS junction of the photoreceptors in the maculae of each eye (Figure 2).
Figure 2.
Spectral-domain OCT scans of patient 2 (left eye) demonstrate a reduction of cystoid macular edema and retinal thickness while on treatment with topical dorzolamide 2% (TID) following two and five months of treatment.
Microperimetry testing with the Polar 3 pattern program showed areas of dense scotomas and markedly subnormal individual point sensitivities that were below the 99% confidence limit of normal sensitivity. The overall average test score was 3.0 dB OD and 1.6 dB OS. The patient showed stable foveal fixation in each eye (90% of eye movements within 2 degrees around the projected fixation target OD and 90% OS).
At the baseline visit, P-R CS was substantially reduced in both eyes, as compared to normal.25 The P-R CS loss in the left eye (0.80 log units) was greater than that in the right eye (0.65 log units). Measurement of grating CS in the right eye showed a loss of approximately 1.0 log unit, as compared to normal. Grating CS could not be performed in the left eye.
Treatment with topical dorzolamide 2% three times a day in each eye was initiated. After 2 months, the patient reported a moderate subjective improvement of his central vision and also noticed that objects were brighter in the distance. Additionally, the patient noted that his vision was less “hazy.” No changes in his peripheral, night, or color vision were subjectively observed.
BCVA improved to 0.40 log MAR (20/50−1) OD and to 0.60 log MAR (20/80+1) OS. The patient’s manifest refraction was essentially similar to the baseline visit. Ocular pressures were 11 mmHg OD and 12 mmHg OS. No changes were observed in color vision screening with the Ishihara color plates. The anterior segment and dilated fundus examinations were similar to baseline.
SD-OCT examination showed a mild to moderate decrease in the retinal thickness and both the size and number of the macular cysts in each eye when compared to the baseline scans (Figure 2). The central 1-mm foveal subfield thickness decreased from 390 μm and 395 μm (at the baseline visit) to 276 μm and 261 μm, in the right and left eyes, respectively, (at the 2 months follow-up visit) (Table).
The overall average test score on MP testing was similar to baseline in each eye (3.3 dB OD and 1.5 dB OS), which was still reduced below the lower limit of normal at 14.1 dB. The patient’s fixation was stable, with 92% of eye movements were within 2 degrees around the projected fixation target OD and 91% OS.
Following two months of treatment, P-R CS was unchanged. However, grating CS improved by approximately 0.25 log units. Although grating CS improved, the grating CS value was still approximately 0.7 log units below normal.
After 5 months of treatment, the patient’s BCVA was maintained at 0.44 log MAR (20/50−2) OD and to 0.60 log MAR (20/80−1) OS. SD-OCT exam showed a further marked reduction of the cystoid macular edema (OS>OD) with almost entire resolution in each eye. Of note, in each eye, the SD-OCT scans showed the appearance of a foveal depression, although the foveal depression was still shallow and broad (Figure 2). The central 1-mm foveal subfield thickness further decreased to 248 μm OD and 253 μm OS (Table).
MP testing showed mean sensitivity values with the Polar 3 pattern program of 1.1 dB OS. The patient showed a mild improvement in his ocular fixation as 96% of eye movements were within 2 degrees around the projected fixation target OS. He could not complete the MP testing on his right eye due to eye fatigue.
Following 5 months of treatment, P-R CS remained the same in the right eye, whereas a loss of 0.15 log units was found in the left eye. There was a small loss in grating CS in the right eye (approximately 0.20 log units). The grating CS value following 5 months of treatment (0.68 log units) was very similar to that measured at the baseline visit (0.64 log units). The patient subjectively reported that the “haze” that was present before treatment was initiated had returned.
Discussion
Cystoid macular edema (CME) consists of the intraretinal accumulation of fluid within cystic spaces of the macula. In our current series, we demonstrated that at least some patients with choroideremia can have a positive response to treatment with topical dorzolamide formulation, which was evident by an improvement of their cystoid macular edema on SD-OCT exam and mild improvement in their central vision.
A recent report by Genead and Fishman,4 showed the presence of variable degrees of CME in 63% of their study cohort of choroideremia patients by SD-OCT examination. Additionally, this report also documented the presence of thickening of the retina in this group of patients without any statistically significant age-related dependencies. This finding is consistent with 2 previous studies27,28 that used time domain-OCT testing on a single choroideremia patient and 21 carriers. The authors4speculated that these findings may be attributed to either macular edema or the presence of retinal glial cell proliferation as has been previously described by MacDonald and associates.29
Our study showed an improvement in central macular thickening due to treatment with topical dorzolamide in macular regions where the SD-OCT scans showed no or only small microcystic changes. The increased macular thickness in these regions may be attributed to accumulation of extracellular and/or intracellular retinal fluid that had not formed cystoid spaces. This speculation needs verification by the study of a greater number of patients in future clinical therapeutic trials in such patients.
Our findings document that the cystoid macular edema seen in patients with choroideremia may favorably respond to treatment with a topical CAI. A precise explanation for such a favorable response, at least some such patients, is not readily apparent. However, it may depend on the residual pumping function of the retinal pigment epithelial cells in individual patients. It is also conceivable that continued treatment with dorzolamide could result in larger improvements of visual function in this cohort. Nonetheless, it may be prudent to treat the cystoid macular edema present in choroideremia patients to possibly reduce the occurrence of later-onset atrophic macular lesions, which can result from the presence of chronic macular edema.
Although there were clear improvements in retinal thickness for both patients over the course of treatment, the effects of treatment on visual acuity, microperimetric sensitivity, P-R CS, and grating CS were less or not apparent. For patient 1, visual acuity improved in both eyes by more than 0.2 log MAR over the course of treatment. However, patient 2 had a smaller improvement in acuity in the left eye (approximately 0.1 log MAR) and essentially no improvement in the right eye. Microperimetric sensitivity was substantially reduced for both patients at baseline (more than a 1 log unit loss, as compared to normal)24 and the changes due to treatment were negligible. Similarly, P-R CS was substantially subnormal at baseline for both patients and there was no clinically significant change due to treatment. From the baseline visit to the first follow-up, both patients showed an improvement in grating CS when compared to the baseline visit. However, both patients also showed small losses in grating CS between the first and second follow-up visits. The fluctuation in grating CS in these patients may have been due, at least in part, to fatigue and lapses in attention.
In conclusion, the present study demonstrates that treatment of CME in patients with choroideremia with topical dorzolamide 2% can reduce central macular thickness on SD-OCT testing with a potential improvement for visual acuity. By comparison, the effect of treatment on microperimetric sensitivity, P-R CS, and grating CS was less apparent in these patients.
Summary statement.
The use of dorzolamide 2% eye drops was found to reduce cystoid macular edema and macular thickness in patients with choroideremia observed by spectral-domain OCT.
Acknowledgements
Supported by funds from the Foundation Fighting Blindness, Owings Mills, Maryland; Grant Healthcare Foundation, Lake Forest, Illinois; NIH research grant EY019510 (JM); NIH core grant EYO1792; and an unrestricted departmental grant from Research to Prevent Blindness.
Footnotes
None of the authors have any proprietary interest in this work.
Copyright Transfer: “In consideration of the journal Retinal Cases & Brief Reports® taking action in reviewing and editing our submission, which represents an original article, the authors undersigned hereby transfers, assigns, or otherwise conveys all copyright ownership to the Ophthalmic Communications Society, Inc. in the event that such work is published in the journal Retinal Cases & Brief Reports”
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andres DA, Seabra MC, Brown MS, Armstrong SA, Smeland TE, Cremers FP, et al. cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell. 1993;73(6):1091–1099. doi: 10.1016/0092-8674(93)90639-8. [DOI] [PubMed] [Google Scholar]
- 2.Seabra MC. New insights into the pathogenesis of choroideremia: a tale of two REPs. Ophthalmic Genet. 1996;17(2):43–46. doi: 10.3109/13816819609057869. [DOI] [PubMed] [Google Scholar]
- 3.Roberts MF, Fishman GA, Roberts DK, Heckenlively JR, Weleber RG, Anderson RJ, et al. Retrospective, longitudinal, and cross sectional study of visual acuity impairment in choroideraemia. Br J Ophthalmol. 2002;86(6):658–662. doi: 10.1136/bjo.86.6.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genead MA, Fishman GA. Cystic macular oedema on spectral-domain optical coherence tomography in choroideremia patients without cystic changes on fundus examination. Eye (Lond) 2011;25(1):84–90. doi: 10.1038/eye.2010.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajali M, Fishman GA, Anderson RJ. The prevalence of cystoid macular oedema in retinitis pigmentosa patients determined by optical coherence tomography. Br J Ophthalmol. 2008;92(8):1065–1068. doi: 10.1136/bjo.2008.138560. [DOI] [PubMed] [Google Scholar]
- 6.Walia S, Fishman GA, Hajali M. Prevalence of cystic macular lesions in patients with Usher II syndrome. Eye (Lond) 2009;23(5):1206–1209. doi: 10.1038/eye.2008.105. [DOI] [PubMed] [Google Scholar]
- 7.Genead MA, Fishman GA, Walia S. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch Ophthalmol. 2010;128(2):190–197. doi: 10.1001/archophthalmol.2009.398. [DOI] [PubMed] [Google Scholar]
- 8.Sohn EH, Chen FK, Rubin GS, Moore AT, Webster AR, MacLaren RE. Macular function assessed by microperimetry in patients with enhanced S-cone syndrome. Ophthalmology. 2010;117(6):1199–1206. doi: 10.1016/j.ophtha.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 9.Genead MA, Fishman GA, McAnany JJ. Efficacy of topical dorzolamide for treatment of cystic macular lesions in a patient with enhanced S-cone syndrome. Doc Ophthalmol. 2010;121(3):231–240. doi: 10.1007/s10633-010-9247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira TL, Andrade RE, Muccioli C, Sallum J, Belfort R., Jr Cystoid macular edema in gyrate atrophy of the choroid and retina: a fluorescein angiography and optical coherence tomography evaluation. Am J Ophthalmol. 2005;140(1):147–149. doi: 10.1016/j.ajo.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 11.Grover S, Apushkin MA, Fishman GA. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 2006;141(5):850–858. doi: 10.1016/j.ajo.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Genead MA, Fishman GA. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with retinitis pigmentosa and usher syndrome. Arch Ophthalmol. 2010;128(9):1146–1150. doi: 10.1001/archophthalmol.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bastos AL, Freitas de PB, Boas O Villas, Ramiro AC. Use of topical dorzolamide for patients with X-linked juvenile retinoschisis: case report. Arq Bras Oftalmol. 2008;71(2):286–290. doi: 10.1590/s0004-27492008000200030. [DOI] [PubMed] [Google Scholar]
- 14.Iannaccone A, Fung KH, Eyestone ME, Stone EM. Treatment of adult-onset acute macular retinoschisis in enhanced s-cone syndrome with oral acetazolamide. Am J Ophthalmol. 2009;147(2):307–312. doi: 10.1016/j.ajo.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko TH, Fujimoto JG, Schuman JS, Paunescu LA, Kowalevicz AM, Hartl I, et al. Comparison of ultrahigh- and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology. 2005;112(11):1922.e1–15. doi: 10.1016/j.ophtha.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholda C, Wirtitsch M, Hermann B, Unterhuber A, Ergun E, Sattmann H, et al. Ultrahigh resolution optical coherence tomography of macular holes. Retina. 2006;26(9):1034–1041. doi: 10.1097/01.iae.0000254898.80552.e7. [DOI] [PubMed] [Google Scholar]
- 17.Baba T, Yamamoto S, Arai M, Arai E, Sugawara T, Mitamura Y, et al. Correlation of visual recovery and presence of photoreceptor inner/outer segment junction in optical coherence images after successful macular hole repair. Retina. 2008;28(3):453–458. doi: 10.1097/IAE.0b013e3181571398. [DOI] [PubMed] [Google Scholar]
- 18.Midena E, Vujosevic S, Convento E, Manfre’ A, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91(11):1499–1503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rohrschneider K, Bültmann S, Glück R, Kruse FE, Fendrich T, Völcker HE. Scanning laser ophthalmoscope fundus perimetry before and after laser photocoagulation for clinically significant diabetic macular edema. Am J Ophthalmol. 2000;129(1):27–32. doi: 10.1016/s0002-9394(99)00270-6. [DOI] [PubMed] [Google Scholar]
- 20.Sunness JS, Applegate CA, Haselwood D, Rubin GS. Fixation patterns and reading rates in eyes with central scotomas from advanced atrophic age-related macular degeneration and Stargardt disease. Ophthalmology. 1996;103(9):1458–1466. doi: 10.1016/s0161-6420(96)30483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szlyk JP, Seiple W, Fishman GA, Alexander KR, Grover S, Mahler CL. Perceived and actual performance of daily tasks: relationship to visual function tests in patients with retinitis pigmentosa. Ophthalmology. 2001;108:65–75. doi: 10.1016/s0161-6420(00)00413-9. [DOI] [PubMed] [Google Scholar]
- 22.Mones JB, Rubin G. Contrast sensitvity as an outcome measure in patients with subfoveal choroidal neovascularisation due to age-related macular degeneration. Eye. 2005;19:1142–1150. doi: 10.1038/sj.eye.6701717. [DOI] [PubMed] [Google Scholar]
- 23.Csaky KGC, Richman EA, Ferris FL. 3rd Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49:479–489. doi: 10.1167/iovs.07-1132. [DOI] [PubMed] [Google Scholar]
- 24.Anastasakis A, McAnany JJ, Fishman GA, Seiple WH. Clinical value, normative retinal sensitivity values, and intrasession repeatability using a spectral-domain OCT/SLO microperimeter. Eye (Lond) 2011;25(2):245–251. doi: 10.1038/eye.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mäntyjärvi M, Laitinen T. Normal values for the Pelli-Robson contrast sensitivity test. J Cataract Refract Surg. 2001;27:261–266. doi: 10.1016/s0886-3350(00)00562-9. [DOI] [PubMed] [Google Scholar]
- 26.Quinn CJ. Cystoid macular edema. Optom Clin. 1996;5(1):111–130. [PubMed] [Google Scholar]
- 27.Mura M, Sereda C, Jablonski MM, MacDonald IM, Iannaccone A. Clinical and functional findings in choroideremia due to complete deletion of the CHM gene. Arch Ophthalmol. 2007;125(8):1107–1113. doi: 10.1001/archopht.125.8.1107. [DOI] [PubMed] [Google Scholar]
- 28.Jacobson SG, Cideciyan AV, Sumaroka A, Aleman TS, Schwartz SB, Windsor EA, et al. Remodeling of the human retina in choroideremia: rab escort protein 1 (REP-1) mutations. Invest Ophthalmol Vis Sci. 2006;47(9):4113–4120. doi: 10.1167/iovs.06-0424. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald IM, Russell L, Chan CC. Choroideremia: new findings from ocular pathology and review of recent literature. Surv Ophthalmol. 2009;54(3):401–407. doi: 10.1016/j.survophthal.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]