Abstract
After a series of serendipitous discoveries of pharmacological treatments for mania and depression several decades ago, relatively little progress has been made for novel hypothesis-driven drug development in mood disorders. Multifactorial etiologies of, and lack of a full understanding of, the core neurobiology of these conditions clearly have contributed to these development challenges. There are, however, relatively novel targets that have raised opportunities for progress in the field, such as glutamate and cholinergic receptor modulators, circadian regulators, and enzyme inhibitors, for alternative treatment. This review will discuss these promising new treatments in mood disorders, the underlying mechanisms of action, and critical issues of their clinical application. For these new treatments to be successful in clinical practice, it is also important to design innovative clinical trials that identify the specific actions of new drugs, and, ideally, to develop biomarkers for monitoring individualized treatment response. It is predicted that future drug development will identify new agents targeting the molecular mechanisms involved in the pathophysiology of mood disorders.
Keywords: mood disorders, clinical pharmacology, clinical trials, neurotransmission, circadian, signal transduction
INTRODUCTION
The major mood disorders—bipolar disorder (acute mania and bipolar depression) and major depressive disorder—represent a spectrum of brain disorders with multiple etiologies, including genetic vulnerability, environmental stress, dysregulation of neurotransmission, abnormal neuroplasticity, and altered gene expression (Chen et al, 2010; Krishnan and Nestler, 2010; Pittenger and Duman, 2008). However, currently available pharmacological agents for mood disorders were often discovered serendipitously, with the mechanisms of action only partially understood after the initial demonstration of clinical effects. In the sections below, we briefly review the available pharmacological treatment of mood disorders and their proposed mechanisms of action. Obviously, future drug development needs to have a greater understanding of mechanisms with hypothesis-driven clinical trials based on preclinical findings. Recent treatment development has already moved toward this direction, although gaps remain between preclinical findings and clinical trial outcomes. In this review, we primarily summarize those newly developed treatments with some supportive data at the level of clinical trials. Finally, we will discuss outstanding issues that need to be considered for treatment development in mood disorders.
Anti-manic and Mood-Stabilizing Medications
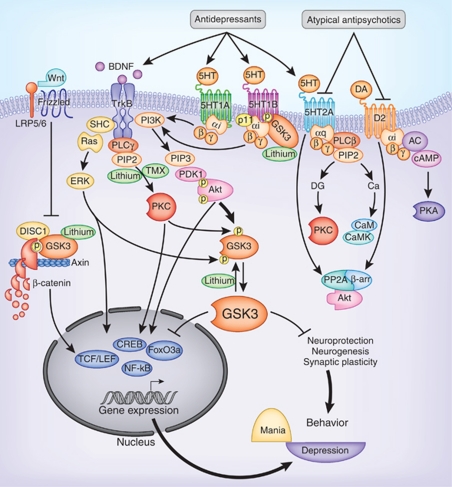
Lithium was the first psychotropic with a specific indication for bipolar disorder. Its efficacy in acute mania (Cade, 1949), especially mania with typical clinical features (ie, euphoric mood, grandiosity), has been confirmed in many clinical trials (Goodwin and Jamison, 2007). Anti-manic efficacy tends to be reduced in mixed states, rapid cycling, multiple prior episodes, or comorbid substance use disorders. As a small ion, lithium freely penetrates into neurons and is likely to interact with multiple intracellular molecules. The precise mechanism of therapeutic action of lithium remains to be elusive, but the candidate cellular targets of lithium appear to be enzymes such as inositol phosphatases (Berridge et al, 1982) and glycogen synthase kinase-3 (GSK3) (Klein and Melton, 1996) (Figure 1).
Figure 1.
Signal transduction pathways mediating the actions of lithium and monoamine-regulating drugs. Lithium directly inhibits GSK3 and facilitates the phosphorylation of GSK3 at the N-terminal serine. Lithium also inhibits inositol phosphatases to block phosphatidylinositol signaling. Antidepressants facilitate serotonin action on serotonin receptors as well as facilitate neurotrophic receptor activity. Atypical antipsychotics block both serotonin 2A and dopamine D2 receptors. Activation of these monoamine receptors causes the activation or inhibition of Akt, PKC, or Erk through different signaling pathways. Akt and PKC phosphorylate GSK3 at the N-terminal serine and Erk phosphorylates GSK3 at the C-terminal serine, which cause GSK3 inactivation. Tamoxifen inhibits PKC. These signaling cascades directly or indirectly regulate gene expression and neuroplasticity that have an impact in mood regulation. AC, adenylyl cyclase; Ca, calcium; CaM, calmodulin; CaMK, calmodulin-dependent protein kinase; cAMP, cyclic adenosine monophosphate; CREB, cAMP-responsive element-binding protein; DG, diacylglycerol; DISC1, disrupted in schizophrenia-1; ERK, extracellular signal-regulated kinase; FoxO, forkhead ‘O' transcription factor; GSK3, glycogen synthase kinase-3; IP3, inositol trisphosphate; LRP5/6, low-density lipoprotein receptor-related protein-5/6; MEK, mitogen-activated protein kinase; NF-κB, nuclear factor-κ-B; PDK1, phosphoinositide-dependent protein kinase-1; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2); PIP3, phosphatidylinositol-(3,4,5)-trisphosphate (PtdIns(3,4,5)P3); PKA, protein kinase-A; PKC, protein kinase-C; PLC, phospholipase-C; PP1, protein phosphatase-1; PP2, protein phosphatase-2, TMX, tamoxifen; TrkB, tyrosine kinase-B; β-arr, β-arrestin.
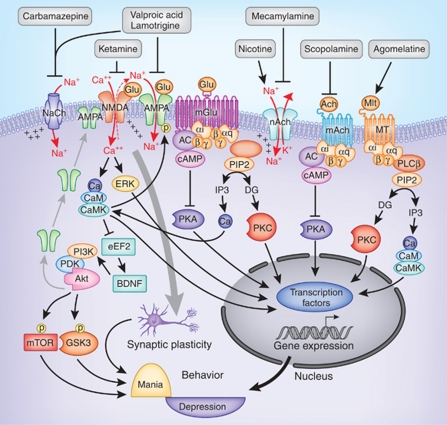
The introduction of antiepileptics as a treatment option for bipolar disorder resulted, in part, from preclinical models of limbic kindling, and behavioral sensitization resembled the longitudinal course of illness progression in bipolar disorder (Goddard, 1967). Early proof-of-concept studies (Okuma et al, 1979, 1981; Post, 1990a, 1990b, Post et al., 1982) and subsequent clinical trials have led to two FDA-approved antiepileptics for treatment of acute mania, divalproex sodium (Bowden et al, 2000; Freeman et al, 1992; Pope et al, 1991) and extended-release carbamazepine (Weisler et al, 2005). Both are effective in euphoric mania, like lithium, but also mixed mania where lithium appears to be somewhat less effective. Despite a different medication class, valproic acid and carbamazepine may directly or indirectly affect similar signaling mechanisms targeted by lithium (Gurvich and Klein, 2002). Lithium, valproic acid, and carbamazepine have common effects on the depletion of the phosphoinositide precursor inositol (Williams et al, 2002). All three drugs also reduce brain arachidonic acid, although by targeting different lipid metabolic processes (Rao et al, 2008). However, the significance of these common cellular effects in their anti-manic action remains unknown. Besides their common intracellular actions, valproic acid and carbamazepine block sodium channels to reduce neuron excitability (Figure 2), which is a proposed mechanism for their antiepileptic action (Macdonald and Kelly, 1995). Although it has not acquired regulatory approval, two placebo-controlled clinical trials have shown that phenytoin, another sodium channel blocker, also has an anti-manic and bipolar prophylactic effect as add-on treatment (Mishory et al, 2003, 2000). However, not all antiepileptic drugs have noticeable anti-manic effects (Gajwani et al, 2005), leaving the direct association of their antiepileptic effects with bipolar disorder inconclusive. Valproic acid also has been reported to inhibit GABA transaminase (Rosenberg, 2007), reduce the synaptosomal α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunits GLuR1 and GluR2, and AMPA receptor trafficking (Du et al, 2008, 2004) and increase the turnover of vesicular glutamate transporter (Kang et al, 2005); these results suggest a role for valproic acid in modulating glutamate neurotransmission and synaptic plasticity (Figure 2). Valproic acid is also a weak histone deacetylase (HDAC) inhibitor that facilitates histone acetylation and enhances the expression of many genes (Phiel et al, 2001). Carbamazepine appears to have certain mechanisms of action distinctive from valproic acid (Eyal et al, 2004; Mai et al, 2002), which remain to be fully investigated.
Figure 2.
Regulation of intracellular signaling by antiepileptics and modulators of glutamate, acetylcholine, and melatonin systems. The antiepileptic drugs are either sodium channel blockers or have a direct effect on AMPA receptors. Ketamine is an antagonist of NMDA receptors. Activation of inotropic glutamate receptors increases intracellular sodium and calcium to regulate downstream protein kinases such as CaMK, Akt, and Erk. Blocking NMDA receptors by ketamine can induce the activation of Akt, which activates the mTOR pathway and inhibits GSK3. Ketamine also increases BDNF synthesis induced by elongation factor-2. Nicotine activates and mecamylamine blocks nicotinic acetylcholine receptors; scopolamine is an antagonist of muscarinic receptors; and agomelatine activates melatonin receptors. Metabolic glutamate, muscarinic acetylcholine, and melatonin receptors regulate G-protein-coupled signaling pathways such as PKA, PKC, and CaMK. AMPA, 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl) propanoic acid receptor; BDNF, brain-derived neurotrophic factor; CaMK, calcium/calmodulin-dependent protein kinase; eEF2, elongation factor-2; mAch, muscarinic acetylcholine receptor; mGlu, metabolic glutamate receptor; MT, melatonin receptor; mTOR, mammalian target of rapamycin; nAch, nicotinic acetylcholine receptor; NaCh, sodium channel; NMDA, N-methyl--aspartic acid receptor.
Lamotrigine is the only other antiepileptic with clear benefit for bipolar disorder and is approved by FDA for maintenance treatment (Bowden et al, 2003; Calabrese et al, 2003). Lamotrigine is not an effective anti-manic agent, but the lack of acute effect in mania may be attributable to the slow dosage titration that is required to prevent its serious adverse effects, including Stevens–Johnson syndrome (Schmidt and Kramer, 1994). Lamotrigine has demonstrated efficiency for maintenance mood stabilization, especially for preventing relapse into depression (Calabrese et al, 2003). Although only approved by FDA for the maintenance phase of bipolar-I disorder (Bowden et al, 2003; Calabrese et al, 2003), additional studies (Frye et al, 2000; van der Loos et al, 2009) and a recent meta-analysis of clinical trials (Geddes et al, 2009) have suggested a specific effect of lamotrigine in bipolar depression. Besides blocking sodium channels (Cheung et al, 1992; Prica et al, 2008; Zona and Avoli, 1997), lamotrigine potently inhibits presynaptic glutamate neurotransmission by direct inhibition of glutamate release (Leach et al, 1986). In addition, lamotrigine may also modulate glutamate neurotransmission by increasing the membrane-surface expression of AMPA receptor subunits (Du et al, 2007) and inhibition of postsynaptic AMPA receptor-induced currents (Lee et al, 2008). More recently, lamotrigine has been found to selectively suppress α4β2 nicotinic acetylcholine receptor-mediated currents in freshly dissociated ventral tegmental dopamine neurons, suggesting that lamotrigine may inhibit nicotinic acetylcholine receptor function in this brain area (Zheng et al, 2010). Glutamate and acetylcholine receptor ligands both represent newer targets for antidepressant drug development (see additional discussion later in this review).
Antidepressants
The earliest drugs discovered to be effective for the treatment of depression include monoamine oxidase inhibitors (MAOIs) (Youdim and Bakhle, 2006) and tricyclic antidepressants (TCAs) (Klerman and Cole, 1965). More recent medications, including serotonin-selective reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), and a few atypical antidepressants, such as mirtazapine and nefazodone, have improved side-effect profiles but are no more effective than MAOIs and TCAs. All these drugs share similar mechanisms of enhancing or otherwise modulating serotonin and norepinephrine neurotransmission. Therefore, currently available antidepressants largely have a limited set of mechanisms and may contribute partially to inadequate remission rates for major depression found in contemporary clinical trials.
As evidenced by the NIMH-funded Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (Rush et al, 2006), remission rates with current antidepressant treatment were not robust. The STAR*D study enrolled 3671 patients to four sequential levels of treatment based on non-remission. The findings of the study were striking; only about 1/3 of participants remitted at the first-level treatment with citalopram. There were only modest differences between the individual treatments at each subsequent level; the remission rates for Levels 1–4 were 36.8%, 30.6%, 13.7%, and 13.0%, respectively, indicating diminishing responses across the four levels. Although 67% of the participants remitted, about half relapsed over a 1-year follow-up with increased rates at successive levels of care (40.1%, 55.3%, 64.6%, and 71.1% relapse for Levels 1–4, respectively). This landmark study demonstrated several salient facts on the available antidepressants: (1) Depression is difficult to treat to remission; (2) sustained remission with any available option or even multiple treatment steps remain unacceptably low and hard to maintain; (3) intolerance rates are unacceptably high; and (4) most notably, modulating monoamine neurotransmission alone is not sufficient for sustained treatment of depression for the majority of patients (Shelton et al, 2010).
The impressive lack of response/remission with antidepressants is even more striking in bipolar depression than in major depression (Frye, 2011). In the NIMH-funded 26-week Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) study (Sachs et al, 2007), bipolar depressed patients treated with a mood stabilizer were randomized to adjunctive antidepressant therapy (paroxetine or bupropion) or placebo, but there were no significant differences among groups in the rates of durable recovery, defined as eight consecutive weeks of euthymia without switch to mania/hypomania. A recent randomized trial comparing paroxetine monotherapy and quetiapine in bipolar depression also suggested that paroxetine was less effective in improving depression and was more likely to cause a switch to mania/hypomania (McElroy et al, 2010). Finally, a recent meta-analysis of 15 randomized, double-blind trials comparing acute antidepressant with either placebo or active comparator in bipolar-I and II depressed patients demonstrated non-significant benefits of antidepressant therapy over placebo (p<0.09) in rates of remission (Sidor and Macqueen, 2011). In current clinical practice, antidepressant monotherapy is not recommended for bipolar depression, particularly bipolar-I, owing to the concern of its mania-inducing effect (Frye et al, 2009). In fact, bipolar depression has been an exclusion criterion in most antidepressant clinical trials, which may have contributed to the unsure efficacy of any new antidepressant in the treatment of bipolar depression. The general non-responsiveness of bipolar depression to the available monoamine-modulating antidepressant treatment and the risk of inducing mania suggest that the neuropathology of bipolar depression likely involves mechanisms beyond monoamine neurotransmission, which need to be identified to develop effective treatment.
Antipsychotics in Mood Disorders
Conventional antipsychotics block dopamine D2 receptors and have acute anti-manic and anti-psychotic effects in acutely ill bipolar patients (Tohen and Vieta, 2009). Among them, chlorpromazine is the only one that has FDA indication for treatment of bipolar mania. Most atypical antipsychotics available in the United States, except for clozapine, paliperidone, iloperidone, and lurasidone, have received FDA approval for bipolar mania.
Some atypical antipsychotics, either as monotherapy or augmentation, have also shown efficacy in ameliorating symptoms of bipolar depression and treatment-resistant major depression. Quetiapine has demonstrated efficacy in bipolar depression (Calabrese et al, 2005) and has received FDA indication as monotherapy for this condition. Olanzapine plus fluoxetine is an FDA-approved combination treatment for bipolar depression (Tohen et al, 2003). Aripiprazole and quetiapine have shown antidepressant efficacy as augmentation treatment to antidepressant in major depressive disorder (Bauer et al, 2009; Berman et al, 2007; Marcus et al, 2008) and have recently received FDA approval for this indication.
The mechanisms of atypical antipsychotics in the treatment of mood disorders remain unclear. These agents not only are antagonists of dopamine D2 receptors (as with the typical antipsychotics), but also block type-2 serotonin (5-HT2) receptors, particularly 5-HT2A and 5-HT2C receptors (Markowitz et al, 1999; Meltzer et al, 1989). Blockade of 5-HT2A receptors is a mechanism of action of certain antidepressants, particularly trazodone and nefazodone. The contribution of 5-HT2A/2C blockade to the antidepressant actions of atypical antipsychotics is unclear, but may involve their effect on forebrain norepinephrine and dopamine neurotransmission (Zhang et al, 2000). Additionally, aripiprazole, ziprasidone, and asenapine are 5-HT1A receptor partial agonists, which have also been shown to enhance the release of norepinphrine and dopamine (Ghanbari et al, 2009; Gobert et al, 1999; Hajos-Korcsok et al, 1999). Although the exact mechanism remains elusive, the apparent superior efficacy of these drugs to conventional antipsychotics as mood-stabilizing agents suggests that this effect occurs through actions beyond a D2 receptor antagonistic action (Blier and Szabo, 2005) (Figure 1).
In summary, a group of anti-manic agents have been effectively used to control acute mania, and the efficacy of those clinically used anti-manic treatments are supported by meta-analysis of available clinical data (Yildiz et al, 2011). Evidently, current treatment of depression for both major depressive disorder and bipolar depression is limited, and the efficacy of treatment is not satisfactory. This problem may be attributable to the focus in antidepressant development to date primarily on the monoamine-modulating mechanism of action. Therefore, new treatments for depression are needed, particularly antidepressants that have new mechanisms of action and target a broader set of depressive symptomatology. In the next few sections, we will review pharmacological treatments that have promising efficacy in depression or mania, and discuss issues in concern when they are advanced to clinical implications.
NEW TREATMENT DEVELOPMENT IN MOOD DISORDERS
Improved Treatment Targeting Monoamine Neurotransmission
For decades, monoamine-regulating drugs are the only class of antidepressants available; thus, there is no doubt that balanced monoamine neurotransmission is important in mood regulation. However, the majority of these drugs, including MAOIs, TCAs, SSRIs, SNRIs, and the α2-adrenergic antagonist, mirtazapine, globally alter the synaptic neurotransmission of serotonin and/or norepinephrine, and are non-selective to each type of serotonin and norepinephrine receptors. At least 13 subtypes of serotonin receptors and five subtypes of noradrenergic receptors have been identified. These monoamine receptors have different and specific functions in defined brain regions. Therefore, unless the role of each of these receptors in mood regulation is fully characterized, the most effective treatment of depression by targeting monoamine neurotransmission may not be developed.
5-HT1A receptors have been studied extensively for their roles in regulating mood and anxiety in both animal and human studies (Akimova et al, 2009; Leonardo and Hen, 2008; Parks et al, 1998; Polter and Li, 2010b; Price and Drevets, 2010; Ramboz et al, 1998; Savitz et al, 2009). However, trials of 5-HT1A receptor full or partial agonists for depression have been unsuccessful, with only buspirone, a 5-HT1A receptor partial agonist, receiving FDA approval as an anxiolytic agent (Goldberg, 1979; Goldberg and Finnerty, 1979). In fact, the delayed onset of serotonin reuptake inhibitor antidepressants is thought to be partly due to the time required to desensitize the presynaptic 5-HT1A autoreceptors (Albert and Lemonde, 2004; Blier and Ward, 2003; Martin et al, 1990, 1991) that express in the serotonin neurons (Bockaert et al, 2006) and negatively regulate serotonin release (Sprouse and Aghajanian, 1987). Thus, blockade of 5-HT1A receptors may accelerate response to SSRIs (Arborelius, 1999) instead of enhancing the overall responsiveness to antidepressants (Shelton et al, 2010).
Although remaining to be thoroughly investigated, 5-HT1B and 5-HT4 receptors are potential targets for new antidepressants (Lucas, 2009; Ruf and Bhagwagar, 2009), particularly with evidence showing their intimate association (Svenningsson et al, 2006; Warner-Schmidt et al, 2009) and brain colocalization (Egeland et al, 2011) with a small adaptor protein p11 (Donato, 1999; Marenholz et al, 2004). Expression of p11 is decreased in the cingulate cortex in animal models of depression (Svenningsson et al, 2006) and in postmortem brains of depressed suicide subjects (Anisman et al, 2008). Additionally, mice with neuron-targeted deletion of p11 show a depressive phenotype (Svenningsson et al, 2006). Conversely, upregulation of p11 expression can be induced by antidepressant treatment, electroconvulsive therapy, and brain-derived neurotrophic factor (BDNF) (Svenningsson et al, 2006; Warner-Schmidt et al, 2010), and behaviors of transgenic mice with p11 overexpression mimic the effects of antidepressants (Svenningsson et al, 2006). The association of p11 with 5-HT1B and 5-HT4 receptors causes an increase in the cell-surface expression of these receptors (Svenningsson et al, 2006; Warner-Schmidt et al, 2009), raising the possibility that the behavioral effect of p11 is mediated by enhancing serotonin-regulated neuron activity through these receptor subtypes (Svenningsson and Greengard, 2007).
Noticeably, functions of 5-HT1B receptors are complex owing to its autoreceptor and heteroreceptor expression in different types of neurons (McDevitt et al, 2011; Sari, 2004), but agents targeting specific subtypes of 5-HT1B receptors are not yet available. Interestingly, in several earlier studies, 5-HT1B receptor activity and associated behaviors were found to be regulated by the mood stabilizer lithium (Januel et al, 2002; Massot et al, 1999; Redrobe and Bourin, 1999), which appeared to be a selective effect to 5-HT1B receptors, as lithium did not elicit similar regulation on 5-HT1A receptors (Redrobe and Bourin, 1999). From various molecular approaches, it is now clear that the selective effect of lithium on 5-HT1B receptors is likely the result of inhibition of an intracellular protein kinase, GSK3 (Figure 1), as GSK3 selectively interacts and differentially facilitates 5-HT1B receptor-regulated G-protein signaling, serotonin release, and associated behaviors (Chen et al, 2009, 2011). As discussed in more detail later in this review, GSK3 has been increasingly recognized as a protein kinase involved in mood regulation, and it is a potential therapeutic target of several classes of drugs used in the treatment of mood disorders (Li and Jope, 2010b). Therefore, 5-HT1B receptors act as an anchor of serotonin signaling to mood regulation through interaction with p11 and GSK3. This unique characteristic of 5-HT1B receptors calls for developing agents that facilitate p11 or disrupt GSK3 interaction with 5-HT1B receptors, as these specific protein interactions may modify 5-HT1BR function for the treatment of depression.
5-HT4 receptor-selective agonists have been tested in preclinical animal models for their antidepressant effect. In several tests measuring mood-related behaviors, the 5-HT(4) agonist RS67333 was found to elicit a strong antidepressant-like action and the effect is accompanied by rapid induction of 5-HT1A receptor desensitization, CREB expression, and neurogenesis, events that are typically induced by chronic antidepressant treatment in animals (Lucas et al, 2007). In another study, the 5-HT4 receptor partial agonist, SL650155, was also found to reduce depressive behaviors in rats as well as to increase the levels of active CREB and BDNF (Tamburella et al, 2009). However, the clinical usefulness of 5-HT4 receptor agonists in depression remains to be determined with proper clinical trials.
Besides several 5-HT2A and 5-HT2C receptor antagonists, such as the antidepressant, nefazodone (Fontaine, 1993), and most atypical antipsychotics (Meltzer, 1999), having clinically evidenced antidepressant effect, more recently, a considerable amount of evidence also supports the treatment of depression by blocking 5-HT7 receptors. In animal studies, blocking or inactivating 5-HT7 receptors results in an antidepressant-like effect in models of depression (Guscott et al, 2005; Wesolowska et al, 2006a, 2006b), potentiates the physiological and behavioral actions of monoamine reuptake inhibitors (Bonaventure et al, 2007; Wesolowska et al, 2007), and normalizes circadian rhythm (Duncan et al, 2004; Glass et al, 2003; Lovenberg et al, 1993; Sprouse et al, 2005) and sleep (Bonaventure et al, 2007; Hedlund et al, 2005; Shelton et al, 2009; Thomas et al, 2003). Clinically, several atypical antipsychotics with antidepressant properties, such as amisulpride and lurasidone, have a strong 5-HT7 receptor antagonistic effect (Stahl, 2010), supporting further investigation of putative 5-HT7 receptor antagonists for treatment of depression.
Noticeably, most existing antidepressants modulate serotonin and norepinephrine neurotransmission, and these drugs often do not typically improve depressive symptoms related to impaired dopamine action (Dunlop and Nemeroff, 2007). Earlier studies have demonstrated that amphetamine and other stimulants that increase dopamine release are effective in increasing concentration, energy, and enjoyment in depressed patients, although these agents do not have indications in the treatment of depression owing to their abuse potential. MAOIs block metabolism and increase the synaptic availability of synaptic dopamine, norepinephrine, and serotonin (Amsterdam and Bodkin, 2006; Feiger et al, 2006); as well bupropion increases synaptic dopamine and norepinephrine through increases in presynaptic release (Gobbi et al, 2003; Tomarken et al, 2004; Tremblay and Blier, 2006). In addition, newer triple reuptake inhibitors that block dopamine, serotonin, and norepinephrine transporters have been developed in the hope that these agents may enhance antidepressant effects and shorten the onset of treatment response. In a phase-II clinical study of depressed patients, the triple reuptake inhibitor DOV 216,303 with in vitro IC50 values of approximately 78, 14, and 20 nM at dopamine, serotonin, and norepinephrine transportors, respectively, was compared with citalopram for its safety and tolerability. Both DOV 216,303 (50 mg, b.i.d.) and citalopram (20 mg, b.i.d.) reduced baseline depressive symptoms within a 2-week treatment period (Skolnick et al, 2006). This pilot trial did not evaluate the superiority of the triple vs the mono reuptake inhibitor; thus, the better and early onset of effect cannot be evaluated. It should be noted that, although triple reuptake inhibitors have an acute antidepressant effect in an animal behavior test (Popik et al, 2006; Skolnick et al, 2006), chronic administration of triple reuptake inhibitors may actually dampen, instead of increasing, dopamine release (Prins et al, 2011), a phenomenon associated with the reciprocal interactions among all three monoamine neurotransmitters. Therefore, there may be limitations with triple reuptake inhibitors in the treatment of depression.
Alternatively, direct activation of selective dopamine receptors may have a specific therapeutic value in treating depression. Pramipexole, a partial D2/D3 agonist approved for Parkinson's disease, has shown antidepressant efficacy as monotherapy in major depressive disorder, and the effect is superior to placebo and equivalent to fluoxetine (Corrigan et al, 2000). It has also been evaluated in bipolar depression in two 6-week, randomized, double-blind, placebo-controlled studies as adjunct treatment to mood stabilizers (Goldberg et al, 2004; Zarate et al, 2004b). Dosed well below the average dosing for Parkinson's disease (1.7 vs 4.5 mg), the response rates were significantly higher in both studies for pramipexole vs placebo (67 vs 20% and 60 vs 9%). In a neuroimaging analysis of bipolar-II depression, pramipexole was seen to reduce normalized metabolism in frontal cortical areas (Mah et al, 2010), where cerebral metabolic activity is reportedly elevated in the depressed state of mood disorders (Drevets, 1999; Drevets et al, 1992; Mah et al, 2007). The findings are particularly important as they implicate dopamine receptor-targeting agents in depressive symptoms presented in both major depression and bipolar depression. Future clinical studies comparing the efficacy of dopamine agents to serotonergic and adrenergic agents in depression are in need, particularly to identify agents effective in both major depression and bipolar depression, as well as their onset of action, side-effect profile, and abuse potential.
Modulation of Glutamatergic Neurotransmission in the Treatment of Mood Disorders
Glutamate is the major excitatory neurotransmitter of the brain (Curtis and Watkins, 1961). It is synthesized from glutamine in glutamate neurons, released into synapses to activate pre- and postsynaptic glutamate receptors, and metabolized to glutamine in glial cells to be re-used for glutamate synthesis (Chaudhry et al, 2002). The association of glutamate neurotransmission with mood disorders has been increasingly recognized in the last decade (Paul and Skolnick, 2003). Earlier studies showed that patients with mood disorders have increased glutamate levels in the peripheral blood compartments (Altamura et al, 1995, 1993; Kim et al, 1982a, 1982b; Mauri et al, 1998). Postmortem brain studies also found increased glutamate levels in the frontal cortex of patients with bipolar disorder and major depression (Hashimoto et al, 2007), whereas reduced glutamate and increased glutamine were found in the cerebrospinal fluid (Frye et al, 2007b; Levine et al, 2000). The altered glutamate neurotransmission in mood disorders is further demonstrated in magnetic resonance spectroscopy as recently reviewed by Yuksel and Ongur (2010). In general, the overall glutamate and glutamine signals tend to be decreased in major depressive disorder and elevated in bipolar disorder, and depressed patients show an overall pattern of a reduced glutamine/glutamate ratio. Although data are not completely consistent, it is likely that perturbed glutamate neurotransmission is a feature associated with mood disorders.
As discussed earlier, the mood-stabilizing agents, valproic acid and lamotrigine, have glutamate-modulating property. In addition, monoamine-regulating antidepressants were also found to normalize brain glutamate activity (Bonanno et al, 2005; Michael-Titus et al, 2000) and peripheral glutamate level (Maes et al, 1998; Mauri et al, 1998). More recent clinical trials have continued to support the antidepressant efficacy of glutamate-modulating drugs. Riluzole, a neuroprotective agent with FDA approval for amyotrophic lateral sclerosis (ALS), inhibits presynaptic glutamate release (Benavides et al, 1985; Doble, 1996). In two preliminary open-label studies, riluzole demonstrated a significant antidepressant effect as either monotherapy or an add-on to a prior antidepressant therapy in major depressive disorder (Sanacora et al, 2007; Zarate et al, 2004a). In lithium-resistant bipolar depression, open-label addition of riluzole also showed significant clinical improvement in depressive symptoms (Zarate et al, 2005). In another open-label study on bipolar depression, the antidepressant effect of riluzole was associated with an increase in the glutamine/glutamate ratio in magnetic resonance spectroscopy, further suggesting that normalizing glutamate turnover by riluzole is involved in the treatment of depression (Brennan et al, 2010). Additionally, in a randomized, double-blind, multicenter, placebo-controlled study of 75 subjects with bipolar disorder, addition of N-acetylcysteine to usual medication over 24 weeks was reported to improve depression in the bipolar maintenance phase (Berk et al, 2008). Although the antioxidant dietary supplement likely has multiple actions, modulating glutamate exchange to eventually reduce the synaptic release of glutamate is one of its recognized actions (Moran et al, 2005).
Therefore, biochemical studies and clinical trials both suggest that modulating glutamate neurotransmission is one of the promising alternative therapeutics in mood disorders, an effect that may occur in the depressive phase of both major depressive and bipolar disorder. Glutamate activates three types of inotropic receptors with high affinity to N-methyl--aspartate (NMDA), AMPA, and kainic acid (Dingledine et al, 1999), and a group of G-protein-coupled metabolic glutamate receptors (Conn, 2003) (Figure 2).
Evidence of NMDA receptors as a therapeutic target of depression can be traced back to 1959 when cycloserine, a partial agonist of the glycine recognition site of NMDA receptors, was reported to have an antidepressant effect (Crane, 1959). A preliminary study with amantadine, a weak NMDA antagonist, also found antidepressant efficacy (Vale et al, 1971). The proof-of-concept clinical study, however, was demonstrated more recently with the NMDA receptor antagonist ketamine. In the first pilot study, depressed patients who received intravenous infusion of ketamine evidenced significant improvement in depressive symptoms within 72 h of ketamine infusion (Berman et al, 2000). The beneficial effect of ketamine in treatment-resistant depression was further demonstrated in a landmark double-blind, randomized, placebo-controlled clinical trial (Zarate et al, 2006a), which involved 18 male and female participants with major depressive disorder who had experienced insufficient response with at least two different adequate antidepressant trials. Following a 2-week drug-free period, the participants received intravenous infusions of either a saline solution or ketamine hydrochloride (0.5 mg/kg) 1 week apart in a crossover assignment. The participants were rated at baseline and at 40, 80, 110, and 230 min, and 1, 2, 3, and 7 days, after each infusion. The results were striking as participants experienced a rapid and robust antidepressant effect within 110 min of the infusion, and the effect continued through day 3. The study resulted in a large effect size (Cohen's d=1.46), with 71% meeting the response and 29% meeting the remission criteria during the ketamine treatment week. In another randomized, placebo-controlled, double-blind, crossover, add-on study of treatment-resistant bipolar depression, intravenous ketamine infusion, when added to the mood stabilizer treatment, showed a robust and rapid antidepressant effect after a single intravenous dose (Diazgranados et al, 2010a). Therefore, ketamine has a rapid antidepressant effect in both major depressive and bipolar disorders.
Although data from ketamine trials strongly support the view that NMDA receptor blockade may exert a rapid and robust antidepressant action, a recent trial of the oral form of another NMDA receptor antagonist, memantine, did not show an antidepressant effect in major depressive disorder (Zarate et al, 2006b). Intravenous drug delivery, although less useful in an out-patient setting, could be beneficial for hospitalized depressed patients who require rapid response to treatment, and for emergency management of suicidality in depressed patients, a condition that has shown response to ketamine infusion (DiazGranados et al, 2010b). In addition, as ketamine is also an anesthetic that does not elevate the seizure threshold, supplementing standard anesthetics with low-dose ketamine (within the dose range found effective in depression trials) may facilitate a seizure response and an antidepressant effect during electroconvulsive therapy for treatment-resistant depression. Several case reports have suggested this application with ketamine (Kranaster et al, 2011; McDaniel et al, 2006; Okamoto et al, 2010; Ostroff et al, 2005), but clinical efficacy remains to be demonstrated through randomized clinical trials. It should be noted that the antidepressant effect of ketamine is rapid albeit transient. A pilot study attempting to continue the effect of ketamine using oral riluzole unfortunately failed to show sustained therapeutic benefit (Mathew et al, 2010). Therefore, the clinical implications of NMDA receptor antagonists for depressant remain to be fully evaluated. Ketamine infusions may cause a brief (ie, an hour or two) duration of mild perceptual distortions, which may be related to a well-known property of ketamine in inducing psychotic symptoms, especially hallucinations (Berti et al, 2009; Giannini et al, 2000). Therefore, intravenous administration of ketamine requires close monitoring in clinical settings.
In a recent proof-of-concept clinical trial, CP101606, an antagonist of the brain-enriched NR2B subunit of NMDA receptors, was found to elicit an antidepressant effect when added to paroxetine in patients with major depressive disorder who failed to respond to two earlier antidepressants (Preskorn et al, 2008). An intravenous injection of CP101606 reduced the depressive symptoms within 5 days of administration, and ‘low-dose' CP101606 appears to have less a psychotomimetic effect than ketamine, suggesting an improved side-effect profile with highly selective NMDA receptor antagonists (Koller and Urwyler, 2010).
Blocking NMDA receptors by systemic administration of ketamine or other NMDA receptor antagonists markedly increases glutamate release in the medial prefrontal cortex (Lopez-Gil et al, 2007; Moghaddam et al, 1997) and concomitantly increases the firing rate of pyramidal neurons (Jackson et al, 2004), suggesting that the action of NMDA receptor antagonists is to enhance prefrontal cortical activity. The antidepressant effect of NMDA receptor antagonists requires the action of AMPA receptors, as AMPA receptor antagonists are able to abolish the behavioral effects of NMDA receptor antagonists (Maeng et al, 2008; Moghaddam et al, 1997).
Animal studies of intracellular mechanisms of NMDA receptor antagonist have revealed that ketamine rapidly activates the mammalian target of rapamycin (mTOR) pathway in the prefrontal cortex, leading to increased levels of the postsynaptic proteins PSD95 and the GluR1 AMPA receptor subunit, and the presynaptic protein synapsin-I, as well as an increased number and function of new spine synapses (Li et al, 2010a). Conversely, blockade of mTOR signaling completely abolished ketamine-induced synaptogenesis and several behaviors representing depression and anxiety (Li et al, 2010a, 2011). In another brief report, ketamine was found to inhibit GSK3, and the antidepressant-like behavioral effect of ketamine in animals depends on the inhibition of GSK3 through an inhibitory phosphorylation (Beurel et al, 2011). Noticeably, both activation of mTOR and inhibition of GSK3 are linked to Akt signaling (Cross et al, 1995; Scott et al, 1998), which can also be activated by ketamine (Li et al, 2010a). A more recent study also found that the rapid antidepressant effect of ketamine in animals depends on BDNF protein synthesis induced by the elongation factor when neurons are at the resting state, an effect likely mediated by suppressing the calcium-dependent calmodulin kinases (Autry et al, 2011). Although the signaling network from the transient blocking of NMDA receptors to a rapid behavior effect remains to be elucidated, current findings suggest the involvement of suppression on calcium-dependent signaling followed by activation of neurotrophic signaling pathways (Figure 2).
Besides NMDA receptor antagonists, potential antidepressant effects of AMPA receptor modulators has also been proposed; AMPA receptor allosteric potentiators have shown potent antidepressant action in preclinical studies (Alt et al, 2006). The AMPA receptor agents have been reported to have a neurogenic and neuroprotective action, which may contribute to their effect in mood regulation (Zarate and Manji, 2008). In animal behavioral studies, several metabolic glutamate receptor antagonists selective for the metabolic glutamate receptors mGlu2/mGLu3 (Chaki et al, 2004; Yoshimizu et al, 2006), mGlu5 (Li et al, 2006; Molina-Hernandez et al, 2006), and mGlu7 (O'Mahony et al, 2010; Wieronska et al, 2010) have shown promising antidepressant effect. It has been proposed that metabolic glutamate receptor ligands may impact a neural network that downregulates NMDA receptor function and enhances AMPA receptor signaling (Pilc et al, 2008).
Modulators of Acetylcholine Receptors in the Treatment of Depression
The cholinergic hypothesis of depression is an earlier model that proposed an association of a predominantly cholinergic over an adrenergic tone for depression, and the reverse for mania (Janowsky et al, 1972). The hypothesis was supported by a series of studies on animals and of humans showing that cholinergic challenge, such as with the acetylcholinesterase inhibitor physostigmine, causes depressive symptoms (El-Yousef et al, 1973; Riemann et al, 1994; Rosic and Bignami, 1970; Steinberg et al, 1997). However, potential use of anticholinergic drugs as antidepressants is much less developed, despite some evidences showing that agents modulating cholinergic receptors have mood-regulating properties. While promising new drugs targeting cholinergic receptors are discussed in other chapters of this review issue, below we reveal cholinergic drugs that have undergone trials as antidepressant treatment.
In early clinical trials evaluating the role of the cholinergic system in cognitive function in depressed patients, investigators noticed a reduction of depressive symptoms following administration of the muscarinic cholinergic receptor antagonist scopolamine (Gillin et al, 1991; Newhouse et al, 1988). The antidepressant efficacy of scopolamine has been replicated in two recent clinical trials: one includes both major depressive disorder and bipolar depression (Furey and Drevets, 2006), and the other is focused on major depressive disorder (Drevets and Furey, 2010). Importantly, the trials used an intravenous infusion of scopolamine in three intermittent periods, and each infusion elicited a potent antidepressant effect. The effect was prolonged even after scopolamine had been crossed over to placebo. This suggests that scopolamine has an acute and sustained antidepressant effect that differs from monoamine modulators. Noticeably, the acute antidepressant effect of scopolamine is similar to that observed with the glutamate receptor antagonist ketamine (Berman et al, 2000; Zarate et al, 2006a), which also has an acute onset of action after intravenous infusion. As delayed onset of therapeutic action is a major drawback of monoamine-modulating antidepressants, rapid antidepressant efficacy after intravenous administration may find unique applications in the treatment of depression in the emergency setting. However, muscarinic receptor antagonists have unavoidable negative effects in cognition and memory (Molchan et al, 1992; Sunderland et al, 1985; Vitiello et al, 1997), especially in elder patients, thus clinical application of muscarinic receptor antagonists for the treatment of depression appears to be pessimistic, unless age, prodromal cognitive deficit, and pseudodementia of depression have been carefully taken into consideration.
Scopolamine is a non-selective muscarinic receptor antagonist, whereas there are five subtypes of muscarinic receptors, among which M1, M3, and M5 couple to Gq protein, which mediates receptor activation to phospholipase-C/protein kinase-C (PKC) and calcium signaling, whereas M2 and M4 couple to the inhibitory G-protein (Gi) protein, which inhibits adenylyl cyclase and reduces cAMP production (Brann et al, 1993) (Figure 2). In earlier small clinical trials, the central M1 muscarinic receptor-selective antagonist biperiden was found to have an antidepressant effect (Beckmann and Moises, 1982; Kasper et al, 1981), but the effect was not replicated in a later trial (Gillin et al, 1995). Therefore, available data are not sufficient to project which subtype of muscarinic receptor has a major role in the antidepressant effect of anticholinergic drugs, and which one contributes to the cognitive side effect.
The role of nicotinic cholinergic receptors in depression and treatment has recently been reinforced by the large body of studies on cigarette smoking and cessation. Epidemiological analyses have shown that people with major depressive disorder have a higher rate of smoking than the general population (Kalman and Smith, 2005). People with depression also have more difficulties to stop smoking, and are likely to relapse to a depressive episode during smoking cessation (Dani and Harris, 2005; Hughes, 2007). These analyses appear to suggest that continued cigarette use may help ameliorate depressive symptoms. The direct contribution of nicotine in ameliorating depression has further been demonstrated in a clinical trial in mildly depressed non-smokers, where chronic transdermal nicotine administration for 4 weeks, but not acute nicotine administration, was found to reduce depressive symptoms (McClernon et al, 2006).
Nicotinic acetylcholine receptors are pentameric ligand-gated ion channels (Figure 2) that are grouped into α-subunits (α2–α10) and β-subunits (β2–4). In the neural system, the α- and β-subunits form heteromeric receptors that bind to agonists with high affinity (in nM concentrations), among which α4β2 receptors account for >90% of the high-affinity nicotinic receptors in the brain (Whiting and Lindstrom, 1986). The low-affinity receptors are homomeric α-receptors that bind to agonists with lower affinity (μM concentrations). The physiological contributions of nicotinic receptors to neurotransmission, signaling, and behavior are not completely understood, but their predominant role is thought to be in modulating the neurotransmission of dopamine, glutamate, GABA, and norepinephrine (Benowitz, 2009). Owing to the prominent role of nicotine in addiction, a large body of research has been focused on the dopaminergic modulating effect of nicotinic receptors. Systemic administration of nicotine was found to induce dopamine neuron firing and long-lasting dopamine release from the ventral tegmental area (VTA) onto nucleus accumbens (Gao et al, 2010; Imperato et al, 1986; Schilstrom et al, 1998). This effect of nicotine is mediated by either the β2 or the α7 subunit of nicotine receptors, which initially causes the rapid enhancement of glutamate neurotransmission, which contributes to an induction of glutamatergic synaptic long-term potentiation in VTA dopamine neurons (Gao et al, 2010; Mansvelder et al, 2002; Mansvelder and McGehee, 2000). With the increasingly recognized roles of dopamine and glutamate in mood disorders, the well-recognized modulating effect of nicotine on dopamine/glutamate neurotransmission could contribute to the antidepressant action of nicotine.
The potential antidepressant effect of activating nicotine receptors, however, has been challenged by an earlier preclinical study showing that not only nicotine, but the nicotinic receptor antagonists mecamylamine and varenicline also potentiated the acute antidepressant-like effect of monoamine reuptake inhibitors in the forced swim test in mice (Popik et al, 2003). As a single-agent treatment, however, studies on animals did not find an acute antidepressant effect of nicotine or selective nicotinic receptor agonists; instead, the antidepressant-like effects of the nicotinic receptor antagonist mecamylamine have been replicated in several animal behavioral studies (Andreasen and Redrobe, 2009; Mineur et al, 2007; Rabenstein et al, 2006). However, clinical trial data demonstrating an antidepressant effect of mecamylamine in humans are still lacking, except one report showing that 8-week mecamylamine treatment augmented the antidepressant effect of SSRI in patients who are refractory to SSRI monotherapy (George et al, 2008), but the effect was not significantly different between mecamylamine and placebo augmentation. Several trials of mecamylamine monotherapy and augmentation in depression have been conducted (Clinicaltrial.gov), which are expected to provide conclusive information, although data have not yet been reported. If confirmed for treatment of depression, nicotinic receptor antagonists could be promising as unlike scopolamine, there is little evidence showing a cognitive impairment effect when mecamylamine is administered alone (Ellis et al, 2006; Voss et al, 2010).
The question remains to be addressed is why nicotine receptor activation by nicotine and blocking by mecamylamine both appear to have some antidepressant effect in preclinical or clinical studies. It has been hypothesized that desensitization of nicotinic receptors after prolonged activation may be responsible for the antidepressant effect of nicotine (Mineur and Picciotto, 2010). Additionally, the antidepressant effect of a nicotinic receptor antagonist is likely to be receptor subtype-selective; for example, the α4β2 receptor antagonist varenicline, a drug with indication in smoking cessation, has shown acute antidepressant effect in animals (Rollema et al, 2009). In an open-label study of 14 subjects with depression and nicotine dependence, varenicline augmentation to antidepressants or mood stabilizers was reported to be associated with significant improvement in mood (Philip et al, 2009); however, effects on mood in studies investigating the smoking cessation effect of varenicline are inconsistent. Most likely that balanced nicotinic receptor activity is the key for mood regulation, as shown in several preclinical studies investigating the antidepressant efficacy of cytosine, a partial agonist at the α4β2 nicotinic receptors (Mineur et al, 2009; Mineur et al, 2007). In animals, the nicotinic receptor agonist cytisine not only reduces immobility in forced swim and tail suspension tests, but it also changes the novelty-suppressed feeding and light–dark box preference, tests that represent both anxious and depressive behaviors. Therefore, the clinical efficacy of nicotinic receptor modulators in depression remains to be investigated in large clinical trials, whereas better understanding of the pharmacological mechanisms of action of each nicotinic receptor subtype will be an important aspect for successfully applying nicotinic receptor modulators, either agonists or antagonists, in the treatment of depression.
Sleep and Circadian Regulation in the Treatment of Mood Disorders
Sleep disturbance has long been known as a core symptom of both depression and mania. A typical symptom of sleep disturbance in mania is feeling less need for sleep. In depression, insomnia and hypersomnia are frequent symptoms, with the latter considered characteristic of atypical depression and bipolar depression. It is also evident that some types of mood disturbances present with a seasonal pattern, as exemplified by seasonal depression and bipolar mania. Sleep is partly regulated by both homeostatic balance and the endogenous circadian clock (Fuller et al, 2006). However, sleep is not the only sign of circadian dysregulation in mood disorders. Patients with mood disorders also undergo disturbance of other circadian-regulated functions such as weight change, metabolic syndrome, hormone dysregulation, and cardiovascular diseases.
The circadian clock is an endogenous biological system that determines the temporal pattern of many physiological processes such as sleep, temperature, feeding, hormone release, and rhythmic activities of the heart and other organs (Bass and Takahashi, 2010). The primary circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Welsh et al, 2010), where light signals from the retina, endogenous hormones, and neurotransmitters, as well as environmental stimuli, converge to reset the phase of the circadian clock. Inside the SCN and throughout the body, a cluster of circadian genes turn on and off in response to transcriptional and translational feedback regulation, as well as external signals to reset the on/off switch (Cermakian and Sassone-Corsi, 2000; Dunlap, 1999; Reppert and Weaver, 2002). Among the most studied circadian genes are positive activators BMAL1 and CLOCK/NPAS2. They regulate the transcriptional expression of Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2), which feedback-suppress their own expression by negatively regulating BMAL1 and CLOCK/NPAS2. Per and Cry transcription can also be acutely upregulated in response to light at night such that light exposure at early night extends the current circadian cycle and light exposure at late night advances the next cycle (Ashmore and Sehgal, 2003). In addition, a secondary feedback loop is formed when CLOCK–BMAL1 activate the transcription of a nuclear orphan receptor Rev-erbα whose protein product feeds back to repress Bmal1 transcription.
Genetic and animal studies have recently provided evidence showing that variation of circadian genes can be etiologic to mood disorders. A single-nucleotide polymorphism (SNP) in the 3'-flanking region of CLOCK (3111T/C; rs1801260) has been found in more than one study to be associated with bipolar disorder (Benedetti et al, 2007, 2008, 2003; Lee et al, 2010). Recent studies testing behavioral changes in animal models have also revealed that manic-like and drug-seeking behaviors are observed in mice carrying a mutant dominant-negative CLOCK gene whose protein products cannot activate transcription (Roybal et al, 2007). Therefore, circadian regulation is increasingly considered a therapeutic component in mood disorders.
Improving sleep is an important treatment component in mood disorders. For example, sleep deprivation has a transient antidepressant effect (Hemmeter et al, 2010). But this approach has limited therapeutic value as patients would rapidly return to depression after cessation of a one-time treatment. Light therapy was initially used for treating seasonal depression to normalize light–dark cycle, but has also been increasingly found beneficial to other types of depression (Golden et al, 2005; Rosenthal et al, 1984), possibly through effects on normalizing circadian rhythms. In fact, most mood-stabilizing agents, such as atypical antipsychotics, valproate, and some antidepressants, have sleep-normalizing effects, although the mechanisms of action vary largely. Lithium also normalizes circadian rhythms, which may be related to its therapeutic effects (Klemfuss, 1992). As noted earlier, lithium is an inhibitor of GSK3. In Drosophila, the ortholog for GSK3, Shaggy, was first reported to phosphorylate the circadian gene Timeless, resulting in premature nuclear translocation of the PERIOD/TIMELESS heterodimer and a shortened period of the Drosophila circadian locomotor activity cycle (Martinek et al, 2001). In mammalian tissues, the activity of GSK3β shows a circadian rhythm, and the dynamically regulated GSK3β was found to interact with and regulate PER2 translocation into the nucleus and Per2 gene expression (Iitaka et al, 2005). GSK3β was also found to phosphorylate and stabilize a negative component of the secondary autoregulatory feedback loop (Rev-erbα) in cultured cells, and lithium treatment leads to rapid proteasomal degradation of Rev-erbα and activation of BMAL1 (Yin et al, 2006). Although deleting GSK3 or inhibition of GSK3 activity might alter circadian rhythm in a sophisticated pattern that is still not completely understood (Hirota et al, 2008; Iitaka et al, 2005; Kaladchibachi et al, 2007; Martinek et al, 2001), it has been shown that some circadian gene-regulating effects of GSK3 can be suppressed by lithium, suggesting that normalizing the circadian clock, at least partly through inhibition of GSK3β, may contribute to the mood-stabilizing effect of lithium. The mood-regulating effect of GSK3 will be discussed later in more detail, to support the development of selective GSK3-inhibiting agents for the therapeutics of mood disorders; among the potential effects of GSK3 inhibitors, normalizing circadian rhythms could be particularly interesting and important.
As dysregulation of circadian rhythm has been recognized as a major contributor or a sequel of mood disturbance, the promising clinical trial data on the high-affinity melatonin receptor agonist plus the 5-HT2C receptor antagonist agomelatine have raised significant interest in the hope that this represents a new mechanism of pharmacological treatment of depression. In clinical trials testing its efficacy in major depressive disorders, agomelatine has demonstrated significant short-term (6–8 weeks) (Kennedy and Rizvi, 2010; Loo et al, 2002a, 2002b; Stahl et al, 2010; Zajecka et al, 2010) and sustained (6 months) (Goodwin et al, 2009) antidepressant effects relative to placebo, as well as evidence of relapse prevention. Although agomelatine has a short half-life of 2 h, this does not seem to prevent it from reducing the clinical symptoms of depression. Instead, the onset of the antidepressant action of agomelatine appeared to be early (at 1–2 weeks) when compared with venlafaxine or sertraline (Kasper et al, 2010; Kennedy et al, 2008). Additionally, agomelatine is found to restore disturbed sleep–wake patterns early in treatment (Kasper et al, 2010; Quera-Salva et al, 2007), which can be an additional benefit to patients with depression.
The primary pharmacological action of agomelatine, although not assessed as a major outcome clinically, is to phase advance circadian rhythms and improve sleep quality (Quera-Salva et al, 2010; Van Reeth et al, 1998). A multi-synaptic pathway from the retina to the pineal gland through the SCN drives rhythmic melatonin synthesis and release, which are negatively regulated by photic stimulation at night (McNulty et al, 1994; Morgan et al, 1994). The resulting nocturnal peak in melatonin acts on the melatonin receptors residing in the SCN. The MT1 and MT2 melatonin receptors couple to the Gi (Stankov et al, 1992); activation of these receptors therefore results in the inhibition of cAMP production and reduction of SCN neuron firing (Figure 2). Agomelatine has been shown to activate the MT1 and MT2 receptors in the SCN to normalize the circadian rhythm (Ying et al, 1996). Besides being an agonist of MT1 and MT2, agomelatine has also been found to be a 5-HT2C receptor antagonist with moderate receptor affinity (Millan et al, 2010). As neither melatonin itself nor putative 5-HT2C receptor antagonists have significant antidepressant efficacy, there is a possibility that a joint action on melatonin receptors and 5-HT2C receptors is necessary to elicit an antidepressant effect by agomelatine.
Currently, agomelatine has been tested primarily as an antidepressant in major depressive disorder. With its circadian-normalizing and 5-HT2C receptor-blocking effects, the efficacy of agomelatine in alleviating symptoms of bipolar depression is worthy of future clinical trials. In a placebo-controlled trial of ambulatory type-I bipolar disorder patients, another melatonin-mimicking drug ramelteon was reported to improve the global ratings of depressive symptoms (McElroy et al, 2011), although ramelteon seems to have limited efficacy in insomnia in bipolar disorder (McElroy et al, 2011; Schaffer et al, 2011). Therefore, the mechanisms of melatonin-mimetic drugs in the treatment of mood disorders remain to be further investigated, as normalizing circadian rhythm may not be the sole action of these drugs.
In the completed clinical trials, agomelatine showed little impact in treatment-induced sexual dysfunction or weight gain (Goodwin et al, 2009; Kennedy and Rizvi, 2010). These favorable side-effect profiles would have significant clinical values as these are major drawbacks of many existing antidepressants and mood-stabilizing agents. However, one should be cautious about this impression when the physiological action of either melatonin receptors or 5-HT2C receptors is taken into account. Melatonin receptors are also expressed in the pituitary and peripheral endocrine tissues. Although melatonin supplementation does not seem to change diurnal hormone levels, there is evidence showing that melatonin-mimetic drugs may change the levels of prolactin (Richardson and Wang-Weigand, 2009), which could raise a concern of reproductive dysfunction. The weight gain effect of atypical antipsychotics has been thought to be associated with their antagonistic action on 5-HT2C receptors (De Luca et al, 2007), but this adverse effect of atypical antipsychotics did not become a significant concern until they had been marketed for years. Additionally, 5-HT2C receptor antagonist-induced weight gain appears to be associated with genetic variations (De Luca et al, 2007), which would require replication studies with a sufficient sample size to confirm this effect. Therefore, it might be premature to conclude a neutral effect on sexual function and weight by agomelatine until longitudinal observation data are made available.
The wakefulness-promoting agent modafinil has indications for excessive sleepiness associated with narcolepsy, obstructive sleep apnea, and shift work disorder (Minzenberg and Carter, 2008; Myrick et al, 2004). Although stimulant-like in its actions, the exact mechanism of action is unknown, and unlike conventional stimulants, modafinil appears to have low abuse liability (Myrick et al, 2004). Adjunctive modafinil 100–200 mg daily, in a placebo-controlled study has been shown to be effective in reducing depressive symptoms in bipolar disorder (Frye et al, 2007a). This initial study was the impetus for a larger controlled evaluation of armodafinil, the R-enantomier of modafinil, in bipolar depression (Calabrese et al, 2010). In this study, patients who received armodafinil along with lithium, valproic acid, or olanzapine showed greater improvement in depressive symptoms as seen in the total score of the Inventory of Depressive Symptomatology—Clinician Rated version (IDS-C30) (15.8±11.57) compared with placebo treatment (−12.8±12.54). With this rating scale, the improvement of depressive symptoms appeared to be superior after 2 weeks of armodafinil treatment, which may suggest an early onset of effect. Adverse events reported with armodafinil include frequent insomnia, but the study did not conduct formal tests on circadian measures.
Although the exact mechanism of action remains to be elucidated, modafinil has pharmacological action as an enhancer of norepinephrine and dopamine release (Mitchell et al, 2008; Wisor and Eriksson, 2005), and it may also elicit a wake-promoting effect by enhancing histamine and glutamate neurotransmission (Minzenberg and Carter, 2008). In animal studies, the wake-promoting effect of modafinil does not adjust the circadian phase nor does it alter light- and novel wheel-induced phase shifts (Webb et al, 2006). Instead, it decreases the amount of novel wheel-stimulated running, such that less activity is required for the same size phase shift. Therefore, modafinil may increase the sensitivity of the circadian pacemaker to non-photic stimuli, and when combined with behavioral strategies, may have potential for promoting circadian clock-resetting (Webb et al, 2006). If this observation is further supported in additional animal and human studies, application of modafinil as an adjunctive to a non-photic circadian regulator, such as a melatonergic agonist or an exercise programme, should be tested with a design to enhance rhythmic circadian regulation in patients with depression, particularly in the context of bipolar disorder.
Targeting Signal Transduction for Mood Stabilization
Activation or blocking neurotransmitter receptors results in an altered intracellular signal transduction process, which proceeds to regulation of neuron activity, gene expression, and other downstream effects (Figures 1 and 2). A signal transduction protein kinase that has drawn significant interest in mood disorder research is PKC, a large family of serine/threonine kinases with 12 isoforms in mammals (Mellor and Parker, 1998) (Figures 1 and 2). Since the first report of elevated PKC activity in platelets of patients during bipolar mania (Friedman et al, 1993), the association of this enzyme with bipolar disorder has been demonstrated from different aspects of preclinical and clinical studies that have been comprehensively summarized in several expert reviews (DiazGranados and Zarate, 2008; Manji and Chen, 2002; Manji and Lenox, 1999; Zarate and Manji, 2009). Particularly important is that both lithium and valproic acid have a PKC-inhibitory effect (Chen et al, 1994; Hahn et al, 2005; Wang and Friedman, 1989), suggesting a role of PKC as a therapeutic target in the treatment of bipolar mania. Additionally, several isoforms of PKC can be activated by Gq protein-coupled receptors such as α1-adrenergic receptor, M1/3 muscarinic cholinergic receptors, and metabolic glutamate receptors (discussed in the above sections). PKC is also a downstream acceptor of tyrosine kinase receptors that are activated by growth factors and neurotrophins (Rankin et al, 2008; Zirrgiebel et al, 1995). Although not discussed in detail in this review, it is widely known that neurotrophins have major contributions in the pathophysiology of mood disorders (Duman and Monteggia, 2006; Martinowich et al, 2007; Post, 2007; Warner-Schmidt and Duman, 2008).
As tamoxifen, an antiestrogen agent for breast cancer suppression, has a PKC-inhibitory property (O'Brian et al, 1985), this clinically available agent has been tested for its efficacy in bipolar mania in several pilot clinical studies. Among them, two studies investigated the efficacy of tamoxifen monotherapy in double-blind, placebo-controlled clinical trials (n=16 and n=66, respectively) (Yildiz et al, 2008; Zarate et al, 2007). Although the sample sizes were small, 3-week tamoxifen treatment was associated with significant improvement in manic symptoms. Other studies have also tested the effect of tamoxifen as an adjunct to lithium or divalproex (Amrollahi et al, 2011; Bebchuk et al, 2000; Kulkarni et al, 2006), all of which demonstrated superior anti-manic effect of tamoxifen.
In their original study, O'Brian et al (1985) reported that tamoxifen inhibits the activity of partially purified PKC extracted from brain tissue. Although tamoxifen did not directly interfere with the catalytic unit of the enzyme, it was suggested that the lipophilic tamoxifen competes with phospholipid for the regulatory domain of the enzyme (O'Brian et al, 1985; Su et al, 1985) (Figure 1). The PKC-inhibitory effect of tamoxifen in intact cells (Horgan et al, 1986) is relatively selective as it has no inhibitory effect on cAMP-dependent protein kinase-A (Spacey et al, 1990), indicating that inhibition of PKC is a potential mechanism of tamoxifen action.
Clinical trial of tamoxifen for bipolar mania is perhaps the first proof-of-concept investigation of protein kinase inhibitors in the treatment of mood disorders, although several issues about this hypothesized treatment remain to be resolved. The obvious antiestrogen effect of tamoxifen cannot be ruled out in clinical studies unless selective PKC inhibitors are found to have similar anti-manic actions. The secondary effect of hormonal changes after tamoxifen treatment in mood regulation also remains to be clarified in studies using other antiestrogenic agents that lack PKC-inhibitory action as comparative control. Additionally, the adverse effect of the antiestrogen in disrupting female hormone regulation and developing malignancy set the limit on using this agent in the general population for treating bipolar mania. Its long-term mood-stabilizing effect has not been investigated, possibly because of a concern for its disruptive action in hormonal regulation. Despite the logistical concerns about tamoxifen, current data from tamoxifen studies encourage developing PKC inhibitors for potential clinical application in mood disorders.
Over 50 years of successful clinical use and mechanistic studies of lithium have further suggested that regulation of intracellular signaling beyond membrane receptors contributes to the mood-stabilizing actions of lithium. Among many investigated lithium actions, GSK3 has arisen as a promising therapeutic target of mood disorders. GSK3 (Embi et al, 1980), including GSK3α and GSK3β, are paralogous protein kinases that are universally expressed. In brain, GSK3 regulates many aspects of neuronal function such as gene expression, neurogenesis, synaptic plasticity, neuronal structure, and neuronal survival and death (Doble and Woodgett, 2003; Frame and Cohen, 2001; Jope and Johnson, 2004). Unlike many other protein kinases, both GSK3 isoforms are constitutively active (ie, partially active in the absence of activation), and they are regulated predominantly in an inhibitory manner (Doble and Woodgett, 2003) by several protein kinases (Cross et al, 1995; Fang et al, 2000; Goode et al, 1992; Li et al, 2000) that phosphorylate GSK3 at an N-terminal serine residue, serine-21 of GSK3α and serine-9 of GSK3β (Stambolic and Woodgett, 1994; Sutherland and Cohen, 1994; Sutherland et al, 1993) (Figure 1).
GSK3 was identified as a site of lithium action in 1996 when two independent research groups (Klein and Melton, 1996; Stambolic et al, 1996) reported that lithium selectively competes with magnesium that is required for the kinase activity of GSK3 (Ryves and Harwood, 2001). Importantly, the direct inhibition by lithium, which has a low potency at an IC50 of 2 mM, can be robustly enhanced by increasing the inhibitory phosphorylation of the N-terminal serine of GSK3 in animal brains and human peripheral blood mononuclear cells (PBMCs) at therapeutically relevant lithium concentrations (Chalecka-Franaszek and Chuang, 1999; De Sarno et al, 2002; Li et al, 2007a), including evidence that the level of phospho-serine-9 of GSK3β is eightfold higher in bipolar patients stabilized on lithium treatment than in healthy controls who are not exposed to lithium (Li et al, 2007a). This secondary effect of lithium on GSK3 could be mediated by the inhibition of the upstream regulator Akt, inhibition of protein phosphatase-1, or disruption of a β-arrestin/Akt/PP2A complex (Beaulieu et al, 2008a; Chalecka-Franaszek and Chuang, 1999; Pan et al, 2011; Zhang et al, 2003).
GSK3 is not only a biological target of lithium, it is also one of the few identified molecules that can be inhibited by many existing pharmacological agents used in mood disorders. These include the anticonvulsant mood stabilizer valproate (Aubry et al, 2009; Chen et al, 1999; De Sarno et al, 2002; Kim et al, 2005; Kozlovsky et al, 2006; Lamarre and Desrosiers, 2008; Phiel et al, 2001; Werstuck et al, 2004), most atypical antipsychotics (Alimohamad et al, 2005; Li et al, 2007b; Roh et al, 2007), and the monoamine reuptake inhibitor antidepressants fluoxetine and imipramine (Beaulieu et al, 2008b; Li et al, 2004). All these agents indirectly increase the level of phospho-serine of GSK3, resulting in the inactive status of the enzyme (Li and Jope, 2010b). These pharmacological studies on animals have recently been reinforced in the clinical treatment of bipolar mania, where an 8-week combination treatment of acutely manic patients with lithium or valproic acid plus an atypical antipsychotic caused a significant increase in serine phosphorylation of GSK3 in PBMCs (Li et al, 2010c). Therefore, inhibition of GSK3 is a common mechanism of action shared by a variety of drugs that treat mood disorders, and GSK3 serves as a target for both anti-manic and anti-depressive treatments.
The common action of mood disorder treatments on GSK3 concurs with evidence showing that GSK3 is regulated by neuromodulators involved in mood disorders. BDNF, a neurotrophin upregulated by antidepressants (Duman and Monteggia, 2006; Schmidt and Duman, 2007), phosphorylates the N-terminal serine of GSK3 by activating Akt (Cross et al, 1995; Mai et al, 2002), resulting in the inhibition of GSK3 (Johnson-Farley et al, 2006; Mai et al, 2002). GSK3 is also under the regulation of serotonin and dopamine. In the mouse brain, enhancing serotonergic activity by d-fenfluramine or activation of 5-HT1A receptors increases the serine phosphorylation of GSK3 in several brain regions (Li et al, 2004). Conversely, in serotonin-deficient mice that carry mutation of the tryptophan hydroxylase-2 gene associated with major depression (Zhang et al, 2005), serine phosphorylation of GSK3 is low and GSK3 activity is elevated (Beaulieu et al, 2008b). By contrast, elevation of extracellular dopamine in dopamine transporter-knockout mice was shown to reduce the serine phosphorylation of GSK3 in the striatum, an effect that was reversed by the administration of a dopamine D2 receptor antagonist (Beaulieu et al, 2004). Regulation of GSK3 by D2 receptors involves a protein complex including the scaffolding protein β-arrestin2, protein phosphatase-2 (PP2A), and Akt (Beaulieu et al, 2005), where Akt is inactivated and was incapable of phosphorylating and inhibiting GSK3.
Besides being regulated by phosphorylation, GSK3 activity can be buffered by its association with protein partners. An example of this is its association with the axin protein complex (Behrens et al, 1998; Rubinfeld et al, 1996) where it phosphorylates β-catenin and facilitates β-catenin degradation by the proteasome (Davies et al, 2001; Henderson, 2000). Activation of Wnt, another signaling pathway that is associated with mood disorders (Gould et al, 2007; Inkster et al, 2010; Zandi et al, 2008), results in the dissociation of the axin complex (Doble et al, 2007), which terminates the protein complex-dependent action of GSK3.
In animal studies, GSK3 has been shown to be an important regulator of mood-related behaviors. Transgenic mice overexpressing constitutively active GSK3β show hyperactivity in the open field test and increased acoustic startle response (Prickaerts et al, 2006), suggesting that excessive GSK3β could be a precipitating factor in heightened locomotor activity and sensory responses. The behavioral effects of active GSK3 have been further characterized recently (Polter et al, 2010a) in mice with serine-to-alanine mutations that block the inhibitory serine phosphorylation of both GSK3α and GSK3β (McManus et al, 2005). A striking feature of these mice is that they show increased susceptibility to both amphetamine-induced hyperactivity and stress-induced depressive-like behaviors. This reinforces the postulation that abnormal activation of GSK3 under conditions resembling lack of regulation by neuromodulators is a risk factor for developing mood-related behavioral disturbances. Indeed, a postmortem study of suicide subjects reported increased activity of GSK3β in depressed but not in non-depressed samples (Karege et al, 2007). In PBMCs from a small group of human subjects, the phosphorylated serine of both GSK3α and GSK3β was found to be lower in symptomatic bipolar patients than healthy controls (Polter et al, 2010a), and the reduction is significantly correlated with the severity of manic and depressive symptoms. Therefore, human studies support evidence from animal behavior studies indicating that GSK3 activity is affected in mood disorders.
Conversely, GSK3β haploinsufficient (lacking one copy of the gene encoding GSK3β) mice have reduced immobility in the forced swim test, increased exploratory activity (O'Brien et al, 2004), and reduced amphetamine-induced hyperactivity (Beaulieu et al, 2004). Reducing GSK3β in these animals is also effective in normalizing the impaired tail suspension behavior in serotonin-deficient mice that otherwise have increased GSK3 activity (Beaulieu et al, 2008b). Similarly, GSK3α-knockout mice show decreased exploratory activity, decreased immobility time in the forced swim test, and reduced aggressive behavior (Kaidanovich-Beilin et al, 2009), suggesting that inhibition of both GSK3α and GSK3β is important in behavior regulation. Significantly, these data also indicate that targeting inhibition of GSK3 may achieve mood stabilization, preventing the behavioral disturbance of not only mania but also depression.
Therefore, ample data from pharmacological, neurochemical, and behavioral studies provide strong evidence that GSK3 is a highly promising therapeutic target in the treatment of mood disorders. However, while lithium inhibits GSK3, it also has other intracellular effects; therefore, inhibition of GSK3 may be a component of the intracellular lithium actions, but it may not explain the full effect of lithium in mood disorders. Tests of selective GSK3 inhibitors are needed. Currently available GSK3 inhibitors are mostly small-molecule ATP competitors (Cohen and Goedert, 2004; Martinez et al, 2006; Meijer et al, 2004). Among them, AR-A014418, SB216763, indirubin-3-monoxime, alsterpaullone, TDZD, NP031115, and BIP-135 have been reported to either cause an antidepressant-like behavioral effect or ameliorate hyperactivity, or have both effects in animal behavior tests (Beaulieu et al, 2004; Chen et al, 2011; Gould et al, 2004; Pan et al, 2011; Rosa et al, 2008), suggesting that these GSK3-selective inhibitors have mood-regulating effects.
However, there may be legitimate difficulties in developing therapeutic agents by inhibiting protein kinases. Protein kinase inhibitors may have non-targeting effect as their selectivity is relative to their affinity to one or more protein kinases. As for GSK3, its wide distribution in non-neural tissues may cause side effects. GSK3 also have significant physiological functions as a constitutively active protein kinase. Extreme suppression of brain GSK3 activity may therefore perturb normal brain function regulated by GSK3. To increase the selectivity of GSK3 inhibitors, substrate-mimicking peptides that block GSK3 access to its substrates is an alternative approach. A small peptide GSK3 inhibitor, L803-mts, when administered through intracerebroventricular injection in rats, has been shown to reduce immobility in the forced swim test (Kaidanovich-Beilin et al, 2004). More recently, virus-guided GSK3β-shRNA, when directly delivered into the hippocampus, is shown to exhibit an antidepressant effect in an animal model of chronic depression (Omata et al, 2011), suggesting a brain regional effect of GSK3 inhibitors. All these GSK3 inhibitors have only been tested in animal models, whereas the clinical implications of these GSK3-targeting approaches remain to be evaluated for their efficiency and safety in mood stabilization.
FUTURE DIRECTIONS
Most currently available pharmacological treatments of mood disorders are related to mechanisms of action discovered decades ago. After a long fallow period, there has been important recent progress in testing drugs with novel mechanisms of action. However, substantial obstacles in advancing the pharmacological therapy of mood disorders remain to be addressed. Ideally, new drug development needs to be advanced to (1) design effective clinical trials to test the specific effects of drugs; (2) identify biomarkers for treatment selection and outcome prediction; and (3) target specific molecular mechanisms involved in mood disorder pathophysiology.
As noted by Gelenberg et al (2008), modifications of traditional clinical trial designs are needed for mood disorders, particularly studies in depression. These changes include (1) broadening the inclusion criteria to involve patients typically treated in the clinical settings in which a drug is likely to be used; (2) the use of measures (eg, the Montgomery–Åsberg Depression Rating Scale (Montgomery and Asberg, 1979) or the Quick Inventory of Depressive Symptomatology (Rush et al, 2003)) that are more sensitive to change; and (3) conducting moderator analyses to identify specific subgroups most likely to respond to a given treatment; moderators of treatment response may include clinical characteristics, genetics, or other biomarkers (Kraemer et al, 2003). Given the heterogeneity of mood disorders, a study design with broad inclusion of all types of depression (major depression, dysthymia, bipolar-I and II depression, depression with substantial medical comorbidity such as diabetes) may delineate clinical response patterns with increased translational appeal. Furthermore, owing to the multiple etiologies of mood disorders, it is to be expected that a single treatment will not target the entire spectrum of conditions. Traditionally, clinical trials of mood disorders use standardized depression and mania rating scales that assess core mood symptoms, which do not provide a comprehensive assessment of the spectrum of mood symptoms. When new drugs targeting different receptor pharmacology or molecular mechanisms are developed, classical assessment of mood disorders may not be sufficient to identify new treatment with unique mechanisms as hypothesized or tested in preclinical studies.
Several examples of successfully using dimensional rather than traditional assessment of mood have emerged in clinical trials. For example, Tomarken et al (2004) compared the effects of bupropion vs placebo by using the Mood and Anxiety Symptom Questionnaire, derived from the tripartite of Clark and Watson (1991), which assesses mood in three dimensions: positive affect (corresponding to mood symptoms such as low energy, motivation, and enjoyment), general distress (more closely related to the psychological symptoms of anxiety as seen in generalized anxiety disorder), and somatic anxiety (physiological arousal, which is found in panic disorder). Bupropion exerted a more robust effect on the positive affective dimension than either general distress or somatic anxiety, which is consistent with its effects in the catecholamine system (Shelton and Tomarken, 2001). Similar effects have been shown with modafinil augmentation trial in antidepressant non-responders (Fava et al, 2005, 2007); modafinil was shown to improve symptoms of fatigue and daytime somnolence in treatment-resistant depression. By contrast, Tang et al (2009) showed that the SSRI paroxetine has robust effects on the personality/temperament dimensions of neuroticism (an enduring trait of negative emotional reactions to real or anticipated stress) and extraversion relative to placebo. In fact, the drug–placebo differences on the personality dimensions were much more robust than that seen with the Hamilton Rating Scale for Depression (Hamilton, 1960). The effects of an SSRI on the neuroticism dimension may be consistent with the constraining effects of the SSRI class on the amygdala response to perceived threat (Sheline et al, 2001). Therefore, clinical assessments targeting the hypothesized mechanism of drug action should be routinely included in mood disorder clinical trials for the proof-of-concept new drug development with a unique mechanism of action.
Another outstanding need in mood disorder treatment is to identify biological tests with predictive ability to assist treatment selection for individual patients who may have different response to drugs. Pharmacogenomic approaches have produced only very modest associations accounting for a very little proportion of the variance in outcome (Binder and Holsboer, 2006; Keers and Aitchison, 2010). Alternative biomarker approaches targeting specific mechanisms of action of treatments may be more successful. For example, several studies (Sen et al, 2008; Yasui-Furukori et al, 2011) have shown that the plasma level of BDNF increases in response to antidepressant treatment, which occurs as early as 2 weeks of treatment (Yasui-Furukori et al, 2011). The findings in human samples are valid as they are consistent with findings in brains of animals treated with antidepressants (Duman and Monteggia, 2006). If a correlation between the increase in serum BDNF and the degree of clinical response to antidepressant treatment can be determined, BDNF measurement may serve as a surrogate marker to predict individual response to monoamine-regulating antidepressants. When new treatments with different mechanisms are under development, earlier biomarker identification even during the clinical trial period may facilitate biomarker application in the prediction of treatment response to each group of drugs with specific mechanisms. Genetic and peripheral biomarkers, when combined with a selected brain-imaging test, would be a valuable subjective indicator of treatment response in addition to clinical assessment.
As reviewed in this article, various new drugs that target non-monoamine systems have been tested in preclinical and clinical studies as alternative treatment for mood disorders. It is notable that most drugs in clinical trials act at cell-surface neurotransmitter receptors, which may regulate several signal transduction pathways (Figures 1 and 2) and elicit more than one physiological response. Alternatively, drugs specifically targeting signal transduction and other intracellular elements may emerge as important therapeutic approaches. In preclinical studies, many intracellular molecules have shown prominent roles in the neuropathology of mood disorders (see Table 1 for some examples), and some may have potential to be developed as therapeutic targets for mood disorders. However, outstanding challenges remain as most these intracellular molecules are ubiquitously expressed in both neural and non-neural tissues, and activation or inhibition of some has a brain region-specific effect. It is more likely that before successfully developing drugs targeting these important mood-regulating molecules, research is needed to understand the neuron- and brain region-specific function of each one of them. This review did not discuss these molecules in detail as drugs targeting them have not been investigated for clinical implication, but this is one of the promising future areas of research for advanced treatment of mood disorders.
Table 1. Representative Cellular Molecules with Potential Roles as Mood Disorder Therapeutic Targets.
Acknowledgments
We thank Dr Karen L Gamble for critical reading of a major section of the review, and Dr Richard S Jope for scientific comments.
Xiaohua Li, MD, PhD: Received grant support from NIMH, the American Foundation for Suicide Prevention (AFSP), Corcept, Ortho-McNeil-Janssen, Otsuka, and Novartis.
Review activities (such as data and safety monitoring boards): VA Research Tuscaloosa.
Mark A Frye, MD: Received grant support from NIMH, NIAAA, the National Alliance for Schizophrenia and Depression (NARSAD), the Mayo Foundation, and Pfizer.
Consultant: Dainippon Sumittomo Pharma, Merck, and Sepracor.
CME supported activity: Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly and Co., GlaxoSmithKline, Merck, Otsuka Pharmaceuticals, Pfizer, and Sanofi-Aventis.
Speakers' bureau: None.
Richard C Shelton, MD: Received research support from NIMH, AHRQ, NARSAD, Bristol-Myers Squibb, Eli Lilly and Company, Euthymics Bioscience, Forest Pharmaceuticals, Janssen Pharmaceutica, Novartis Pharmaceuticals, Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., Repligen Corp., and St Jude Medical.
Consultation: Eli Lilly and Company, Cyberonics Inc., Evotec AG, Forest Pharmaceuticals, Gideon Richter PLC, Janssen Pharmaceutica, Medronic Inc., Otsuka Pharmaceuticals, Pamlab Inc., Pfizer Inc., Repligen Inc., and Sierra Neuropharmaceuticals.
Review activities (such as data and safety monitoring boards, statistical analysis, endpoint committees, etc.): Pfizer Inc., Medtronic Inc., and Cyberonics Inc.
References
- Akimova E, Lanzenberger R, Kasper S. The serotonin-1A receptor in anxiety disorders. Biol Psychiatry. 2009;66:627–635. doi: 10.1016/j.biopsych.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- Alexander B, Warner-Schmidt J, Eriksson T, Tamminga C, Arango-Lievano M, Ghose S, et al. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci Transl Med. 2010;2:54ra76. doi: 10.1126/scitranslmed.3001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Alt A, Nisenbaum ES, Bleakman D, Witkin JM. A role for AMPA receptors in mood disorders. Biochem Pharmacol. 2006;71:1273–1288. doi: 10.1016/j.bcp.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Altamura C, Maes M, Dai J, Meltzer HY. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur Neuropsychopharmacol. 1995;5 (Suppl:71–75. doi: 10.1016/0924-977x(95)00033-l. [DOI] [PubMed] [Google Scholar]
- Altamura CA, Mauri MC, Ferrara A, Moro AR, D'Andrea G, Zamberlan F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry. 1993;150:1731–1733. doi: 10.1176/ajp.150.11.1731. [DOI] [PubMed] [Google Scholar]
- Amrollahi Z, Rezaei F, Salehi B, Modabbernia AH, Maroufi A, Esfandiari GR, et al. Double-blind, randomized, placebo-controlled 6-week study on the efficacy and safety of the tamoxifen adjunctive to lithium in acute bipolar mania. J Affect Disord. 2011;129:327–331. doi: 10.1016/j.jad.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Bodkin JA. Selegiline transdermal system in the prevention of relapse of major depressive disorder: a 52-week, double-blind, placebo-substitution, parallel-group clinical trial. J Clin Psychopharmacol. 2006;26:579–586. doi: 10.1097/01.jcp.0000239794.37073.70. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: role of strain, test and sex. Behav Pharmacol. 2009;20:286–295. doi: 10.1097/FBP.0b013e32832c713e. [DOI] [PubMed] [Google Scholar]
- Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, et al. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- Arborelius L. 5-HT1A receptor antagonists as putative adjuvants to antidepressants: preclinical and clinical evidence. IDrugs. 1999;2:121–128. [PubMed] [Google Scholar]
- Ashmore LJ, Sehgal A. A fly's eye view of circadian entrainment. J Biol Rhythms. 2003;18:206–216. doi: 10.1177/0748730403018003003. [DOI] [PubMed] [Google Scholar]
- Aubry JM, Schwald M, Ballmann E, Karege F. Early effects of mood stabilizers on the Akt/GSK-3beta signaling pathway and on cell survival and proliferation. Psychopharmacology (Berl) 2009;205:419–429. doi: 10.1007/s00213-009-1551-2. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. 2011NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses Nature 47591–95.In mouse models, ketamine and other NMDAR antagonists produce fast-acting behavioral antidepressant-like effects that depend on the rapid synthesis of brain-derived neurotrophic factor. Ketamine-mediated blockade of NMDAR at rest deactivates eukaryotic elongation factor 2 kinase (CaMKIII), resulting in reduced eEF2 phosphorylation and de-suppression of translation of brain-derived neurotrophic factor, whereas inhibition of CaMKIII induces fast-acting behavioral antidepressant-like effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Pretorius HW, Constant EL, Earley WR, Szamosi J, Brecher M. Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry. 2009;70:540–549. doi: 10.4088/jcp.08m04629. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008a;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. 2004Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade Proc Natl Acad Sci USA 1015099–5104.In the mouse striatum, increased DA neurotransmission arising either from administration of amphetamine or from the lack of the DA transporter results in inactivation of Akt and concomitant activation of GSK-3alpha and GSK-3beta. These biochemical changes are effectively reversed either by inhibition of DA synthesis, D2 receptor blockade, or administration of lithium salts. Furthermore, pharmacological or genetic inhibition of GSK-3 significantly reduces DA-dependent locomotor behaviors. [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. 2008bRole of GSK3 beta in behavioral abnormalities induced by serotonin deficiency Proc Natl Acad Sci USA 1051333–1338.Knock-in mice expressing a rare human variant (R441H) mutant of the brain tryptophan hydroxylase 2 have markedly reduced brain 5-HT production and behavioral abnormalities in tests assessing 5-HT-mediated emotional states. The reduction in brain 5-HT levels in these mice is accompanied by activation of GSK3beta, while inactivation of GSK3beta using pharmacological or genetic approaches alleviates the aberrant behaviors produced by 5-HT deficiency. [Google Scholar]
- Bebchuk JM, Arfken CL, Dolan-Manji S, Murphy J, Hasanat K, Manji HK. A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch Gen Psychiatry. 2000;57:95–97. doi: 10.1001/archpsyc.57.1.95. [DOI] [PubMed] [Google Scholar]
- Beckmann H, Moises HW. The cholinolytic biperiden in depression. An acute placebo controlled study. Arch Psychiatr Nervenkr. 1982;231:213–220. doi: 10.1007/BF00343291. [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- Benavides J, Camelin JC, Mitrani N, Flamand F, Uzan A, Legrand JJ, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission—II. Biochemical properties. Neuropharmacology. 1985;24:1085–1092. doi: 10.1016/0028-3908(85)90196-0. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Radaelli D, Bernasconi A, Dallaspezia S, Falini A, Scotti G, et al. Clock genes beyond the clock: CLOCK genotype biases neural correlates of moral valence decision in depressed patients. Genes Brain Behav. 2008;7:20–25. doi: 10.1111/j.1601-183X.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Serretti A, Colombo C, Barbini B, Lorenzi C, Campori E, et al. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123B:23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:843–853. doi: 10.4088/jcp.v68n0604. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR.1982Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands Biochem J 206587–595.Lithium inhibits myo-inositol 1-phosphatase, which amplifies the agonist-dependent accumulation of myo-inositol 1-phosphate, but lowers the level of myo-inositol that leads to a decrease in the concentration of phosphatidylinositol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berti M, Baciarello M, Troglio R, Fanelli G. Clinical uses of low-dose ketamine in patients undergoing surgery. Curr Drug Targets. 2009;10:707–715. doi: 10.2174/138945009788982496. [DOI] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS.2011Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice Mol Psychiatrye-pub ahead of print 19 April 2011. [DOI] [PMC free article] [PubMed]
- Binder EB, Holsboer F. Pharmacogenomics and antidepressant drugs. Ann Med. 2006;38:82–94. doi: 10.1080/07853890600551045. [DOI] [PubMed] [Google Scholar]
- Blier P, Szabo ST. Potential mechanisms of action of atypical antipsychotic medications in treatment-resistant depression and anxiety. J Clin Psychiatry. 2005;66 (Suppl 8:30–40. [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression. Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Bonanno G, Giambelli R, Raiteri L, Tiraboschi E, Zappettini S, Musazzi L, et al. Chronic antidepressants reduce depolarization-evoked glutamate release and protein interactions favoring formation of SNARE complex in hippocampus. J Neurosci. 2005;25:3270–3279. doi: 10.1523/JNEUROSCI.5033-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Kelly L, Aluisio L, Shelton J, Lord B, Galici R, et al. Selective blockade of 5-hydroxytryptamine (5-HT)7 receptors enhances 5-HT transmission, antidepressant-like behavior, and rapid eye movement sleep suppression induced by citalopram in rodents. J Pharmacol Exp Ther. 2007;321:690–698. doi: 10.1124/jpet.107.119404. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Calabrese JR, Sachs G, Yatham LN, Asghar SA, Hompland M, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Arch Gen Psychiatry. 2003;60:392–400. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- Brann MR, Ellis J, Jorgensen H, Hill-Eubanks D, Jones SV. Muscarinic acetylcholine receptor subtypes: localization and structure/function. Prog Brain Res. 1993;98:121–127. doi: 10.1016/s0079-6123(08)62388-2. [DOI] [PubMed] [Google Scholar]
- Brennan BP, Hudson JI, Jensen JE, McCarthy J, Roberts JL, Prescot AP, et al. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cade JF.1949Lithium salts in the treatment of psychotic excitement Med J Aust 2349–352.The first case report of anti-manic effect of lithium in ten bipolar manic patients. As comparison, lithium had no therapeutic effect on psychosis and did not induce depression. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Bowden CL, Sachs G, Yatham LN, Behnke K, Mehtonen OP, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64:1013–1024. doi: 10.4088/jcp.v64n0906. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Keck PE, Jr, Macfadden W, Minkwitz M, Ketter TA, Weisler RH, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162:1351–1360. doi: 10.1176/appi.ajp.162.7.1351. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Ketter TA, Youakim JM, Tiller JM, Yang R, Frye MA. Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder: a randomized, multicenter, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry. 2010;71:1363–1370. doi: 10.4088/JCP.09m05900gry. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Cermakian N, Sassone-Corsi P. Multilevel regulation of the circadian clock. Nat Rev Mol Cell Biol. 2000;1:59–67. doi: 10.1038/35036078. [DOI] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46:457–467. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc Natl Acad Sci USA. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Edwards RH. The glutamine commute: take the N line and transfer to the A. J Cell Biol. 2002;157:349–355. doi: 10.1083/jcb.200201070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Henter ID, Manji HK. Translational research in bipolar disorder: emerging insights from genetically based models. Mol Psychiatry. 2010;15:883–895. doi: 10.1038/mp.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Manji HK, Hawver DB, Wright CB, Potter WZ. Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J Neurochem. 1994;63:2361–2364. doi: 10.1046/j.1471-4159.1994.63062361.x. [DOI] [PubMed] [Google Scholar]
- Chen L, Salinas GD, Li X.2009Regulation of serotonin 1B receptor by glycogen synthase kinase-3 Mol Pharmacol 761150–1161.GSK3beta directly interacts with 5-HT1B receptors, but not 5-HT1A receptors, at the intracellular loop-2, and the interaction is required for agonist-induced inhibition of cyclic AMP production and surface receptor expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Zhou W, Chen P, Gaisina I, Yang S, Li X.2011Glycogen synthase kinase-3beta is a functional modulator of serotonin 1B receptors Mol Pharmacol 79974–986.GSK3beta selectively modulates 5-HT1B receptor-regulated Gialpha signaling, but not recruitment of beta-arrestin2. Inhibition of GSK3beta differentially affects behavioral effect of 5-HT1B receptor activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H, Kamp D, Harris E. An in vitro investigation of the action of lamotrigine on neuronal voltage-activated sodium channels. Epilepsy Res. 1992;13:107–112. doi: 10.1016/0920-1211(92)90065-2. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann NY Acad Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL.2000Comparison of pramipexole, fluoxetine, and placebo in patients with major depression Depress Anxiety 1158–65.In a randomized, double-blind, parallel-group study, pramipexole was tested in 174 patients with major depression, and was compared to fluoxetine and placebo. By week-8 endpoint, patients receiving pramipexole had significant improvement over baseline compared to the placebo group, and significant improvement was also seen at other time points. [DOI] [PubMed] [Google Scholar]
- Crane GE. Cycloserine as an antidepressant agent. Am J Psychiatry. 1959;115:1025–1026. doi: 10.1176/ajp.115.11.1025. [DOI] [PubMed] [Google Scholar]
- Creson TK, Hao Y, Engel S, Shen Y, Hamidi A, Zhuo M, et al. The anterior cingulate ERK pathway contributes to regulation of behavioral excitement and hedonic activity. Bipolar Disord. 2009;11:339–350. doi: 10.1111/j.1399-5618.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Watkins JC. Analogues of glutamic and gamma-amino-n-butyric acids having potent actions on mammalian neurones. Nature. 1961;191:1010–1011. doi: 10.1038/1911010a0. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA. Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- Davies G, Jiang WG, Mason MD. The interaction between beta-catenin, GSK3beta and APC after motogen induced cell–cell dissociation, and their involvement in signal transduction pathways in prostate cancer. Int J Oncol. 2001;18:843–847. doi: 10.3892/ijo.18.4.843. [DOI] [PubMed] [Google Scholar]
- De Luca V, Mueller DJ, de Bartolomeis A, Kennedy JL. Association of the HTR2C gene and antipsychotic induced weight gain: a meta-analysis. Int J Neuropsychopharmacol. 2007;10:697–704. doi: 10.1017/S1461145707007547. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS.2002Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium Neuropharmacology 431158–1164.This study demonstrated the first evidence that therapeutic-relevant lithium treatment in mice increases phospho-Ser9–GSK3beta in brain. Additionally, treatment of mice with valproate and other HDAC inhibitors also increase phospho-Ser9–GSK3beta in brain. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl--aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010a;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl--aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010b;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiazGranados N, Zarate CA., Jr A review of the preclinical and clinical evidence for protein kinase C as a target for drug development for bipolar disorder. Curr Psychiatry Rep. 2008;10:510–519. doi: 10.1007/s11920-008-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47 (6 Suppl 4:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116 (Part 7:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical–amygdalar metabolism in major depression. Ann NY Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Furey ML. Replication of scopolamine's antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Creson TK, Wu LJ, Ren M, Gray NA, Falke C, et al. 2008The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior J Neurosci 2868–79.Chronic treatment of rats with therapeutically relevant concentrations of lithium or valproate reduced hippocampal synaptosomal GluR1 levels and surface GluR1 distribution, an effect mediated by reducing GluR1 phosphorylation at a specific PKA site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Gray NA, Falke CA, Chen W, Yuan P, Szabo ST, et al. Modulation of synaptic plasticity by antimanic agents: the role of AMPA glutamate receptor subunit 1 synaptic expression. J Neurosci. 2004;24:6578–6589. doi: 10.1523/JNEUROSCI.1258-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Suzuki K, Wei Y, Wang Y, Blumenthal R, Chen Z, et al. 2007The anticonvulsants lamotrigine, riluzole, and valproate differentially regulate AMPA receptor membrane localization: relationship to clinical effects in mood disorders Neuropsychopharmacology 32793–802.Lamotrigine and riluzole significantly enhanced the surface expression of GluR1 and GluR2, which is in contrast to the reduced surface expression of GluR1 and GluR2 by valproate. Concomitantly, lamotrigine and riluzole, as well as the traditional antidepressant imipramine, increased PKA-dependent GluR1 phosphorylation. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Grear KE, Hoskins MA. Aging and SB-269970-A, a selective 5-HT7 receptor antagonist, attenuate circadian phase advances induced by microinjections of serotonergic drugs in the hamster dorsal raphe nucleus. Brain Res. 2004;1008:40–48. doi: 10.1016/j.brainres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Co-expression of serotonin 5-HT(1B) and 5-HT(4) receptors in p11 containing cells in cerebral cortex, hippocampus, caudate-putamen and cerebellum. Neuropharmacology. 2011;61:442–450. doi: 10.1016/j.neuropharm.2011.01.046. [DOI] [PubMed] [Google Scholar]
- Elfving B, Plougmann PH, Wegener G. Differential brain, but not serum VEGF levels in a genetic rat model of depression. Neurosci Lett. 2010;474:13–16. doi: 10.1016/j.neulet.2010.02.063. [DOI] [PubMed] [Google Scholar]
- Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, et al. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–189. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- El-Yousef MK, Janowsky DS, Davis JM, Rosenblatt JE. Induction of severe depression by physostigmine in marihuana intoxicated individuals. Br J Addict Alcohol Other Drugs. 1973;68:321–325. doi: 10.1111/j.1360-0443.1973.tb01264.x. [DOI] [PubMed] [Google Scholar]
- Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107:519–527. [PubMed] [Google Scholar]
- Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2009;14:448–461. doi: 10.1038/sj.mp.4002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal S, Yagen B, Sobol E, Altschuler Y, Shmuel M, Bialer M. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia. 2004;45:737–744. doi: 10.1111/j.0013-9580.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- Fang X, Yu SX, Lu Y, Bast RC, Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA. 2000;97:11960–11965. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Alpert J, Nierenberg AA, Mischoulon D, Otto MW, Zajecka J, et al. A fouble-blind, randomized trial of St John's wort, fluoxetine, and placebo in major depressive disorder. J Clin Psychopharmacol. 2005;25:441–447. doi: 10.1097/01.jcp.0000178416.60426.29. [DOI] [PubMed] [Google Scholar]
- Fava M, Thase ME, DeBattista C, Doghramji K, Arora S, Hughes RJ. Modafinil augmentation of selective serotonin reuptake inhibitor therapy in MDD partial responders with persistent fatigue and sleepiness. Ann Clin Psychiatry. 2007;19:153–159. doi: 10.1080/10401230701464858. [DOI] [PubMed] [Google Scholar]
- Feiger AD, Rickels K, Rynn MA, Zimbroff DL, Robinson DS. Selegiline transdermal system for the treatment of major depressive disorder: an 8-week, double-blind, placebo-controlled, flexible-dose titration trial. J Clin Psychiatry. 2006;67:1354–1361. doi: 10.4088/jcp.v67n0905. [DOI] [PubMed] [Google Scholar]
- Fontaine R. Novel serotonergic mechanisms and clinical experience with nefazodone. Clin Neuropharmacol. 1993;16 (Suppl 3:S45–S50. [PubMed] [Google Scholar]
- Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359 (Part 1:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TW, Clothier JL, Pazzaglia P, Lesem MD, Swann AC. A double-blind comparison of valproate and lithium in the treatment of acute mania. Am J Psychiatry. 1992;149:108–111. doi: 10.1176/ajp.149.1.108. [DOI] [PubMed] [Google Scholar]
- Friedman E, Hoau Yan W, Levinson D, Connell TA, Singh H.1993Altered platelet protein kinase C activity in bipolar affective disorder, manic episode Biol Psychiatry 33520–525.Protein kinase C (PKC) activity and translocation in response to serotonin were investigated in platelets obtained from bipolar disorder subjects before and during lithium treatment. Ratios of platelet membrane-bound to cytosolic PKC activities were elevated in the manic subjects, and serotonin-elicited platelet PKC translocation was enhanced in those subjects. Lithium treatment for up to 2 weeks resulted in a reduction in cytosolic and membrane-associated PKC activities and in an attenuated PKC translocation in response to serotonin. [DOI] [PubMed] [Google Scholar]
- Frye MA. Clinical practice. Bipolar disorder—a focus on depression. N Engl J Med. 2011;364:51–59. doi: 10.1056/NEJMcp1000402. [DOI] [PubMed] [Google Scholar]
- Frye MA, Grunze H, Suppes T, McElroy SL, Keck PE, Jr, Walden J, et al. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry. 2007a;164:1242–1249. doi: 10.1176/appi.ajp.2007.06060981. [DOI] [PubMed] [Google Scholar]
- Frye MA, Helleman G, McElroy SL, Altshuler LL, Black DO, Keck PE, Jr, et al. Correlates of treatment-emergent mania associated with antidepressant treatment in bipolar depression. Am J Psychiatry. 2009;166:164–172. doi: 10.1176/appi.ajp.2008.08030322. [DOI] [PubMed] [Google Scholar]
- Frye MA, Ketter TA, Kimbrell TA, Dunn RT, Speer AM, Osuch EA, et al. A placebo controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607–614. doi: 10.1097/00004714-200012000-00004. [DOI] [PubMed] [Google Scholar]
- Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry. 2007b;61:162–166. doi: 10.1016/j.biopsych.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep–wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21:482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajwani P, Forsthoff A, Muzina D, Amann B, Gao K, Elhaj O, et al. Antiepileptic drugs in mood-disordered patients. Epilepsia. 2005;46 (Suppl 4:38–44. doi: 10.1111/j.1528-1167.2005.463008.x. [DOI] [PubMed] [Google Scholar]
- Gao M, Jin Y, Yang K, Zhang D, Lukas RJ, Wu J. Mechanisms involved in systemic nicotine-induced glutamatergic synaptic plasticity on dopamine neurons in the ventral tegmental area. J Neurosci. 2010;30:13814–13825. doi: 10.1523/JNEUROSCI.1943-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194:4–9. doi: 10.1192/bjp.bp.107.048504. [DOI] [PubMed] [Google Scholar]
- Gelenberg AJ, Thase ME, Meyer RE, Goodwin FK, Katz MM, Kraemer HC, et al. The history and current state of antidepressant clinical trial design: a call to action for proof-of-concept studies. J Clin Psychiatry. 2008;69:1513–1528. doi: 10.4088/jcp.v69n1001. [DOI] [PubMed] [Google Scholar]
- George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol. 2008;28:340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- Ghanbari R, El Mansari M, Shahid M, Blier P. Electrophysiological characterization of the effects of asenapine at 5-HT(1A), 5-HT(2A), alpha(2)-adrenergic and D(2) receptors in the rat brain. Eur Neuropsychopharmacol. 2009;19:177–187. doi: 10.1016/j.euroneuro.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Giannini AJ, Underwood NA, Condon M. Acute ketamine intoxication treated by haloperidol: a preliminary study. Am J Ther. 2000;7:389–391. doi: 10.1097/00045391-200007060-00008. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Lauriello J, Kelsoe JR, Rapaport M, Golshan S, Kenny WM, et al. No antidepressant effect of biperiden compared with placebo in depression: a double-blind 6-week clinical trial. Psychiatry Res. 1995;58:99–105. doi: 10.1016/0165-1781(95)02700-7. [DOI] [PubMed] [Google Scholar]
- Gillin JC, Sutton L, Ruiz C, Darko D, Golshan S, Risch SC, et al. The effects of scopolamine on sleep and mood in depressed patients with a history of alcoholism and a normal comparison group. Biol Psychiatry. 1991;30:157–169. doi: 10.1016/0006-3223(91)90170-q. [DOI] [PubMed] [Google Scholar]
- Glass JD, Grossman GH, Farnbauch L, DiNardo L. Midbrain raphe modulation of nonphotic circadian clock resetting and 5-HT release in the mammalian suprachiasmatic nucleus. J Neurosci. 2003;23:7451–7460. doi: 10.1523/JNEUROSCI.23-20-07451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Slater S, Boucher N, Debonnel G, Blier P. Neurochemical and psychotropic effects of bupropion in healthy male subjects. J Clin Psychopharmacol. 2003;23:233–239. doi: 10.1097/01.jcp.0000084023.22282.03. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet JM, Cistarelli L, Melon C, Millan MJ. Buspirone modulates basal and fluoxetine-stimulated dialysate levels of dopamine, noradrenaline and serotonin in the frontal cortex of freely moving rats: activation of serotonin1A receptors and blockade of alpha2-adrenergic receptors underlie its actions. Neuroscience. 1999;93:1251–1262. doi: 10.1016/s0306-4522(99)00211-0. [DOI] [PubMed] [Google Scholar]
- Goddard GV. Development of epileptic seizures through brain stimulation at low intensity. Nature. 1967;214:1020–1021. doi: 10.1038/2141020a0. [DOI] [PubMed] [Google Scholar]
- Goldberg HL. Buspirone—a new antianxiety agent not chemically related to any presently marketed drugs [proceedings] Psychopharmacol Bull. 1979;15:90–92. [PubMed] [Google Scholar]
- Goldberg HL, Finnerty RJ. The comparative efficacy of buspirone and diazepam in the treatment of anxiety. Am J Psychiatry. 1979;136:1184–1187. doi: 10.1176/ajp.136.9.1184. [DOI] [PubMed] [Google Scholar]
- Goldberg JF, Burdick KE, Endick CJ. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry. 2004;161:564–566. doi: 10.1176/appi.ajp.161.3.564. [DOI] [PubMed] [Google Scholar]
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry. 2005;162:656–662. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992;267:16878–16882. [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR.2007Manic–Depressive Illness: Bipolar Disorders and Recurrent Depression2nd edn.Oxford University Press: New York [Google Scholar]
- Goodwin GM, Emsley R, Rembry S, Rouillon F.2009Agomelatine prevents relapse in patients with major depressive disorder without evidence of a discontinuation syndrome: a 24-week randomized, double-blind, placebo-controlled trial J Clin Psychiatry 701128–1137.In the trial, patients with DSM-IV-TR major depressive disorder who responded to an 8- or 10-week course of agomelatine were randomly assigned to receive continuation treatment with agomelatine (n=165) or placebo (n=174) during a 24-week, randomized, double-blind treatment period. During the 6-month evaluation period, the incidence of relapse (using the Kaplan–Meier method of survival analysis) was significantly lower in patients who continued agomelatine treatment than in those switched to placebo (P=0.0001). Agomelatine was also superior to placebo in preventing relapse in the subset of patients with baseline 17-item Hamilton Depression Rating Scale total score ⩾25. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O'Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, O'Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34:2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvich N, Klein PS. Lithium and valproic acid: parallels and contrasts in diverse signaling contexts. Pharmacol Ther. 2002;96:45–66. doi: 10.1016/s0163-7258(02)00299-1. [DOI] [PubMed] [Google Scholar]
- Guscott M, Bristow LJ, Hadingham K, Rosahl TW, Beer MS, Stanton JA, et al. 2005Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression Neuropharmacology 48492–502.5-HT7 receptor knockout mice have significantly decreased immobility in the Porsolt swim test, treatment of wild type mice with SB-258719, a selective 5-HT7 receptor antagonist, produces a significant decrease in immobility in the dark (or active) cycle, and circadian rhythm phase shifts to 8-OH-DPAT are attenuated in the 5-HT7 receptor knockout mice. The study provides the first direct evidence that 5-HT7 receptor antagonists should be investigated for efficacy in the treatment of depression. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Umapathy, Wang HY, Koneru R, Levinson DF, Friedman E. Lithium and valproic acid treatments reduce PKC activation and receptor-G protein coupling in platelets of bipolar manic patients. J Psychiatr Res. 2005;39:355–363. doi: 10.1016/j.jpsychires.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Hajos-Korcsok E, McQuade R, Sharp T. Influence of 5-HT1A receptors on central noradrenergic activity: microdialysis studies using (+/−)-MDL 73005EF and its enantiomers. Neuropharmacology. 1999;38:299–306. doi: 10.1016/s0028-3908(98)00175-0. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Hemmeter UM, Hemmeter-Spernal J, Krieg JC. Sleep deprivation in depression. Expert Rev Neurother. 2010;10:1101–1115. doi: 10.1586/ern.10.83. [DOI] [PubMed] [Google Scholar]
- Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA. A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc Natl Acad Sci USA. 2008;105:20746–20751. doi: 10.1073/pnas.0811410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan K, Cooke E, Hallett MB, Mansel RE. Inhibition of protein kinase C mediated signal transduction by tamoxifen. Importance for antitumour activity. Biochem Pharmacol. 1986;35:4463–4465. doi: 10.1016/0006-2952(86)90764-1. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Depression during tobacco abstinence. Nicotine Tob Res. 2007;9:443–446. doi: 10.1080/14622200701243185. [DOI] [PubMed] [Google Scholar]
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem. 2005;280:29397–29402. doi: 10.1074/jbc.M503526200. [DOI] [PubMed] [Google Scholar]
- Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- Inkster B, Nichols TE, Saemann PG, Auer DP, Holsboer F, Muglia P, et al. Pathway-based approaches to imaging genetics association studies: Wnt signaling, GSK3beta substrates and major depression. Neuroimage. 2010;53:908–917. doi: 10.1016/j.neuroimage.2010.02.065. [DOI] [PubMed] [Google Scholar]
- Jackson ME, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA. 2004;101:8467–8472. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic–adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Januel D, Massot O, Poirier MF, Olie JP, Fillion G. Interaction of lithium with 5-HT(1B) receptors in depressed unipolar patients treated with clomipramine and lithium versus clomipramine and placebo: preliminary results. Psychiatry Res. 2002;111:117–124. doi: 10.1016/s0165-1781(02)00136-1. [DOI] [PubMed] [Google Scholar]
- Johnson-Farley NN, Travkina T, Cowen DS. Cumulative activation of akt and consequent inhibition of glycogen synthase kinase-3 by brain-derived neurotrophic factor and insulin-like growth factor-1 in cultured hippocampal neurons. J Pharmacol Exp Ther. 2006;316:1062–1069. doi: 10.1124/jpet.105.094433. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GVW. The glamour and gloom of glycogen synthase kinase-3. Trehds in Biochemical Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberte C, et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol Brain. 2009;2:35. doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Kaladchibachi SA, Doble B, Anthopoulos N, Woodgett JR, Manoukian AS. Glycogen synthase kinase 3, circadian rhythms, and bipolar disorder: a molecular link in the therapeutic action of lithium. J Circadian Rhythms. 2007;5:3. doi: 10.1186/1740-3391-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nicotine Tob Res. 2005;7:317–333. doi: 10.1080/14622200500125385. [DOI] [PubMed] [Google Scholar]
- Kang TC, Kim DS, Kwak SE, Kim JE, Kim DW, Kang JH, et al. Valproic acid reduces enhanced vesicular glutamate transporter immunoreactivities in the dentate gyrus of the seizure prone gerbil. Neuropharmacology. 2005;49:912–921. doi: 10.1016/j.neuropharm.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, et al. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007;61:240–245. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Kasper S, Hajak G, Wulff K, Hoogendijk WJ, Montejo AL, Smeraldi E, et al. Efficacy of the novel antidepressant agomelatine on the circadian rest–activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry. 2010;71:109–120. doi: 10.4088/JCP.09m05347blu. [DOI] [PubMed] [Google Scholar]
- Kasper S, Moises HW, Beckmann H. The anticholinergic biperiden in depressive disorders. Pharmacopsychiatria. 1981;14:195–198. doi: 10.1055/s-2007-1019597. [DOI] [PubMed] [Google Scholar]
- Keers R, Aitchison KJ. Gender differences in antidepressant drug response. Int Rev Psychiatry. 2010;22:485–500. doi: 10.3109/09540261.2010.496448. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Rizvi S, Fulton K, Rasmussen J. A double-blind comparison of sexual functioning, antidepressant efficacy, and tolerability between agomelatine and venlafaxine XR. J Clin Psychopharmacol. 2008;28:329–333. doi: 10.1097/JCP.0b013e318172b48c. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Rizvi SJ. Agomelatine in the treatment of major depressive disorder: potential for clinical effectiveness. CNS Drugs. 2010;24:479–499. doi: 10.2165/11534420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kim AJ, Shi Y, Austin RC, Werstuck GH. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J Cell Sci. 2005;118 (Part 1:89–99. doi: 10.1242/jcs.01562. [DOI] [PubMed] [Google Scholar]
- Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. Effects of amitriptyline on serum glutamate and free tryptophan in rats. Arch Psychiatr Nervenkr. 1982a;232:391–394. doi: 10.1007/BF00345595. [DOI] [PubMed] [Google Scholar]
- Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr. 1982b;232:299–304. doi: 10.1007/BF00345492. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA.1996A molecular mechanism for the effect of lithium on development Proc Natl Acad Sci USA 938455–8459.Lithium potently inhibits GSK-3 beta activity (Ki=2 mM) in vitro, but is not a general inhibitor of other protein kinases. Lithium treatment phenocopies loss of GSK-3β function in Xenopus and Dictyostelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacol Ther. 1992;56:53–78. doi: 10.1016/0163-7258(92)90037-z. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Cole JO. Clinical pharmacology of imipramine and related antidepressant compounds. Pharmacol Rev. 1965;17:101–141. [PubMed] [Google Scholar]
- Koller M, Urwyler S. Novel N-methyl--aspartate receptor antagonists: a review of compounds patented since 2006. Expert Opin Ther Pat. 2010;20:1683–1702. doi: 10.1517/13543776.2010.533656. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Galloway MP, Duman RS, Russell DS, D'Sa C. Repeated unpredictable stress and antidepressants differentially regulate expression of the bcl-2 family of apoptotic genes in rat cortical, hippocampal, and limbic brain structures. Neuropsychopharmacology. 2008;33:1545–1558. doi: 10.1038/sj.npp.1301527. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Amar S, Belmaker RH, Agam G. Psychotropic drugs affect Ser9-phosphorylated GSK-3beta protein levels in rodent frontal cortex. Int J Neuropsychopharmacol. 2006;9:337–342. doi: 10.1017/S1461145705006097. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects. Arch Gen Psychiatry. 2003;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Kranaster L, Kammerer-Ciernioch J, Hoyer C, Sartorius A.2011Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: a retrospective study Eur Arch Psychiatry Clin Neuroscie-pub ahead of print 19 April 2011. [DOI] [PubMed]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J, Garland KA, Scaffidi A, Headey B, Anderson R, de Castella A, et al. A pilot study of hormone modulation as a new treatment for mania in women with bipolar affective disorder. Psychoneuroendocrinology. 2006;31:543–547. doi: 10.1016/j.psyneuen.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Lamarre M, Desrosiers RR. Upregulation of protein l-isoaspartyl methyltransferase expression by lithium is mediated by glycogen synthase kinase-3 inactivation and beta-catenin stabilization. Neuropharmacology. 2008;55:669–676. doi: 10.1016/j.neuropharm.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Leach MJ, Marden CM, Miller AA. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II Neurochemical studies on the mechanism of action. Epilepsia. 1986;27:490–497. doi: 10.1111/j.1528-1157.1986.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Lee CY, Fu WM, Chen CC, Su MJ, Liou HH.2008Lamotrigine inhibits postsynaptic AMPA receptor and glutamate release in the dentate gyrus Epilepsia 49888–897.Lamotrigine suppresses postsynaptic AMPA receptors and reduces glutamate release in granule cells of dentate gyrus. [DOI] [PubMed] [Google Scholar]
- Lee KY, Song JY, Kim SH, Kim SC, Joo EJ, Ahn YM, et al. Association between CLOCK 3111T/C and preferred circadian phase in Korean patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1196–1201. doi: 10.1016/j.pnpbp.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology. 2008;33:134–140. doi: 10.1038/sj.npp.1301569. [DOI] [PubMed] [Google Scholar]
- Levine J, Panchalingam K, Rapoport A, Gershon S, McClure RJ, Pettegrew JW. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol Psychiatry. 2000;47:586–593. doi: 10.1016/s0006-3223(99)00284-x. [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol. 2000;20:9356–9363. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. 2010amTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists Science 329959–964.Ketamine rapidly activated the mammalian target of rapamycin (mTOR) pathway, leading to increased synaptic signaling proteins and increased number and function of new spine synapses in the prefrontal cortex of rats. Blockade of mTOR signaling via Erk and Akt completely blocked ketamine induction of synaptogenesis and behavioral responses in models of depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl--aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Friedman AB, Zhu W, Wang L, Boswell S, May RS, et al. 2007aLithium regulates glycogen synthase kinase-3beta in human peripheral blood mononuclear cells: implication in the treatment of bipolar disorder Biol Psychiatry 61216–222.The level of phospho-Ser9–GSK3beta in PBMCs responded to agents that modify GSK3-regulating kinases and phosphatases and was increased by in vitro lithium treatment. More important, phospho-Ser9–GSK3beta levels were eightfold higher in PBMCs from lithium-treated bipolar than healthy control subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation. Neuropsychopharmacology. 2010b;35:2143–2154. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Liu M, Cai Z, Wang G, Li X. Regulation of glycogen synthase kinase-3 during bipolar mania treatment. Bipolar Disord. 2010c;12:741–752. doi: 10.1111/j.1399-5618.2010.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Need AB, Baez M, Witkin JM. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J Pharmacol Exp Ther. 2006;319:254–259. doi: 10.1124/jpet.106.103143. [DOI] [PubMed] [Google Scholar]
- Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007b;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS.2004In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain Neuropsychopharmacology 291426–1431.Enhancing endogenous serotonin release by d-fenfluramine, blocking serotonin reuptake by fluoxetine, and activating 5-HT1A receptors all increase phosphor-Ser9–GSK3beta in brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Moussaif M, Kuan CJ, Gargus JJ, Sze JY. Serotonin targets the DAF-16/FOXO signaling pathway to modulate stress responses. Cell Metab. 2006;4:429–440. doi: 10.1016/j.cmet.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Lien R, Flaisher-Grinberg S, Cleary C, Hejny M, Einat H. Behavioral effects of Bcl-2 deficiency: implications for affective disorders. Pharmacol Rep. 2008;60:490–498. [PubMed] [Google Scholar]
- Loo H, Dalery J, Macher JP, Payen A. Pilot study comparing in blind the therapeutic effect of two doses of agomelatine, melatoninergic agonist and selective 5HT2C receptors antagonist, in the treatment of major depressive disorders. Encephale. 2002a;28:356–362. [PubMed] [Google Scholar]
- Loo H, Hale A, D'Haenen H.2002bDetermination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT(2C) antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study Int Clin Psychopharmacol 17239–247.In a double-blind trial, three different doses of agomelatine were compared with placebo and paroxetine over an 8-week treatment period. Agomelatine 25 mg demonstrated to be statistically more effective than placebo on the mean final HAM-D total score (Full Analysis Set LOCF); and the result was confirmed by other analyses and criteria (responders, remission, subpopulation of severely depressed patients, Montgomery–Asberg Depression Rating Scale, Clinical Global Impression-Severity of Illness). Agomelatine showed good acceptability with a side-effects profile close to that of placebo. Paroxetine was found to be effective on pivotal analysis and most of the secondary criteria used to validate the study methodology and population. [DOI] [PubMed] [Google Scholar]
- Lopez-Gil X, Babot Z, Amargos-Bosch M, Sunol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- Lucas G. Serotonin receptors, type 4: a new hope. Curr Drug Targets. 2009;10:1085–1095. doi: 10.2174/138945009789735200. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55:712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36 (Suppl 2:S2–12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. 2008Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors Biol Psychiatry 63349–352.Subanesthetic doses of ketamine treatment caused acute and sustained antidepressant-like effects without impairing fear memory retention. Pretreatment with an AMPA receptor antagonist NBQX attenuated both ketamine-induced antidepressant-like behavior and regulation of hippocampal phosphorylated GluR1 AMPA receptors. NMDA antagonists might exert rapid antidepressant-like effects by enhancing AMPA relative to NMDA throughput in critical neuronal circuits. [DOI] [PubMed] [Google Scholar]
- Maes M, Verkerk R, Vandoolaeghe E, Lin A, Scharpe S. Serum levels of excitatory amino acids, serine, glycine, histidine, threonine, taurine, alanine and arginine in treatment-resistant depression: modulation by treatment with antidepressants and prediction of clinical responsivity. Acta Psychiatr Scand. 1998;97:302–308. doi: 10.1111/j.1600-0447.1998.tb10004.x. [DOI] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Jr, Singh J, Duan YF, Luckenbaugh DA, Manji HK, et al. Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biol Psychiatry. 2007;61:765–775. doi: 10.1016/j.biopsych.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Nugent AC, Singh JB, Manji HK, Drevets WC. Neural mechanisms of antidepressant efficacy of the dopamine receptor agonist pramipexole in treatment of bipolar depression. Int J Neuropsychopharmacol. 2010;14:545–551. doi: 10.1017/S1461145710001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai L, Jope RS, Li X. BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J Neurochem. 2002;82:75–83. doi: 10.1046/j.1471-4159.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- Manji HK, Chen G. PKC, MAP kinases and the bcl-2 family of proteins as long-term targets for mood stabilizers. Mol Psychiatry. 2002;7 (Suppl 1:S46–S56. doi: 10.1038/sj.mp.4001018. [DOI] [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Ziskind–Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic–depressive illness. Biol Psychiatry. 1999;46:1328–1351. doi: 10.1016/s0006-3223(99)00235-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mao Z, Liu L, Zhang R, Li X. Lithium reduces FoxO3a transcriptional activity by decreasing its intracellular content. Biol Psychiatry. 2007;62:1423–1430. doi: 10.1016/j.biopsych.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28:156–165. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- Marenholz I, Heizmann CW, Fritz G. S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature) Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Brown CS, Moore TR. Atypical antipsychotics. Part I: pharmacology, pharmacokinetics, and efficacy. Ann Pharmacother. 1999;33:73–85. doi: 10.1345/aph.17215. [DOI] [PubMed] [Google Scholar]
- Martin P, Beninger RJ, Hamon M, Puech AJ. Antidepressant-like action of 8-OH-DPAT, a 5-HT1A agonist, in the learned helplessness paradigm: evidence for a postsynaptic mechanism. Behav Brain Res. 1990;38:135–144. doi: 10.1016/0166-4328(90)90011-3. [DOI] [PubMed] [Google Scholar]
- Martin P, Tissier MH, Adrien J, Puech AJ. Antidepressant-like effects of buspirone mediated by the 5-HT1A post-synaptic receptors in the learned helplessness paradigm. Life Sci. 1991;48:2505–2511. doi: 10.1016/0024-3205(91)90605-b. [DOI] [PubMed] [Google Scholar]
- Martinek S, Inonog S, Manoukian AS, Young MW. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell. 2001;105:769–779. doi: 10.1016/s0092-8674(01)00383-x. [DOI] [PubMed] [Google Scholar]
- Martinez A, Castro A, Medina M. Glycogen Synthase Kinase 3 (GSK-3) and its Inhibitors. John Wiley and Sons Inc.: Hoboken, New Jersey; 2006. [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Massot O, Rousselle JC, Fillion MP, Januel D, Plantefol M, Fillion G. 5-HT1B receptors: a novel target for lithium. Possible involvement in mood disorders. Neuropsychopharmacology. 1999;21:530–541. doi: 10.1016/S0893-133X(99)00042-1. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri MC, Ferrara A, Boscati L, Bravin S, Zamberlan F, Alecci M, et al. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology. 1998;37:124–129. doi: 10.1159/000026491. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology (Berl) 2006;189:125–133. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel WW, Sahota AK, Vyas BV, Laguerta N, Hategan L, Oswald J. Ketamine appears associated with better word recall than etomidate after a course of 6 electroconvulsive therapies. J ECT. 2006;22:103–106. doi: 10.1097/00124509-200606000-00005. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Hiroi R, Mackenzie SM, Robin NC, Cohn A, Kim JJ, et al. Serotonin 1B autoreceptors originating in the caudal dorsal raphe nucleus reduce expression of fear and depression-like behavior. Biol Psychiatry. 2011;69:780–787. doi: 10.1016/j.biopsych.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Weisler RH, Chang W, Olausson B, Paulsson B, Brecher M, et al. A double-blind, placebo-controlled study of quetiapine and paroxetine as monotherapy in adults with bipolar depression (EMBOLDEN II) J Clin Psychiatry. 2010;71:163–174. doi: 10.4088/JCP.08m04942gre. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Winstanley EL, Martens B, Patel NC, Mori N, Moeller D, et al. A randomized, placebo-controlled study of adjunctive ramelteon in ambulatory bipolar I disorder with manic symptoms and sleep disturbance. Int Clin Psychopharmacol. 2011;26:48–53. doi: 10.1097/YIC.0b013e3283400d35. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, et al. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty S, Ross AW, Barrett P, Hastings MH, Morgan PJ. Melatonin regulates the phosphorylation of CREB in ovine pars tuberalis. J Neuroendocrinol. 1994;6:523–532. doi: 10.1111/j.1365-2826.1994.tb00615.x. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ. The extended protein kinase C superfamily. Biochem J. 1998;332 (Part 2:281–292. doi: 10.1042/bj3320281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21 (2 Suppl:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. The ratios of serotonin2 and dopamine2 affinities differentiate atypical and typical antipsychotic drugs. Psychopharmacol Bull. 1989;25:390–392. [PubMed] [Google Scholar]
- Michael-Titus AT, Bains S, Jeetle J, Whelpton R. Imipramine and phenelzine decrease glutamate overflow in the prefrontal cortex—a possible mechanism of neuroprotection in major depression. Neuroscience. 2000;100:681–684. doi: 10.1016/s0306-4522(00)00390-0. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Kamal M, Jockers R, Chanrion B, Labasque M, et al. The melatonergic agonist and clinically active antidepressant, agomelatine, is a neutral antagonist at 5-HT2C receptors. Int J Neuropsychopharmacol. 2010;14:768–783. doi: 10.1017/S1461145710001045. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gundisch D, et al. Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther. 2009;329:377–386. doi: 10.1124/jpet.108.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Somenzi O, Picciotto MR. Cytisine, a partial agonist of high-affinity nicotinic acetylcholine receptors, has antidepressant-like properties in male C57BL/6J mice. Neuropharmacology. 2007;52:1256–1262. doi: 10.1016/j.neuropharm.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Mishory A, Winokur M, Bersudsky Y. Prophylactic effect of phenytoin in bipolar disorder: a controlled study. Bipolar Disord. 2003;5:464–467. doi: 10.1046/j.1399-5618.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- Mishory A, Yaroslavsky Y, Bersudsky Y, Belmaker RH. Phenytoin as an antimanic anticonvulsant: a controlled study. Am J Psychiatry. 2000;157:463–465. doi: 10.1176/appi.ajp.157.3.463. [DOI] [PubMed] [Google Scholar]
- Mitchell HA, Bogenpohl JW, Liles LC, Epstein MP, Bozyczko-Coyne D, Williams M, et al. Behavioral responses of dopamine beta-hydroxylase knockout mice to modafinil suggest a dual noradrenergic-dopaminergic mechanism of action. Pharmacol Biochem Behav. 2008;91:217–222. doi: 10.1016/j.pbb.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molchan SE, Martinez RA, Hill JL, Weingartner HJ, Thompson K, Vitiello B, et al. Increased cognitive sensitivity to scopolamine with age and a perspective on the scopolamine model. Brain Res Brain Res Rev. 1992;17:215–226. doi: 10.1016/0165-0173(92)90017-g. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo MT. Antidepressant-like and anxiolytic-like actions of the mGlu5 receptor antagonist MTEP, microinjected into lateral septal nuclei of male Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1129–1135. doi: 10.1016/j.pnpbp.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PJ, Barrett P, Howell HE, Helliwell R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance—a review of abuse liability issues. Ann Clin Psychiatry. 2004;16:101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM, et al. The effects of acute scopolamine in geriatric depression. Arch Gen Psychiatry. 1988;45:906–912. doi: 10.1001/archpsyc.1988.01800340028004. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian CA, Liskamp RM, Solomon DH, Weinstein IB.1985Inhibition of protein kinase C by tamoxifen Cancer Res 452462–2465.Tamoxifen was found to inhibit rat brain protein kinase C in vitro when activated by Ca2+, phorbol ester, or teleocidin in the presence of phospholipid. Tamoxifen does not inhibit the Ca2+- and phospholipid-independent activity of protein kinase C, indicating that the drug does not interact with the active site of the enzyme; whereas the binding of [3H]phorbol dibutyrate to high-affinity membrane receptors is inhibited by tamoxifen. The study suggests that the growth-inhibitory and cytotoxic effects of tamoxifen may be in part due to its effects on protein kinase C. [PubMed] [Google Scholar]
- O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, et al. 2004Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium J Neurosci 246791–6798.Chronic lithium treatment and GSK3beta haploinsufficiency altered specific behaviors of mice similarly, which was accompanied by increased beta-catenin, a GSK3-regulated protein, in adult brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony CM, Bravo JA, Dinan TG, Cryan JF. Comparison of hippocampal metabotropic glutamate receptor 7 (mGlu7) mRNA levels in two animal models of depression. Neurosci Lett. 2010;482:137–141. doi: 10.1016/j.neulet.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Ohnishi YN, Ohnishi YH, Hokama M, Nomaru H, Yamazaki K, Tominaga Y, et al. FosB is essential for the enhancement of stress tolerance and antagonizes locomotor sensitization by DeltaFosB. Biol Psychiatry. 2011;70:487–495. doi: 10.1016/j.biopsych.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT. 2010;26:223–227. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- Okuma T, Inanaga K, Otsuki S, Sarai K, Takahashi R, Hazama H, et al. Comparison of the antimanic efficacy of carbamazepine and chlorpromazine: a double-blind controlled study. Psychopharmacology (Berl) 1979;66:211–217. doi: 10.1007/BF00428308. [DOI] [PubMed] [Google Scholar]
- Okuma T, Inanaga K, Otsuki S, Sarai K, Takahashi R, Hazama H, et al. A preliminary double-blind study on the efficacy of carbamazepine in prophylaxis of manic-depressive illness. Psychopharmacology (Berl) 1981;73:95–96. doi: 10.1007/BF00431111. [DOI] [PubMed] [Google Scholar]
- Omata N, Chiu CT, Moya PR, Leng Y, Wang Z, Hunsberger JG, et al. 2011Lentivirally mediated GSK-3beta silencing in the hippocampal dentate gyrus induces antidepressant-like effects in stressed mice Int J Neuropsychopharmacol 14711–714.mice subjected to chronic stress, a single pre-injection of lentivirus-expressing GSK-3beta shRNA into the hippocampal dentate gyrus significantly decreased immobility time in both forced swim and tail suspension tests, while the locomotor activity of these mice was unchanged. Therefore, gene silencing GSK-3beta in the hippocampal dentate gyrus of chronically stressed mice has antidepressant-like effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroff R, Gonzales M, Sanacora G. Antidepressant effect of ketamine during ECT. Am J Psychiatry. 2005;162:1385–1386. doi: 10.1176/appi.ajp.162.7.1385. [DOI] [PubMed] [Google Scholar]
- Pan JQ, Lewis MC, Ketterman JK, Clore EL, Riley M, Richards KR, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36:1397–1411. doi: 10.1038/npp.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks CL, Robinson PS, Sibille E, Shenk T, Toth M. Increased anxiety of mice lacking the serotonin1A receptor. Proc Natl Acad Sci USA. 1998;95:10734–10739. doi: 10.1073/pnas.95.18.10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann NY Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS.2001Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen J Biol Chem 27636734–36741.Valproic acid directly inhibits histone deacetylase. At therapeutic levels, valproic acid causes hyperacetylation of histones in cultured cells and activates transcription from diverse exogenous and endogenous promoters. It is proposed that inhibition of histone deacetylase provides a mechanism for valproic acid-induced birth defects and could also explain the efficacy of valproic acid in the treatment of bipolar disorder. [DOI] [PubMed] [Google Scholar]
- Philip NS, Carpenter LL, Tyrka AR, Whiteley LB, Price LH. Varenicline augmentation in depressed smokers: an 8-week, open-label study. J Clin Psychiatry. 2009;70:1026–1031. doi: 10.4088/jcp.08m04441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol. 2008;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Polter A, Yang S, Zmijewska AA, van Groen T, Paik JH, Depinho RA, et al. Forkhead box, class o transcription factors in brain: regulation and behavioral manifestation. Biol Psychiatry. 2009;65:150–159. doi: 10.1016/j.biopsych.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. 2010aDeficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances Neuropsychopharmacology 351761–1774.(1) GSK3alpha/beta(21A/21A/9A/9A)-knock-in mice (with serine-to-alanine mutations to block inhibitory serine phosphorylation of GSK3) displayed increased susceptibility to amphetamine-induced hyperactivity and to stress-induced depressive-like behaviors; (2) serine phosphorylation of GSK3 was reduced during stress-induced behavioral responses in wild-type mouse brain; and (3) serine phosphorylation of GSK3 was also reduced in peripheral blood mononuclear cells from bipolar disorder patients during a mood episode. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter AM, Li X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell Signal. 2010b;22:1406–1412. doi: 10.1016/j.cellsig.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr, McElroy SL, Keck PE, Jr, Hudson JI. Valproate in the treatment of acute mania. A placebo-controlled study. Arch Gen Psychiatry. 1991;48:62–68. doi: 10.1001/archpsyc.1991.01810250064008. [DOI] [PubMed] [Google Scholar]
- Popik P, Kozela E, Krawczyk M.2003Nicotine and nicotinic receptor antagonists potentiate the antidepressant-like effects of imipramine and citalopram Br J Pharmacol 1391196–1202.In the tail-suspension test of mice, nicotine exerted no effect on immobility, but enhanced the anti-immobility effect of citalopram and mipramine. In addition, the nAChR antagonists mecamylamine and dihydro-β-erythroidine unexpectedly potentiated the anti-immobility effect of imipramine; and mecamylamine also increased the effect of citalopram. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Krawczyk M, Golembiowska K, Nowak G, Janowsky A, Skolnick P, et al. Pharmacological profile of the “triple” monoamine neurotransmitter uptake inhibitor, DOV 102,677. Cell Mol Neurobiol. 2006;26:857–873. doi: 10.1007/s10571-006-9012-5. [DOI] [PubMed] [Google Scholar]
- Post RM.1990aNon-lithium treatment for bipolar disorder J Clin Psychiatry 51(Suppl9–16.discussion 17–19. [PubMed] [Google Scholar]
- Post RM. Sensitization and kindling perspectives for the course of affective illness: toward a new treatment with the anticonvulsant carbamazepine. Pharmacopsychiatry. 1990b;23:3–17. doi: 10.1055/s-2007-1014476. [DOI] [PubMed] [Google Scholar]
- Post RM. Role of BDNF in bipolar and unipolar disorder: clinical and theoretical implications. J Psychiatr Res. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Post RM, Uhde TW, Putnam FW, Ballenger JC, Berrettini WH. Kindling and carbamazepine in affective illness. J Nerv Ment Dis. 1982;170:717–731. doi: 10.1097/00005053-198212000-00002. [DOI] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl--aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Prica C, Hascoet M, Bourin M. Antidepressant-like effect of lamotrigine is reversed by veratrine: a possible role of sodium channels in bipolar depression. Behav Brain Res. 2008;191:49–54. doi: 10.1016/j.bbr.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, et al. 2006Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania J Neurosci 269022–9029.Mice overexpressing GSK-3beta were found to have increased activity and reactivity, with decreased habituation processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins J, Westphal KG, Korte-Bouws GA, Quinton MS, Schreiber R, Olivier B, et al. The potential and limitations of DOV 216,303 as a triple reuptake inhibitor for the treatment of major depression: a microdialysis study in olfactory bulbectomized rats. Pharmacol Biochem Behav. 2011;97:444–452. doi: 10.1016/j.pbb.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Quera-Salva MA, Vanier B, Laredo J, Hartley S, Chapotot F, Moulin C, et al. Major depressive disorder, sleep EEG and agomelatine: an open-label study. Int J Neuropsychopharmacol. 2007;10:691–696. doi: 10.1017/S1461145707007754. [DOI] [PubMed] [Google Scholar]
- Quera-Salva MA, Lemoine P, Guilleminault C. Impact of the novel antidepressant agomelatine on disturbed sleep–wake cycles in depressed patients. Hum Psychopharmacol. 2010;25:222–229. doi: 10.1002/hup.1112. [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl) 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin SL, Guy CS, Rahimtula M, Mearow KM. Neurotrophin-induced upregulation of p75NTR via a protein kinase C-delta-dependent mechanism. Brain Res. 2008;1217:10–24. doi: 10.1016/j.brainres.2008.03.076. [DOI] [PubMed] [Google Scholar]
- Rao JS, Lee HJ, Rapoport SI, Bazinet RP. Mode of action of mood stabilizers: is the arachidonic acid cascade a common target. Mol Psychiatry. 2008;13:585–596. doi: 10.1038/mp.2008.31. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M. Evidence of the activity of lithium on 5-HT1B receptors in the mouse forced swimming test: comparison with carbamazepine and sodium valproate. Psychopharmacology (Berl) 1999;141:370–377. doi: 10.1007/s002130050846. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Richardson G, Wang-Weigand S. Effects of long-term exposure to ramelteon, a melatonin receptor agonist, on endocrine function in adults with chronic insomnia. Hum Psychopharmacol. 2009;24:103–111. doi: 10.1002/hup.993. [DOI] [PubMed] [Google Scholar]
- Riemann D, Hohagen F, Bahro M, Lis S, Stadmuller G, Gann H, et al. Cholinergic neurotransmission, REM sleep and depression. J Psychosom Res. 1994;38 (Suppl 1:15–25. doi: 10.1016/0022-3999(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Roh MS, Seo MS, Kim Y, Kim SH, Jeon WJ, Ahn YM, et al. Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exp Mol Med. 2007;39:353–360. doi: 10.1038/emm.2007.39. [DOI] [PubMed] [Google Scholar]
- Rollema H, Guanowsky V, Mineur YS, Shrikhande A, Coe JW, Seymour PA, et al. Varenicline has antidepressant-like activity in the forced swim test and augments sertraline's effect. Eur J Pharmacol. 2009;605:114–116. doi: 10.1016/j.ejphar.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AO, Kaster MP, Binfare RW, Morales S, Martin-Aparicio E, Navarro-Rico ML, et al. Antidepressant-like effect of the novel thiadiazolidinone NP031115 in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1549–1556. doi: 10.1016/j.pnpbp.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees. Cell Mol Life Sci. 2007;64:2090–2103. doi: 10.1007/s00018-007-7079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- Rosic N, Bignami G. Depression of two-way avoidance learning and enhancement of passive avoidance learning by small doses of physostigmine. Neuropharmacology. 1970;9:311–316. doi: 10.1016/0028-3908(70)90027-4. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. 2007Mania-like behavior induced by disruption of CLOCK Proc Natl Acad Sci USA 1046406–6411.Mice carrying a mutation in the Clock gene display an overall behavioral profile that is strikingly similar to human mania. Chronic administration of the mood stabilizer lithium returns many of the behavioral responses to wild-type levels. The Clock mutant mice have an increase in dopaminergic activity in the ventral tegmental area, and their behavioral abnormalities are rescued by expressing a functional CLOCK protein in the ventral tegmental area. [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC–beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Ruf BM, Bhagwagar Z. The 5-HT1B receptor: a novel target for the pathophysiology of depression. Curr Drug Targets. 2009;10:1118–1138. doi: 10.2174/138945009789735192. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. 2006Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report Am J Psychiatry 1631905–1917.The landmark STAR*D report concluded that hen more treatment steps are required in MDD patients, lower acute remission rates and higher relapse rates are to be expected. Studies to identify the best multistep treatment sequences for individual patients and the development of more broadly effective treatments are needed. [DOI] [PubMed] [Google Scholar]
- Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, et al. 2007Effectiveness of adjunctive antidepressant treatment for bipolar depression N Engl J Med 3561711–1722.The STEP-BD report concluded that the use of adjunctive, standard antidepressant medication, as compared with the use of mood stabilizers, was not associated with increased efficacy or with increased risk of treatment-emergent affective switch. Longer-term outcome studies are needed to fully assess the benefits and risks of antidepressant therapy for bipolar disorder. [DOI] [PubMed] [Google Scholar]
- Salvadore G, Nugent AC, Chen G, Akula N, Yuan P, Cannon DM, et al. Bcl-2 polymorphism influences gray matter volume in the ventral striatum in healthy humans. Biol Psychiatry. 2009;66:804–807. doi: 10.1016/j.biopsych.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, et al. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol Psychiatry. 2007;61:822–825. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer CB, Schaffer LC, Miller AR, Hang E, Nordahl TE. Efficacy and safety of nonbenzodiazepine hypnotics for chronic insomnia in patients with bipolar disorder. J Affect Disord. 2011;128:305–308. doi: 10.1016/j.jad.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Svensson HM, Svensson TH, Nomikos GG. Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 1998;85:1005–1009. doi: 10.1016/s0306-4522(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Kramer G. The new anticonvulsant drugs. Implications for avoidance of adverse effects. Drug Saf. 1994;11:422–431. doi: 10.2165/00002018-199411060-00004. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC., Jr Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. 1998;95:7772–7777. doi: 10.1073/pnas.95.13.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shelton J, Bonaventure P, Li X, Yun S, Lovenberg T, Dugovic C. 5-HT7 receptor deletion enhances REM sleep suppression induced by selective serotonin reuptake inhibitors, but not by direct stimulation of 5-HT1A receptor. Neuropharmacology. 2009;56:448–454. doi: 10.1016/j.neuropharm.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Osuntokun O, Heinloth AN, Corya SA. Therapeutic options for treatment-resistant depression. CNS Drugs. 2010;24:131–161. doi: 10.2165/11530280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Tomarken AJ. Can recovery from depression be achieved. Psychiatr Serv. 2001;52:1469–1478. doi: 10.1176/appi.ps.52.11.1469. [DOI] [PubMed] [Google Scholar]
- Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72:156–167. doi: 10.4088/JCP.09r05385gre. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Krieter P, Tizzano J, Basile A, Popik P, Czobor P, et al. Preclinical and clinical pharmacology of DOV 216,303, a “triple” reuptake inhibitor. CNS Drug Rev. 2006;12:123–134. doi: 10.1111/j.1527-3458.2006.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacey GD, Bonser RW, Randall RW, Garland LG. Selectivity of protein kinase inhibitors in human intact platelets. Cell Signal. 1990;2:329–338. doi: 10.1016/0898-6568(90)90062-f. [DOI] [PubMed] [Google Scholar]
- Sprouse J, Li X, Stock J, McNeish J, Reynolds L. Circadian rhythm phenotype of 5-HT7 receptor knockout mice: 5-HT and 8-OH-DPAT-induced phase advances of SCN neuronal firing. J Biol Rhythms. 2005;20:122–131. doi: 10.1177/0748730404273432. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Stahl SM. The serotonin-7 receptor as a novel therapeutic target. J Clin Psychiatry. 2010;71:1414–1415. doi: 10.4088/JCP.10bs06601gry. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Fava M, Trivedi MH, Caputo A, Shah A, Post A. Agomelatine in the treatment of major depressive disorder: an 8-week, multicenter, randomized, placebo-controlled trial. J Clin Psychiatry. 2010;71:616–626. doi: 10.4088/JCP.09m05471blu. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303 (Part 3:701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankov B, Biella G, Panara C, Lucini V, Capsoni S, Fauteck J, et al. Melatonin signal transduction and mechanism of action in the central nervous system: using the rabbit cortex as a model. Endocrinology. 1992;130:2152–2159. doi: 10.1210/endo.130.4.1312448. [DOI] [PubMed] [Google Scholar]
- Steinberg BJ, Trestman R, Mitropoulou V, Serby M, Silverman J, Coccaro E, et al. Depressive response to physostigmine challenge in borderline personality disorder patients. Neuropsychopharmacology. 1997;17:264–273. doi: 10.1016/S0893-133X(97)00051-1. [DOI] [PubMed] [Google Scholar]
- Su HD, Mazzei GJ, Vogler WR, Kuo JF. Effect of tamoxifen, a nonsteroidal antiestrogen, on phospholipid/calcium-dependent protein kinase and phosphorylation of its endogenous substrate proteins from the rat brain and ovary. Biochem Pharmacol. 1985;34:3649–3653. doi: 10.1016/0006-2952(85)90225-4. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Tariot PN, Mueller EA, Murphy DL, Weingartner H, Cohen RM. Cognitive and behavioral sensitivity to scopolamine in Alzheimer patients and controls. Psychopharmacol Bull. 1985;21:676–679. [PubMed] [Google Scholar]
- Sutherland C, Cohen P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994;338:37–42. doi: 10.1016/0014-5793(94)80112-6. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296 (Part 1:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. 2006Alterations in 5-HT1B receptor function by p11 in depression-like states Science 31177–80.p11 interacts with 5-HT1B receptor, increases localization of 5-HT1B receptors at the cell surface, and increases 5HT1B receptor function in cells. p11 is increased in rodent brains by antidepressants or electroconvulsive therapy, but decreased in an animal model of depression and in brain tissue from depressed patients. p11-knockout mice exhibit a depression-like phenotype and have reduced responsiveness to 5-HT1B receptor agonists and reduced behavioral reactions to an antidepressant. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Greengard P. p11 (S100A10)—an inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Tamburella A, Micale V, Navarria A, Drago F. Antidepressant properties of the 5-HT4 receptor partial agonist, SL65.0155: behavioral and neurochemical studies in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1205–1210. doi: 10.1016/j.pnpbp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Tang TZ, DeRubeis RJ, Hollon SD, Amsterdam J, Shelton R, Schalet B. Personality change during depression treatment: a placebo-controlled trial. Arch Gen Psychiatry. 2009;66:1322–1330. doi: 10.1001/archgenpsychiatry.2009.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Melotto S, Massagrande M, Gribble AD, Jeffrey P, Stevens AJ, et al. SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br J Pharmacol. 2003;139:705–714. doi: 10.1038/sj.bjp.0705290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohen M, Vieta E. Antipsychotic agents in the treatment of bipolar mania. Bipolar Disord. 2009;11 (Suppl 2:45–54. doi: 10.1111/j.1399-5618.2009.00710.x. [DOI] [PubMed] [Google Scholar]
- Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, et al. Efficacy of olanzapine and olanzapine–fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry. 2003;60:1079–1088. doi: 10.1001/archpsyc.60.11.1079. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Dichter GS, Freid C, Addington S, Shelton RC. Assessing the effects of bupropion SR on mood dimensions of depression. J Affect Disord. 2004;78:235–241. doi: 10.1016/S0165-0327(02)00306-3. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Blier P. Catecholaminergic strategies for the treatment of major depression. Curr Drug Targets. 2006;7:149–158. doi: 10.2174/138945006775515464. [DOI] [PubMed] [Google Scholar]
- Turner CA, Akil H, Watson SJ, Evans SJ. The fibroblast growth factor system and mood disorders. Biol Psychiatry. 2006;59:1128–1135. doi: 10.1016/j.biopsych.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Vale S, Espejel MA, Dominguez JC. Amantadine in depression. Lancet. 1971;2:437. doi: 10.1016/s0140-6736(71)90153-x. [DOI] [PubMed] [Google Scholar]
- van der Loos ML, Mulder PG, Hartong EG, Blom MB, Vergouwen AC, de Keyzer HJ, et al. Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70:223–231. doi: 10.4088/jcp.08m04152. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Olivares E, Turek FW, Granjon L, Mocaer E. Resynchronisation of a diurnal rodent circadian clock accelerated by a melatonin agonist. Neuroreport. 1998;9:1901–1905. doi: 10.1097/00001756-199806010-00043. [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello B, Martin A, Hill J, Mack C, Molchan S, Martinez R, et al. Cognitive and behavioral effects of cholinergic, dopaminergic, and serotonergic blockade in humans. Neuropsychopharmacology. 1997;16:15–24. doi: 10.1016/S0893-133X(96)00134-0. [DOI] [PubMed] [Google Scholar]
- Voss B, Thienel R, Reske M, Habel U, Kircher T. Cognitive performance and cholinergic transmission: influence of muscarinic and nicotinic receptor blockade. Eur Arch Psychiatry Clin Neurosci. 2010;260 (Suppl 2:S106–S110. doi: 10.1007/s00406-010-0160-8. [DOI] [PubMed] [Google Scholar]
- Wang HY, Friedman E. Lithium inhibition of protein kinase C activation-induced serotonin release. Psychopharmacology (Berl) 1989;99:213–218. doi: 10.1007/BF00442810. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Chen EY, Zhang X, Marshall JJ, Morozov A, Svenningsson P, et al. A role for p11 in the antidepressant action of brain-derived neurotrophic factor. Biol Psychiatry. 2010;68:528–535. doi: 10.1016/j.biopsych.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8:14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Flajolet M, Maller A, Chen EY, Qi H, Svenningsson P, et al. 2009Role of p11 in cellular and behavioral effects of 5-HT4 receptor stimulation J Neurosci 291937–1946.The study identified a novel p11-interacting receptor, 5-HTR4, wherein p11 and 5-HTR4 mRNA and protein are co-expressed in brain regions, p11 increases 5-HTR4 surface expression and facilitates 5-HTR4 signaling, and p11 is required for the behavioral antidepressant responses to 5-HTR4 stimulation in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb IC, Pollock MS, Mistlberger RE. Modafinil [2-[(diphenylmethyl)sulfinyl]acetamide] and circadian rhythms in syrian hamsters: assessment of the chronobiotic potential of a novel alerting compound. J Pharmacol Exp Ther. 2006;317:882–889. doi: 10.1124/jpet.105.099010. [DOI] [PubMed] [Google Scholar]
- Weisler RH, Keck PE, Jr, Swann AC, Cutler AJ, Ketter TA, Kalali AH. Extended-release carbamazepine capsules as monotherapy for acute mania in bipolar disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2005;66:323–330. doi: 10.4088/jcp.v66n0308. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werstuck GH, Kim AJ, Brenstrum T, Ohnmacht SA, Panna E, Capretta A. Examining the correlations between GSK-3 inhibitory properties and anti-convulsant efficacy of valproate and valproate-related compounds. Bioorg Med Chem Lett. 2004;14:5465–5467. doi: 10.1016/j.bmcl.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K. Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J Pharmacol. 2006a;553:185–190. doi: 10.1016/j.ejphar.2006.09.064. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006b;51:578–586. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Wesolowska A, Tatarczynska E, Nikiforuk A, Chojnacka-Wojcik E. Enhancement of the anti-immobility action of antidepressants by a selective 5-HT7 receptor antagonist in the forced swimming test in mice. Eur J Pharmacol. 2007;555:43–47. doi: 10.1016/j.ejphar.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Whiting P, Lindstrom J. Pharmacological properties of immuno-isolated neuronal nicotinic receptors. J Neurosci. 1986;6:3061–3069. doi: 10.1523/JNEUROSCI.06-10-03061.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieronska JM, Branski P, Siwek A, Dybala M, Nowak G, Pilc A. GABAergic dysfunction in mGlu7 receptor-deficient mice as reflected by decreased levels of glutamic acid decarboxylase 65 and 67 kDa and increased reelin proteins in the hippocampus. Brain Res. 2010;1334:12–24. doi: 10.1016/j.brainres.2010.03.078. [DOI] [PubMed] [Google Scholar]
- Williams RS, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417:292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- Wisor JP, Eriksson KS. Dopaminergic–adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience. 2005;132:1027–1034. doi: 10.1016/j.neuroscience.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Yasui-Furukori N, Tsuchimine S, Nakagami T, Fujii A, Sato Y, Tomita T, et al. 2011Association between plasma paroxetine concentration and changes in plasma brain-derived neurotrophic factor levels in patients with major depressive disorder Hum Psychopharmacole-pub ahead of print 19 April 2011. [DOI] [PubMed]
- Yildiz A, Guleryuz S, Ankerst DP, Ongur D, Renshaw PF. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65:255–263. doi: 10.1001/archgenpsychiatry.2007.43. [DOI] [PubMed] [Google Scholar]
- Yildiz A, Vieta E, Leucht S, Baldessarini RJ. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36:375–389. doi: 10.1038/npp.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science. 2006;311:1002–1005. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- Ying SW, Rusak B, Delagrange P, Mocaer E, Renard P, Guardiola-Lemaitre B. Melatonin analogues as agonists and antagonists in the circadian system and other brain areas. Eur J Pharmacol. 1996;296:33–42. doi: 10.1016/0014-2999(95)00684-2. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Shimazaki T, Ito A, Chaki S. An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berl) 2006;186:587–593. doi: 10.1007/s00213-006-0390-7. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson's disease and depressive illness. Br J Pharmacol. 2006;147 (Suppl 1:S287–S296. doi: 10.1038/sj.bjp.0706464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajecka J, Schatzberg A, Stahl S, Shah A, Caputo A, Post A. Efficacy and safety of agomelatine in the treatment of major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2010;30:135–144. doi: 10.1097/JCP.0b013e3181d420a7. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Belmonte PL, Willour VL, Goes FS, Badner JA, Simpson SG, et al. Association study of Wnt signaling pathway genes in bipolar disorder. Arch Gen Psychiatry. 2008;65:785–793. doi: 10.1001/archpsyc.65.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Manji HK. The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Exp Neurol. 2008;211:7–10. doi: 10.1016/j.expneurol.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Quiroz J, Sporn J, Denicoff KK, Luckenbaugh D, et al. An open-label trial of riluzole in patients with treatment-resistant major depression. Am J Psychiatry. 2004a;161:171–174. doi: 10.1176/appi.ajp.161.1.171. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, et al. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry. 2004b;56:54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, et al. An open-label trial of the glutamate-modulating agent riluzole in combination with lithium for the treatment of bipolar depression. Biol Psychiatry. 2005;57:430–432. doi: 10.1016/j.biopsych.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. 2006aA randomized trial of an N-methyl--aspartate antagonist in treatment-resistant major depression Arch Gen Psychiatry 63856–864.Eighteen subjects with DSM-IV treatment resistant major depression were given an intravenous infusion of either ketamine hydrochloride (0.5 mg/kg) or placebo with a 1-week crossover. Ketamine treatment significantly improved depression within 2 h after injection, which remained significant throughout a 1-week period. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Quiroz J, Jolkovsky L, Luckenbaugh DA, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9:561–570. doi: 10.1111/j.1399-5618.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006b;163:153–155. doi: 10.1176/appi.ajp.163.1.153. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Manji HK. Protein kinase C inhibitors: rationale for use and potential in the treatment of bipolar disorder. CNS Drugs. 2009;23:569–582. doi: 10.2165/00023210-200923070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem. 2003;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- Zhang W, Perry KW, Wong DT, Potts BD, Bao J, Tollefson GD, et al. Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology. 2000;23:250–262. doi: 10.1016/S0893-133X(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Gainetdinov RR, Beaulieu JM, Sotnikova TD, Burch LH, Williams RB, et al. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron. 2005;45:11–16. doi: 10.1016/j.neuron.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Zheng C, Yang K, Liu Q, Wang MY, Shen J, Valles AS, et al. The anticonvulsive drug lamotrigine blocks neuronal {alpha}4{beta}2 nicotinic acetylcholin. J Pharmacol Exp Ther. 2010;335:401–408. doi: 10.1124/jpet.110.171108. [DOI] [PubMed] [Google Scholar]
- Zheng WH, Kar S, Quirion R. FKHRL1 and its homologs are new targets of nerve growth factor Trk receptor signaling. J Neurochem. 2002;80:1049–1061. doi: 10.1046/j.0022-3042.2002.00783.x. [DOI] [PubMed] [Google Scholar]
- Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G, et al. The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. J Neurosci. 2005;25:4493–4502. doi: 10.1523/JNEUROSCI.4530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Bijur GN, Styles NA, Li X. Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol Brain Res. 2004;126:45–56. doi: 10.1016/j.molbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Zirrgiebel U, Ohga Y, Carter B, Berninger B, Inagaki N, Thoenen H, et al. Characterization of TrkB receptor-mediated signaling pathways in rat cerebellar granule neurons: involvement of protein kinase C in neuronal survival. J Neurochem. 1995;65:2241–2250. doi: 10.1046/j.1471-4159.1995.65052241.x. [DOI] [PubMed] [Google Scholar]
- Zona C, Avoli M. Lamotrigine reduces voltage-gated sodium currents in rat central neurons in culture. Epilepsia. 1997;38:522–525. doi: 10.1111/j.1528-1157.1997.tb01135.x. [DOI] [PubMed] [Google Scholar]