Abstract
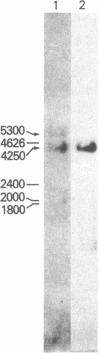
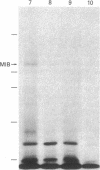
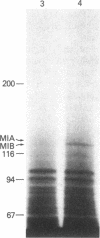
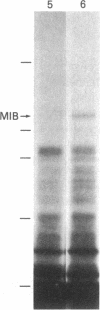
Acanthamoeba castellanii contains two enzymes, myosins IA and IB, that exhibit the catalytic properties of a myosin but possess very unusual physical properties, the most striking of which are their single, low molecular weight heavy chain, their globular shape, and their inability to form bipolar filaments. We have now isolated a putative myosin IB heavy chain gene from Acanthamoeba, using as a heterologous probe a portion of a sarcomeric myosin heavy chain gene from Caenorhabditis elegans. The amoeba genomic clone hybridizes to a 4250-nucleotide RNA species and hybrid-selects an mRNA encoding a 125-kDa polypeptide. This polypeptide comigrates exactly with the heavy chain of purified amoeba myosin IB and is specifically immunoprecipitated with antiserum to myosin IB. We sequenced two restriction enzyme fragments of this gene, and the deduced amino acid sequences show strong homology with the regions of muscle myosins that contain the reactive thiols and the ATP binding site. Our identification of a myosin IB heavy chain gene demonstrates that myosin IB, despite the unusually low molecular weight of its heavy chain, is a true gene product. The sequence results show that, despite its atypical physical properties, myosin IB is clearly related to conventional myosins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albanesi J. P., Fujisaki H., Hammer J. A., 3rd, Korn E. D., Jones R., Sheetz M. P. Monomeric Acanthamoeba myosins I support movement in vitro. J Biol Chem. 1985 Jul 25;260(15):8649–8652. [PubMed] [Google Scholar]
- Albanesi J. P., Fujisaki H., Korn E. D. Localization of the active site and phosphorylation site of Acanthamoeba myosins IA and IB. J Biol Chem. 1984 Nov 25;259(22):14184–14189. [PubMed] [Google Scholar]
- Atkinson M. A., Robinson E. A., Appella E., Korn E. D. Amino acid sequence of the active site of Acanthamoeba myosin II. J Biol Chem. 1986 Feb 5;261(4):1844–1848. [PubMed] [Google Scholar]
- Bajaj M., Blundell T. Evolution and the tertiary structure of proteins. Annu Rev Biophys Bioeng. 1984;13:453–492. doi: 10.1146/annurev.bb.13.060184.002321. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Korn E. D. Purification and characterization of actin-activatable, Ca2+-sensitive myosin II from Acanthamoeba. J Biol Chem. 1981 Mar 10;256(5):2586–2595. [PubMed] [Google Scholar]
- Collins J. H., Kuznicki J., Bowers B., Korn E. D. Comparison of the actin binding and filament formation properties of phosphorylated and dephosphorylated Acanthamoeba myosin II. Biochemistry. 1982 Dec 21;21(26):6910–6915. doi: 10.1021/bi00269a045. [DOI] [PubMed] [Google Scholar]
- Côté G. P., Albanesi J. P., Ueno T., Hammer J. A., 3rd, Korn E. D. Purification from Dictyostelium discoideum of a low-molecular-weight myosin that resembles myosin I from Acanthamoeba castellanii. J Biol Chem. 1985 Apr 25;260(8):4543–4546. [PubMed] [Google Scholar]
- Elzinga M., Collins J. H. Amino acid sequence of a myosin fragment that contains SH-1, SH-2, and Ntau-methylhistidine. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4281–4284. doi: 10.1073/pnas.74.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaki H., Albanesi J. P., Korn E. D. Experimental evidence for the contractile activities of Acanthamoeba myosins IA and IB. J Biol Chem. 1985 Sep 15;260(20):11183–11189. [PubMed] [Google Scholar]
- Hammer J. A., 3rd, Korn E. D., Paterson B. M. Acanthamoeba myosin IA, IB, and II heavy chains are synthesized in vitro from Acanthamoeba messenger RNA. J Biol Chem. 1984 Sep 25;259(18):11157–11159. [PubMed] [Google Scholar]
- Hammer J. A., 3rd, Korn E. D., Paterson B. M. Isolation of a non-muscle myosin heavy chain gene from Acanthamoeba. J Biol Chem. 1986 Feb 5;261(4):1949–1956. [PubMed] [Google Scholar]
- Harrington W. F., Rodgers M. E. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L. Protein structural domains in the Caenorhabditis elegans unc-54 myosin heavy chain gene are not separated by introns. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4253–4257. doi: 10.1073/pnas.80.14.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood R., Yount R. G. Photochemical probes of the active site of myosin. Irradiation of trapped 3'-O-(4-benzoyl)benzoyladenosine 5'-triphosphate labels the 50-kilodalton heavy chain tryptic peptide. J Biol Chem. 1984 Nov 10;259(21):12956–12959. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y., Yount R. G. Identification of an active site peptide of skeletal myosin after photoaffinity labeling with N-(4-azido-2-nitrophenyl)-2-aminoethyl diphosphate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1575–1579. doi: 10.1073/pnas.82.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler E. E., Mahdavi V., Periasamy M., Nadal-Ginard B. Intron positions are conserved in the 5' end region of myosin heavy-chain genes. J Biol Chem. 1985 Jan 10;260(1):468–471. [PubMed] [Google Scholar]
- Szilagyi L., Balint M., Sreter F. A., Gergely J. Photoaffinity labelling with an ATP analog of the N-terminal peptide of myosin. Biochem Biophys Res Commun. 1979 Apr 13;87(3):936–945. doi: 10.1016/0006-291x(79)92047-3. [DOI] [PubMed] [Google Scholar]
- Tong S. W., Elzinga M. The sequence of the NH2-terminal 204-residue fragment of the heavy chain of rabbit skeletal muscle myosin. J Biol Chem. 1983 Nov 10;258(21):13100–13110. [PubMed] [Google Scholar]