Abstract
Objective
The aim of this study was to evaluate the clinical outcome of hepatectomy combined with inferior vena cava (IVC) resection and reconstruction for treatment of invasive liver tumours.
Methods
From February 1995 to September 2010, 2146 patients underwent liver resections in our hospital's hepatopancreatobiliary unit. Of these, 35 (1.6%) patients underwent hepatectomy with IVC resection. These patients were included in this study. Data were analysed from a prospectively collected database.
Results
Resections were carried out for colorectal liver metastasis (CRLM) (n = 21), hepatocellular carcinoma (n = 6), cholangiocarcinoma (n = 3) and other conditions (n = 5). Resections were carried out with total vascular occlusion in 34 patients and without in one patient. In situ hypothermic perfusion was performed in 13 patients; the ante situm technique was used in three patients, and ex vivo resection was used in six patients. There were four early deaths from multiple organ failure. Postoperative complications occurred in 14 patients, three of whom required re-operation. Median overall survival was 29 months and cumulative 5-year survival was 37.7%. Rates of 1-, 2- and 5-year survival were 75.9%, 58.7% and 19.6%, respectively, in CRLM patients.
Conclusions
Aggressive surgical management of liver tumours with IVC involvement offers the only hope for cure in selected patients. Resection by specialist teams affords acceptable perioperative morbidity and mortality rates.
Keywords: colorectal metastases < liver, resection < liver, hepatocellular carcinoma < liver, cholangiocarcinoma < liver, inferior vena cava resection < liver, resection
Introduction
Liver tumours, including hepatocellular carcinoma (HCC), cholangiocarcinoma (CC) and metastatic carcinoma, sometimes directly invade the retrohepatic portion of the inferior vena cava (IVC) because of the anatomic proximity of this vessel. Liver resection remains the only potentially curative treatment for primary and metastatic tumours of the liver. Five-year survival rates are reported to range from 30% to 50% after liver resection for primary hepatic malignancies, metastatic colorectal cancer, and other non-colorectal cancers metastatic to the liver.1–5 Nowadays, chemotherapy is not considered to be a curative option and, if left untreated, these patients often have a median survival of considerably < 12 months.6 Technical innovations such as portal vein embolization, two-stage hepatectomies, re-do hepatectomies and neoadjuvant chemotherapy have all been used to expand the population of patients who may be considered for hepatic resection, and favourable clinical outcomes have been reported.7–9
In the past, patients with hepatic malignancy involving the IVC were considered poor candidates for surgical management and included few 5-year survivors. Suspected involvement of the hepatocaval confluence (HVC) or IVC was long considered a contraindication for liver resection as a result of the risks for intraoperative air embolism or torrential haemorrhage. The development of innovative surgical techniques and increasing acceptance of vascular exclusion procedures are pushing this boundary of hepatic surgery.10–13 Liver resection in patients with IVC involvement is becoming more common with the adoption of these techniques and, when necessary, with the replacement of the IVC using various materials.5,14–17 Most of the data available consist of case reports or small series that emphasize the technical aspects of the procedures.18–31 The longterm outcomes and oncological integrity of such procedures remain unclear.
To our knowledge, this report represents the largest patient series focusing on patient-related outcomes after hepatic resection for malignancy with reconstruction of the IVC to be published.
Materials and methods
All patients who underwent hepatic resection in our hospital's hepatopancreatobiliary unit between February 1995 and September 2010 were included in the study. Data from a prospectively collected database were analysed. Preoperative workup included measurement of serum biochemistry and carcinoembryogenic antigen (CEA) levels, as well as assessment for major and often prolonged high-risk surgery, which currently involves cardiopulmorary exercise testing (CPX). Cross-sectional imaging included staging computed tomography (CT) scans of the chest and abdomen, as well as an iron oxide magnetic resonance imaging (MRI) scan to evaluate hepatic anatomy (Fig. 1). Imaging for all patients considered for surgery was reviewed at the multidisciplinary team meeting and patients who were considered resectable on preoperative imaging were offered surgery.
Figure 1.
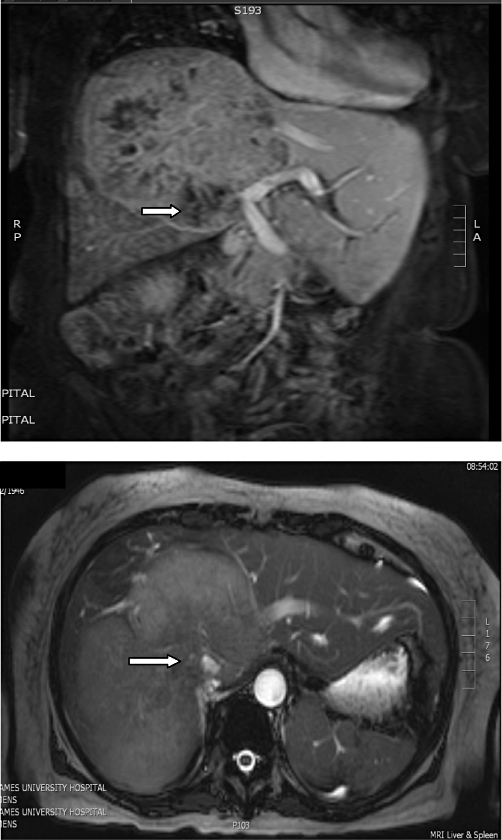
Magnetic resonance imaging of a large tumour with inferior vena cava invasion (white arrows)
Intraoperative ultrasound was used routinely to identify occult tumours. Parenchymal transection was performed using the Cavi-Pulse Ultrasonic Surgical Aspirator (CUSA; Valleylab, Inc., Boulder, CO, USA). Our group has previously reported results with ex vivo, ante situm and in situ hypothermic perfusion techniques.32 When necessary, hepatic pedicle clamping was employed using 15-min cycles with 5-min reperfusion intervals. It is our opinion that anatomical resections are not necessarily indicated if a macroscopic negative surgical margin can be obtained.33,34 The location and number of metastases, and any suspicion of vascular infiltration, were determined with intraoperative ultrasonography at the time of surgery. Experience with liver transplantation has allowed the centre to offer resection for advanced hepatobiliary tumours that require total vascular exclusion for tumour excision. The in situ hypothermic liver preservation technique was used when indicated.35
Selection of procedure
The type of procedure varies according to the location of the tumour and the extent of caval involvement, which is finally evaluated during surgery. When involvement of the IVC is minimal (≤60 ° circumferentially and ≤2 cm longitudinally), control may be established simply by applying a side-biting clamp to the retrohepatic IVC, allowing direct repair, although a patch of bovine pericardium is used to avoid narrowing (Fig. 2). Greater involvement of the IVC mandates resection and replacement with a synthetic graft. In the early part of our experience, we used deleted Dacron® tube grafts (Hemashield®; Meadox Medical, Inc., Oakland, NJ, USA), but latterly we switched to ring-enforced polytetrafluoroethylene (PTFE) tube grafts (Gore-Tex®; W. L. Gore & Associates Inc., Flagstaff, AZ, USA) because they are resistant to compression by abdominal viscera and the regenerating liver (Fig. 3). The initial experience indicated better survival when tumours were peeled off the IVC and thus this remains our policy if the oncological integrity of the procedure is not compromised.32
Figure 2.
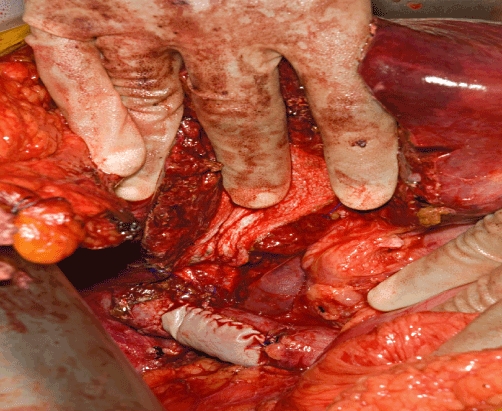
Bovine pericardium patch used to reconstruct the inferior vena cava
Figure 3.
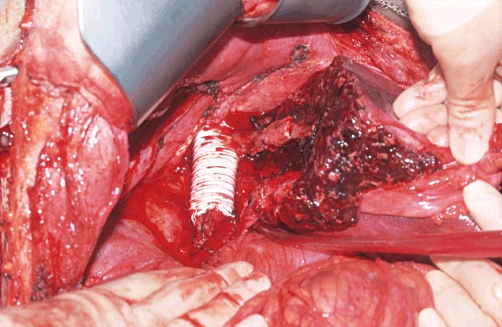
Gore-Tex® graft for inferior vena cava (IVC) reconstruction with use of IVC patch to reconstruct left hepatic vein
Exclusion of the liver with preservation of IVC flow
This technique is useful for small-volume contact in the mid areas of the liver without involvement of the IVC at either the inflow or outflow of the liver. The portal inflow and hepatic veins are dissected and clamped when they are required extrahepatically. The hanging manoeuvre has proved to be useful.36
Standard total vascular exclusion
The technical details of total vascular exclusion (TVE) have previously been published.32 This method involves mobilizing the liver where possible and isolating the suprahepatic and infrahepatic vena cava, as well as the inflow to the liver. The infrahepatic vena cava, hilum and suprahepatic vena cava are serially clamped. A venovenous bypass is used in patients who display haemodynamic intolerance to TVE, despite adequate fluid loading, but this is rarely needed except in elderly subjects.
The ante situm, in situ hypothermic perfusion and ex vivo techniques
Early in the series, the ante situm procedure was used in patients in whom tumours invaded the retrohepatic IVC. Following division of the suprahepatic cava, the liver can be rotated anteriorly to afford good access to the dorsal area of the liver close to the hepatocaval confluence. There is little difference between this technique and that of in situ hypothermic perfusion. The liver is first mobilized as for TVE. A venovenous bypass is systematically installed from the portal and femoral veins to the left internal jugular vein and deleted dual cannulation of the portal vein is performed above the portal triad clamp with a Silastic® (silicone elastomer) catheter (internal diameter: 2.5 mm; external diameter: 4.5 mm). In situ hypothermic perfusion of the liver is then carried out with 2 l of University of Wisconsin solution (UW), chilled to 4 °C. A cavotomy is performed immediately above the inferior caval clamp to drain the cold perfusate. When liver resection and vascular reconstruction are completed, the liver is flushed with albumin to wash out the UW solution via the portal vein. The portal catheter for perfusion is removed and the portotomy and cavotomy are closed. Circulation can then be restored as for TVE. The venovenous bypass is removed as a last step, after haemodynamic stability is restored.
The unit operates an intensive policy of postoperative surveillance and all patients received adjuvant chemotherapy unless they had undergone chemotherapy adjuvant to bowel resection within 12 months of the primary hepatic resection.
Data were reviewed for all patients. These data included information on the mode of presentation, operative and oncological outcomes, the technique of vascular occlusion required for operation and the type of vena cava reconstruction performed. Hospital mortality was defined as death within 30 days of surgery or during the postoperative hospital stay. Postoperative complications were documented according to the International Dindo–Clavien Classification.37 Positive margins were defined as evidence of tumour at within 1 mm of the inked margin. Brisbane terminology was used to define different hepatic resections and the diagnosis was confirmed pathologically in all patients.38,39
Statistical analysis was carried out using spss for Windows Version 17.0 (SPSS, Inc., Chicago, IL, USA). Categorical variables were compared using the chi-squared test or Fisher's exact test, where appropriate. Longterm survival was calculated using the Kaplan–Meier method.
Results
From February 1995 to September 2010, 2146 patients underwent liver resection for tumour in the unit. Of these, 35 (1.6%) patients underwent hepatectomy with IVC resection. Their median age was 57 years (range: 32–84 years). Eighteen of the patients were male. Resections were carried out for colorectal liver metastasis (CRLM) (n = 21), HCC (n = 6), CC (n = 3) and other conditions (n = 5).
Surgical procedures
Major hepatectomy (of at least three segments) was carried out in 30 patients and the caudate lobe was resected in 17 patients (Table 1). The liver resections carried out included 32 first, two second and one third hepatectomy. Resections were carried out with total vascular occlusion in 34 patients and without in one. In situ hypothermic perfusion was performed in 13 patients, ante situm in three patients and ex vivo in six patients. The median operative time was 255 min (range: 90–660 min) and transfusion was necessary in 19 patients (median: 5 units).
Table 1.
Details of surgery
| Patient | Age, years/sex | Diagnosis | Operation | Largest tumour circumference, mm | Segments resected | Technique | IVC reconstruction | Status (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 55/F | CA | Left hemihepatectomy, right segmentectomy, caudate lobectomy | 50 | I–IV, VII, VIII | TVE, IHP | Gore-Tex® graft | Died (29) |
| 2 | 42/F | CRLM | Caudate resection, right trisectionectomy | 130 | I, IV–VIII | TVE | Direct repair | Died in hospital |
| 3 | 50/M | CRLM | Left metastasectomy, right hemihepatectomy | 120 | III–VIII | TVE | Direct repair | Died (11) |
| 4 | 71/M | CRLM | Right hemihepatectomy | 40 | V–VIII | TVE | Direct repair | Died (20) |
| 5 | 68/M | HCC | Right trisectionectomy | 75 | IV–VIII | TVE, IHP, ex vivo | Gore-Tex® graft | Died in hospital |
| 6 | 56/M | CRLM | Caudate resection, right trisectionectomy | 150 | I, IV–VIII | TVE, IHP, ex vivo | Gore-Tex® graft | Died (5) |
| 7 | 75/M | HCC | Bile duct excision, caudate resection, hepaticojejunostomy, right trisectionectomy | 170 | I, IV–VIII | TVE, IHP | Direct repair | Died (52) |
| 8 | 44/F | CRLM | Excision right adrenal, paracaval mass, liver resection | – | V, VI | TVE, IHP, ante situm | Gore-Tex® graft | Alive (140) |
| 9 | 49/M | CRLM | Right trisectionectomy | 170 | IV–VIII | TVE | Direct repair | Died in hospital |
| 10 | 49/F | CRLM | Bile duct excision, caudate resection, hepaticojejunostomy, left trisectionectomy | 150 | I–V, VIII | TVE, IHP, ex vivo (185 min) | Direct repair | Died (5) |
| 11 | 42/M | CRLM | Left hemihepatectomy, right segmentectomy | 50 | I–IV, VII, VIII | Direct repair | Died (23) | |
| 12 | 61/M | HCC | Caudate resection, right trisectionectomy | 190 | I, IV–VIII | TVE | Direct repair | Died (123) |
| 13 | 43/F | CA | Caudate resection, right trisectionectomy | 170 | I, IV–VIII | Direct repair | Died (10) | |
| 14 | 65/F | Adrenal | Right hemihepatectomy | 70 | V–VIII | TVE | Gore-Tex® graft | Died (1) |
| 15 | 65/M | FNH | Right hemihepatectomy | 50 | V–VIII | TVE | Direct repair | Died (28) |
| 16 | 49/F | CRLM | Nephrectomy, retroperitoneal sarcoma excision, caudate resection | – | I | TVE, IHP, ex vivo (240 min) | Dacron® graft | Died (76) |
| 17 | 73/M | HCC | Radiofrequency ablation, right hemihepatectomy | 50 | IV–VIII | TVE, IHP, ex vivo | Gore-Tex® graft | Died (29) |
| 18 | 55/F | HCC | Right trisectionectomy | 100 | IV–VIII and mets 2/3 | TVE, IHP | Direct repair | Alive (70) |
| 19 | 77/F | CA | Right hemihepatectomy | 120 | V–VIII | TVE, IHP, ante situm | Gore-Tex® graft | Alive (57) |
| 20 | 72/M | CRLM | Right hemihepatectomy | 140 | V–VIII | TVE | Direct repair | Died (19) |
| 21 | 61/M | CRLM | Left metastasectomy, right trisectionectomy | 180 | I, IV–VIII and mets 2/3 | Direct repair | Died (17) | |
| 22 | 57/M | Sarcoma | Right metastatectomy | – | V, VI | TVE | Gore-Tex® graft | Alive (40) |
| 23 | 57/F | CRLM | Caudate resection, right trisectionectomy | 150 | I, IV, V, VII, VIII | TVE, IHP | Bovine pericardium patch with left vein reconstruction | Alive (30) |
| 24 | 52/M | Other | Caudate resection, left metastasectomy, right metastasectomy | 70 | I, III–V | Direct repair | Died (20) | |
| 25 | 62/F | CRLM | Right hemihepatectomy | – | V–VIII | TVE | Direct repair | Alive (24) |
| 26 | 52/F | CRLM | Caudate resection, extended right trisectionectomy | 200 | I, IV–VIII | TVE | Direct repair | Alive (22) |
| 27 | 32/F | Lymphoma | Left hemihepatectomy | 100 | II–IV | TVE | Direct repair | Alive (22) |
| 28 | 51/M | CRLM | Right trisectionectomy | 230 | IV–VIII | TVE | Dacron® graft | Alive (22) |
| 29 | 84/M | CRLM | Left hemihepatectomy | 110 | I–IV | TVE, IHP, ex vivo (120 min) | Direct repair | Alive (20) |
| 30 | 58/F | CRLM | Caudate resection, right hemihepatectomy | 50 | I, V–VIII | TVE | Direct repair | Alive (17) |
| 31 | 79/F | CRLM | Right hemihepatectomy | 130 | V–VIII | TVE | Dacron® graft | Alive (16) |
| 32 | 41/M | HCC | Caudate resection, right trisectionectomy | 220 | I, IV–VIII | TVE, IHP, ante situm | Gore-Tex® graft with left vein anastomosis | Alive (15) |
| 33 | 74/M | CRLM | Left metastasectomy | 15 | IV | TVE | Bovine pericardium patch repair | Alive (14) |
| 34 | 50/F | CRLM | Right metastasectomy | 80 | VI, VII | TVE | Direct repair | Alive (10) |
| 35 | 64/F | CRLM | Right trisectionectomy, segment II metastatectomy | 160 | I, IV–VIII, met 2 | TVE | Direct repair | Alive (6) |
F, female; M, male; CA, cholangiocarcinoma; CRLM, colorectal liver metastasis; HCC, hepatocellular carcinoma; FNH, focal nodular hyperplasia; TVE, total vascular exclusion; IHP, in situ hypothermic perfusion
Histopathological data of IVC involvement
Negative microscopic resection margins (R0) were achieved in 18 patients. Histological data showed the IVC to be involved by tumour in 15 patients, not involved in 15 and of unknown status in five patients. The median largest tumour circumference was 120 mm (range: 15–230 mm).
IVC reconstruction
The IVC underwent segmental resection and reconstruction with synthetic grafts in 12 patients (Table 1). Dacron® and Gore-Tex® were used in three and nine patients, respectively. Direct repair of the IVC with or without a bovine pericardial patch was carried out in 23 patients. All vascular reconstructions were patent at last follow-up. There was no difference in overall survival between patients who underwent direct repair of the IVC and patients in whom graft replacement was necessary (P > 0.05).
In-hospital mortality
Four early deaths from multiple organ failure occurred. The median postoperative hospital stay was 12 days (range: 6–45 days). The patients who died included a 42-year-old woman who underwent hepatectomy with ex vivo resection and re-implantation of segment II and part of segment III with IVC reconstruction, but developed multi-organ failure on the second postoperative day, requiring ventilation and haemodialysis. A laparotomy was performed for suspected abdominal sepsis the following day, but, despite intensive support, the patient died on postoperative day 15. The second death occurred after right trisectionectomy with IVC reconstruction in a 68-year-old man who had received intensive preoperative chemotherapy. The patient died of unexplained sepsis and multiple organ failure. The third patient developed hepatic failure against a background of portal vein thrombosis and eventually died from multiple organ failure on day 15. The fourth patient died in the intensive care unit of multiple organ failure on day 32.
Morbidity
Postoperative complications occurred in 14 patients, three of whom required re-operation. Complications included acute renal failure in three patients, hepatic encephalopathy in three, sepsis in four, haemorrhage in two, superficial wound dehiscence in one, wound infection in one and bile leak in one patient (one patient developed acute renal failure with hepatic encephalopathy). The three patients who needed re-laparotomy did so for haemorrhage in two cases and unexplained sepsis in one. Two of the patients who underwent re-laparotomy died. Table 2 shows the postoperative complication rate according to the International Dindo–Clavien Classification.
Table 2.
International Dindo–Clavien Classification of surgical complications37 in the present series
| Grade | n |
|---|---|
| 1 | 1 (wound infection) |
| 2 | 1 (superficial wound dehiscence) |
| 3a | 1 (bile leak) |
| 3b | 3 (haemorrhage, 2; sepsis, 1) |
| 4a | 6 (renal failure, 3; hepatic encephalopathy, 3) |
| 4b | 4 (multiple organ dysfunction) |
| 5 | 4 (death) |
Survival analysis
Median overall survival was 29 months (95% confidence interval [CI] 2.7–55.2 months). Overall actuarial 5-year survival was 37.7%. The Kaplan–Meier survival curve for all patients undergoing hepatectomy with IVC reconstruction is shown in Fig. 4. The actuarial 1-, 2- and 5-year survival rates were 75.9%, 58.7% and 19.6% for patients with CRLM. A subgroup analysis of patients with colorectal metastases showed a median survival of 28 months (95% CI 20.2–35.8 months). One-year survival rates in HCC and CC patients were 83.3% and 33.3%, respectively. The Kaplan–Meier survival curve for patients with CRLM is shown in Fig. 5. Survival after an R0 resection was significantly better (P = 0.009) (Fig. 6).
Figure 4.
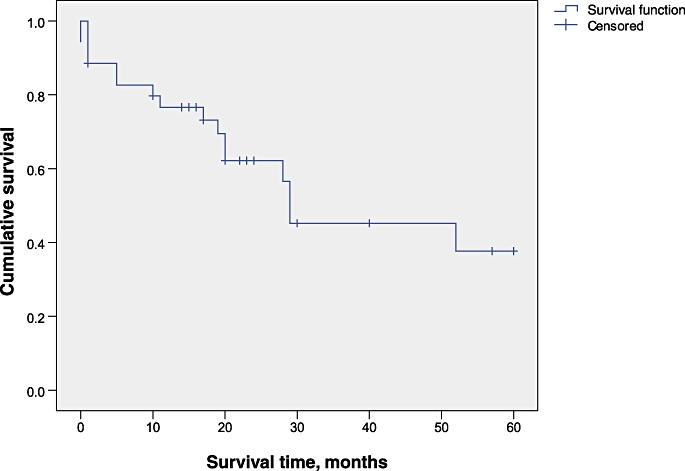
Kaplan–Meier survival curve for all patients undergoing inferior vena cava resection with hepatectomy
Figure 5.
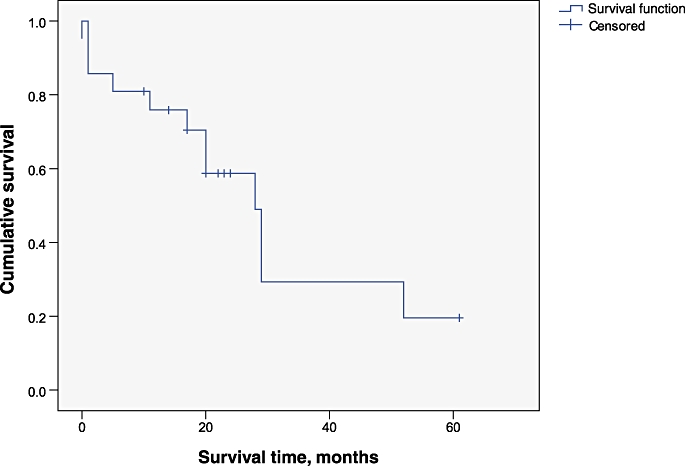
Actuarial survival curves in patients with colorectal liver metastasis
Figure 6.
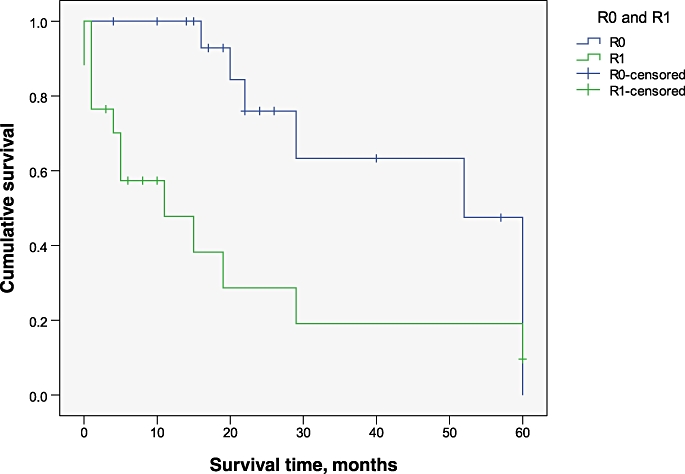
Kaplan–Meier survival curves for patients undergoing R0 and R1 resections. P = 0.009
Discussion
The present study reviewed outcomes in 35 patients who underwent partial hepatectomy with IVC reconstruction and who represent the largest patient series to be reported in the literature to date. Tumours in the central and posterior segments of the liver and occasionally those originating in the kidney, adrenal gland or retroperitoneal soft tissue may extend to involve the IVC or hepatic veins, making resection using standard techniques hazardous.40,41 Consensus on best practice with regard to the selection of patients, survival after these procedures, technique of IVC reconstruction and type of artificial graft for IVC replacement remains unachieved.
It is often difficult to define precisely the extent and nature of contact between the tumour and IVC. Although most institutions use a combination of CT and MRI prior to hepatic resection, neither technique has proven to be accurate in diagnosing caval involvement.42–45 Even when IVC invasion is strongly suggested by conventional radiological studies, the surgeon should endeavour to peel the tumour from the IVC as this is possible more often than not. In our experience, outcomes are superior if the IVC is not actually invaded.32
When involvement of the IVC is below the level of the hepatic veins and there is sufficient room to place a vascular clamp above the tumour but below the hepatic veins, liver blood flow can be maintained during resection and reconstruction of the IVC. It is our preference to divide the liver parenchyma first and to subsequently place the clamps on the IVC. However, an alternative approach described by Madariaga et al. involves replacing the IVC prior to dividing the liver parenchyma, but this has not gained popular acceptance.46 Either approach minimizes the time required for portal inflow occlusion as normal livers can tolerate 60–90 min of warm ischaemia; however, it would seem prudent to minimize ischaemic time where possible.47,48
In selected patients with tumours that invade the right side of the cava, the hanging manoeuvre can be applied so that the liver parenchyma and IVC are transected and the cava then repaired. In general, as much as possible of the liver is mobilized off the vena cava prior to the transection of the hepatic parenchyma. However, occasionally a large, bulky tumour makes the mobilization of the liver off the vena cava difficult or even hazardous. In such cases, a liver parenchymal transection using an anterior approach can be performed first to expose the IVC without excessive rotation or traction of the liver.
Although in situ hypothermic perfusion and the ante situm technique are applicable in hepatic tumours involving the hepatocaval confluence or the retrohepatic vena cava, the ex vivo bench dissection was used in six patients in the present series. It is our opinion that an ex vivo approach is only applicable in patients who have extreme IVC, hepatocaval confluence and portal triad involvement and more conservative approaches can be used in all other contexts.
Different types of IVC repair can be applied, depending on the extent of IVC infiltration by the tumour. Partial invasion of the IVC wall is repaired by direct suture if the wall is infiltrated for a short segment of <2 cm. A patch of autologous saphenous vein, fascial peritoneum or heterologous material can be used in the presence of extended infiltration of the wall (>2 cm) to prevent lumen stenosis; however, we consider bovine pericardium to be more convenient. Finally, total replacement of the IVC with a vascular prosthesis is indicated if at least one half of the circumference of the IVC appears infiltrated, in the presence of longitudinal infiltration or, rarely, in the presence of an intracaval thrombus.
There are a variety of options for replacing the IVC when primary repair is not possible. Although replacement of the resected IVC with an autogenous vein graft carries advantages with regard to the risk for infection or thrombosis, this option may not be technically feasible, particularly if a relatively long segment of the IVC is to be replaced, and here synthetic grafts are preferable. Dacron® has been used in the past, but has been associated with relatively high thrombosis and stenosis rates and therefore we prefer to use reinforced PTFE grafts for replacing the IVC as they seem to resist compression by the abdominal viscera.32,45,47,48 Although the risk for graft thrombosis may be reduced by postoperative anticoagulation, the value of anticoagulation remains questionable and the required duration of therapy is hard to determine.49 We have used anticoagulants and formed arteriovenous fistulas in the past, but no longer feel these manoeuvres are necessary as the IVC flows are high.
Despite the availability of innovative surgical techniques that render extensive hepatic resection and concomitant IVC replacement feasible, the surgical death and complication rates associated with this type of surgery remain considerable. In preoperative assessment an exercise electrocardiogram or a stress echocardiogram is performed in all patients undergoing workup for major hepatic surgery and CPX is used increasingly. The benefits to these patients of including in the surgical team an anaesthetist experienced in low venous pressure techniques as well as highcentral venous pressure transplant anaesthesia for venovenous bypass cannot be underestimated.
Although disease in this group of patients represents an advanced stage of metastasis and overall survival is still unsatisfactory, this approach – using extensive surgery – is worth attempting because surgical resection currently provides the only hope for cure in this patient group and the technique has been established as relatively safe. At present, metastasis that impinges on the hepatic vasculature must be considered from a technical perspective insofar as it relates to resectability, but it has not been reliably identified as an independent adverse prognostic factor.50,51
In conclusion, the major findings of this study show that in selected patients with hepatobiliary malignancy involving the IVC, the use of specialized vascular techniques can offer potentially prolonged survival. These procedures should be performed in high-volume centres experienced in the use of advanced vascular isolation procedures.
Conflicts of interest
None declared.
References
- 1.Lévi F, Zidani R, Brienza S, Dogliotti L, Perpoint B, Rotarski M, et al. A multicenter evaluation of intensified, ambulatory, chronomodulated chemotherapy with oxaliplatin, 5-fluorouracil, and leucovorin as initial treatment of patients with metastatic colorectal carcinoma: International Organization for Cancer Chronotherapy. Cancer. 1999;85:2532–2540. doi: 10.1002/(sici)1097-0142(19990615)85:12<2532::aid-cncr7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 2.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y. Surgical therapy of hepatic colorectal metastases. CA Cancer J Clin. 1999;49:231–255. doi: 10.3322/canjclin.49.4.231. [DOI] [PubMed] [Google Scholar]
- 4.Song T-J, Wai Kit Ip E, Fong Y. Hepatocellular carcinoma: current surgical management. Gastroenterology. 2004;127(Suppl.):248–260. doi: 10.1053/j.gastro.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Bismuth H, Adam R, Lévi F, Farabos C, Waechter F, Castaing D, et al. Resection of non-resectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–522. doi: 10.1097/00000658-199610000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacchetti S, Perpointl B, Zidani R, Le Bail N, Faggiuolo R, Focan C, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 7.Breny T, Mentha G, Morel P. Total vascular exclusion of the liver for resection of lesions in contact with the cava or hepatic veins. Br J Surg. 1998;85:485–488. doi: 10.1046/j.1365-2168.1998.00659.x. [DOI] [PubMed] [Google Scholar]
- 8.Hemming AW, Reed AI, Langham MR, Jr, Fujita S, Howard RJ. Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg. 2004;239:712–721. doi: 10.1097/01.sla.0000124387.87757.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996;224:155–161. doi: 10.1097/00000658-199608000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma D, Ogata S, Belghiti J. Isolated total caval clamping with ‘preserved remnant liver perfusion’ for combined hepatic and venacaval resection in tumours involving venacava. Surgery. 2007;141:112–116. doi: 10.1016/j.surg.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki S, Makuuchi M, Kakazu T, Miyagawa S, Takayama T, Kosuge T, et al. Resection for multiple metastatic liver tumours after portal embolization. Surgery. 1994;115:674–677. [PubMed] [Google Scholar]
- 12.Azoulay D, Castaing D, Smail A, Adam R, Cailliez V, Laurent A, et al. Resection of non-resectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu Hilal M, Lodge JPA. Pushing back the frontiers of resectability in liver cancer surgery. Eur J Surg Oncol. 2008;34:272–280. doi: 10.1016/j.ejso.2007.07.201. [DOI] [PubMed] [Google Scholar]
- 14.Malassagne B, Cherqui D, Alon R, Brunetti F, Humeres R, Fagniez PL. Safety of selective vascular clamping for major hepatectomies. J Am Coll Surg. 1998;187:482–486. doi: 10.1016/s1072-7515(98)00234-8. [DOI] [PubMed] [Google Scholar]
- 15.Smyrniotis VE, Kostopanagiotou GG, Gamaletsos EL, Vassiliou JG, Voros DC, Fotopoulos AC, et al. Total versus selective vascular exclusion in major liver resections. Am J Surg. 2002;183:173–178. doi: 10.1016/s0002-9610(01)00864-9. [DOI] [PubMed] [Google Scholar]
- 16.Torzilli G, Makuuchi M, Midorikawa Y, Sano K, Inoue K, Takayama T, et al. Liver resection without total vascular exclusion: hazardous or beneficial? An analysis of our experience. Ann Surg. 2001;233:167–175. doi: 10.1097/00000658-200102000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grazi GL, Mazziotti A, Jovine E, Pierangeli F, Ercolani G, Gallucci A, et al. Total vascular exclusion of the liver during hepatic surgery: selective use, extensive use or abuse? Arch Surg. 1997;132:1104–1109. doi: 10.1001/archsurg.1997.01430340058009. [DOI] [PubMed] [Google Scholar]
- 18.O'Malley KJ, Stuart RC, McEntee GP. Combined resection of the inferior vena cava and extended right hepatectomy for leiomyosarcoma of the retrohepatic cava. Br J Surg. 1994;81:845–846. doi: 10.1002/bjs.1800810617. [DOI] [PubMed] [Google Scholar]
- 19.Yagyu T, Shimizu R, Nishida M, Nakashima K, Uchiyama T, Suzuki T. Reconstruction of the hepatic vein to the prosthetic inferior vena cava in right extended hemihepatectomy with ex situ procedure. Surgery. 1994;115:740–744. [PubMed] [Google Scholar]
- 20.Ohwada S, Kawashima Y, Ogawa T. Extended hepatectomy with ePTFE graft vena caval replacement and hepatic vein reconstruction: a case report. Hepatogastroenterology. 1999;46:1151–1155. [PubMed] [Google Scholar]
- 21.Enoki T, Hayashi D, Inokuchi T, Okamura K, Takahashi T, Noshima S, et al. Combined right hepatic and retrohepatic caval resection with reconstruction using a polyfluoroethylene graft for primary leiomyosarcoma of the liver: report of a case. Surg Today. 1999;29:67–70. doi: 10.1007/BF02482973. [DOI] [PubMed] [Google Scholar]
- 22.Zografos GN, Palmer S, Papastratis G, Habib NA. Aggressive surgical management of fibrolamellar hepatocellular carcinoma in puberty. Eur J Surg Oncol. 1997;23:570–572. doi: 10.1016/s0748-7983(97)93493-4. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto Y, Terajima H, Ishikawa Y, Uchinami H, Taura K, Nakajima A. In situ pedicle resection in left trisegmentectomy of the liver combined with reconstruction of the right hepatic vein to an inferior vena caval segment transpositioned from the infrahepatic portion. J Am Coll Surg. 2001;192:137–141. doi: 10.1016/s1072-7515(00)00727-4. [DOI] [PubMed] [Google Scholar]
- 24.Lechaux D, Megevand JM, Raoul JL, Boudjema K. Ex vivo right trisegmentectomy with reconstruction of inferior vena cava and ‘flop’ reimplantation. J Am Coll Surg. 2002;194:842–845. doi: 10.1016/s1072-7515(02)01169-9. [DOI] [PubMed] [Google Scholar]
- 25.Del Campo C, Konok GP. Use of a pericardial xenograft patch in repair of resected retrohepatic vena cava. Can J Surg. 1994;37:59–61. [PubMed] [Google Scholar]
- 26.Starzl TE, Koep LJ, Weil R, 3rd, Lilly JR, Putnam CW, Aldrete JA. Right trisegmentectomy for hepatic neoplasms. Surg Gynecol Obstet. 1980;150:208–214. [PMC free article] [PubMed] [Google Scholar]
- 27.Iwatsuki S, Todo S, Starzl TE. Right trisegmentectomy with a synthetic vena cava graft. Arch Surg. 1988;123:1021–1022. doi: 10.1001/archsurg.1988.01400320107023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumada K, Shimahara Y, Fukui K, Itoh K, Morikawa S, Ozawa K. Extended right hepatic lobectomy: combined resection of inferior vena cava and its reconstruction by PTFE graft (Goretex). Case report. Acta Chir Scand. 1988;254:481–483. [PubMed] [Google Scholar]
- 29.Risher WH, Arensman RM, Ochsner JL, Hollier LH. Retrohepatic vena cava reconstruction with polyfluoroethylene graft. J Vasc Surg. 1990;12:367–370. doi: 10.1067/mva.1990.22151. [DOI] [PubMed] [Google Scholar]
- 30.Miller CM, Schwartz ME, Nishizaki T. Combined hepatic and vena caval resection with autogenous caval graft replacement. Arch Surg. 1991;126:106–108. doi: 10.1001/archsurg.1991.01410250114020. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S, Hishiki S, Nakamura S, Koyano K, Kosaka A. Extension incision forrenal carcinoma including invaded vena cava and right lobe of the liver. Urology. 1992;39:285–288. doi: 10.1016/0090-4295(92)90308-j. [DOI] [PubMed] [Google Scholar]
- 32.Lodge JP, Ammori BJ, Prasad KR, Bellamy MC. Ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann Surg. 2000;231:471–479. doi: 10.1097/00000658-200004000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamady ZZ, Cameron IC, Wyatt J, Prasad RK, Toogood GJ, Lodge JP. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1-cm rule. Eur J Surg Oncol. 2006;32:557–563. doi: 10.1016/j.ejso.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Finch RJ, Malik HZ, Hamady ZZ, Al-Mukhtar A, Adair R, Prasad KR, et al. Effect of type of resection on outcome of hepatic resection for colorectal metastases. Br J Surg. 2007;94:1242–1248. doi: 10.1002/bjs.5640. [DOI] [PubMed] [Google Scholar]
- 35.Fortner JG, Shiu MH, Kinne DW, Kim DK, Castro EB, Watson RC, et al. Major hepatic resection using vascular isolation and hypothermic perfusion. Ann Surg. 1974;180:644–652. doi: 10.1097/00000658-197410000-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging manoeuvre: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109–111. doi: 10.1016/s1072-7515(01)00909-7. [DOI] [PubMed] [Google Scholar]
- 37.Dindo D, Demartines N, Clavien P. Classification of surgical complications. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 39.Strasberg SM. International Hepato-pancreato-biliary Association Terminology Committee survey: the Brisbane 2000 terminology of liver anatomy and resections. HPB. 2000;2:333–339. [Google Scholar]
- 40.Beck SDW, Lalka SG, Donohue JP. Longterm results after inferior vena cava resection during retroperitoneal lymphadenectomy for metastatic germ cell cancer. J Vasc Surg. 1998;28:808–814. doi: 10.1016/s0741-5214(98)70055-2. [DOI] [PubMed] [Google Scholar]
- 41.Bower TC, Cherry KJ, Jr, Toomey BJ, Hallett JW, Panneton JM, et al. Replacement of the inferior vena cava for malignancy: an update. J Vasc Surg. 2000;31:270–281. doi: 10.1016/s0741-5214(00)90158-7. [DOI] [PubMed] [Google Scholar]
- 42.Maeba T, Okano K, Mori S, Karasawa Y, Goda F, Wakabayashi H, et al. Extent of pathologic invasion of the inferior vena cava in resected liver cancer compared with possible caval invasion diagnosed by preoperative images. J Hepatobiliary Pancreat Surg. 2000;7:299–305. doi: 10.1007/s005340070052. [DOI] [PubMed] [Google Scholar]
- 43.Ohwada S, Fukusato T, Kawashima Y, Kobayashi I, Ohya T, Nakamura S, et al. Metastasis and invasion of hepatocellular carcinoma mimicking a right adrenal tumour. Hepatogastroenterology. 1998;45:1104–1110. [PubMed] [Google Scholar]
- 44.Tanaka A, Morimoto T, Ozaki N, Ikai I, Yamamoto Y, Tsunekawa S, et al. Extension of surgical indication for advanced hepatocellular carcinoma: is it possible to prolong life span or improve quality of life? Hepatogastroenterology. 1996;43:1172–1181. [PubMed] [Google Scholar]
- 45.Belt TG, Cohen MD, Smith JA, Cory DA, McKenna S, Weetman R. MRI of Wilms' tumour: promise as the primary imaging method. AJR Am J Roentgenol. 1986;146:955–961. doi: 10.2214/ajr.146.5.955. [DOI] [PubMed] [Google Scholar]
- 46.Madariaga JR, Fung J, Gutierrez J, Bueno J, Iwatsuki S. Liver resection combined with excision of vena cava. J Am Coll Surg. 2000;191:244–250. doi: 10.1016/s1072-7515(00)00362-8. [DOI] [PubMed] [Google Scholar]
- 47.Huguet C, Gavelli A, Bona S. Hepatic resection with ischaemia of the liver exceeding one hour. J Am Coll Surg. 1994;178:454–458. [PubMed] [Google Scholar]
- 48.Sarkar R, Eilber FR, Gelabert HA, Quinones-Baldrich WJ. Prosthetic replacement of the inferior vena cava for malignancy. J Vasc Surg. 1998;28:75–83. doi: 10.1016/s0741-5214(98)70202-2. [DOI] [PubMed] [Google Scholar]
- 49.Arii S, Teramoto K, Kawamura T, Takamatsu S, Sato E, Nakamura N, et al. Significance of hepatic resection combined with inferior vena cava resection and its reconstruction with expanded polytetrafluoroethylene for treatment of liver tumours. J Am Coll Surg. 2003;196:243–249. doi: 10.1016/S1072-7515(02)01616-2. [DOI] [PubMed] [Google Scholar]
- 50.Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668–676. doi: 10.1245/ASO.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 51.Reddy SK, Pawlik TM, Zorzi D, Gleisner AL, Ribero D, Assumpcao L, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol. 2007;14:3481–3491. doi: 10.1245/s10434-007-9522-5. [DOI] [PubMed] [Google Scholar]


