Abstract
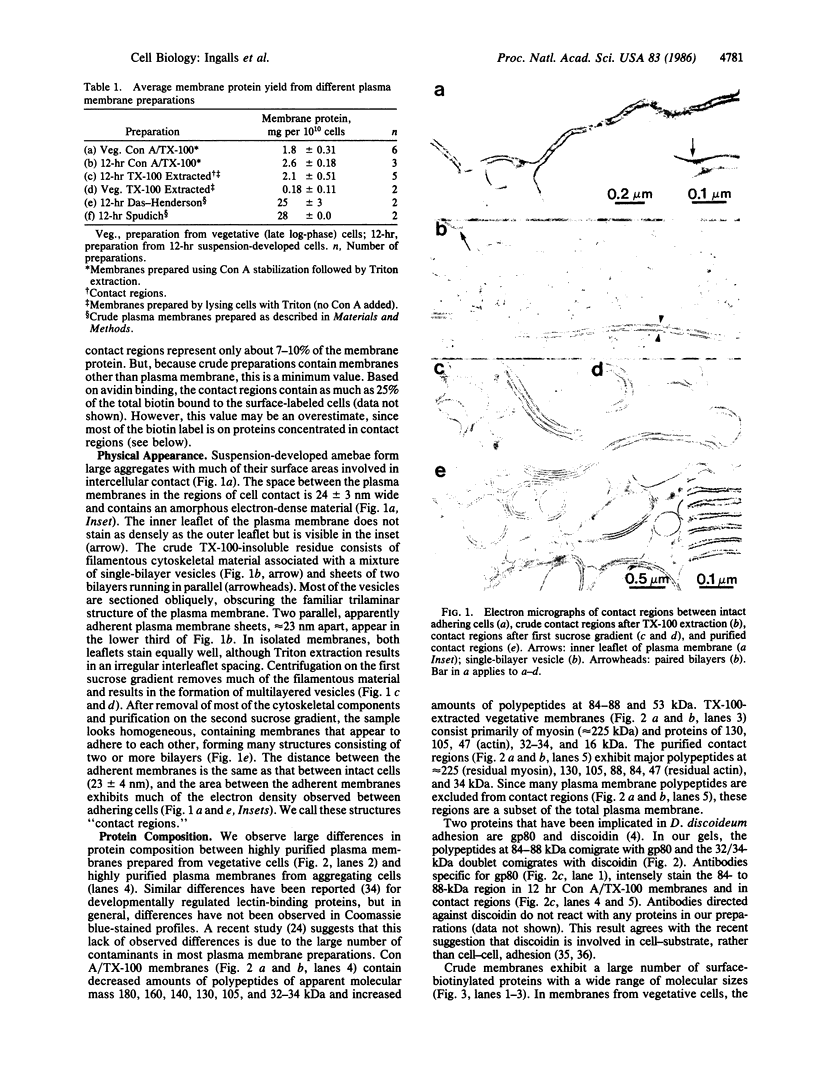
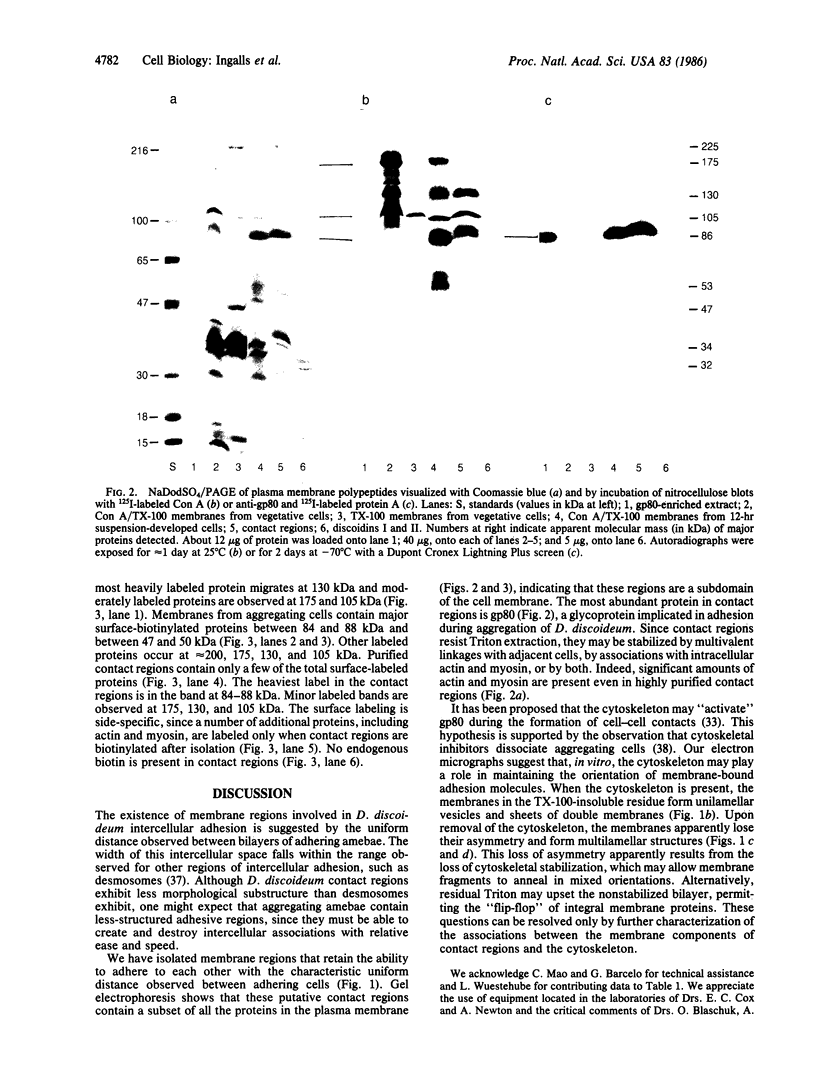
Regions of plasma membrane involved in Dictyostelium discoideum intercellular adhesion resist solubilization with the nonionic detergent Triton X-100. Electron microscopy shows that these regions of the plasma membrane adhere to each other, forming many bi- and multilamellar structures. NaDodSO4/polyacrylamide gels of these regions contain major polypeptides at 225 kDa (residual myosin), 105 kDa, 88 kDa, 84 kDa, 47 kDa (residual actin), and 34 kDa. These membranes contain a subset of the total plasma membrane proteins, as analyzed by labeling of electrophoretically fractionated and blotted membrane proteins with radioiodinated Con A and by electrophoresis of membrane proteins from surface-labeled cells. Antibodies specific for gp80, a glycoprotein implicated in intercellular adhesion, intensely stain the 88-kDa and 84-kDa bands. Since these membrane regions resist Triton extraction, they appear to be stabilized by protein-protein interactions. Such stabilizing interactions may involve multivalent linkages with adjacent cells, or associations with intracellular actin and myosin, or both. Since these membranes appear to represent regions of intercellular contact, we call them "contact regions."
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Davis J. Immunoreactive forms of human erythrocyte ankyrin are localized in mitotic structures in cultured cells and are associated with microtubules in brain. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):647–657. doi: 10.1101/sqb.1982.046.01.061. [DOI] [PubMed] [Google Scholar]
- Beug H., Gerisch G., Kempff S., Riedel V., Cremer G. Specific inhibition of cell contact formation in Dictyostelium by univalent antibodies. Exp Cell Res. 1970 Nov;63(1):147–158. doi: 10.1016/0014-4827(70)90343-5. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappuccinelli P., Ashworth J. M. The effect of inhibitors of microtubule and microfilament function on the cellular slime mould Dictyostelium discoideum. Exp Cell Res. 1976 Dec;103(2):387–393. doi: 10.1016/0014-4827(76)90274-3. [DOI] [PubMed] [Google Scholar]
- Cocucci S. M., Sussman M. RNA in cytoplasmic and nuclear fractions of cellular slime mold amebas. J Cell Biol. 1970 May;45(2):399–407. doi: 10.1083/jcb.45.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman D. S., Leichtling B. H., Rickenberg H. V. Phosphoproteins in Dictyostelium discoideum. J Supramol Struct Cell Biochem. 1981;15(4):369–385. doi: 10.1002/jsscb.1981.380150407. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Isolation of concanavalin A caps during various stages of formation and their association with actin and myosin. J Cell Biol. 1979 Mar;80(3):751–758. doi: 10.1083/jcb.80.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon R. F., Goodenough D. A. Five-hour half-life of mouse liver gap-junction protein. J Cell Biol. 1981 Aug;90(2):521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabius H. J., Springer W. R., Barondes S. H. Receptor for the cell binding site of discoidin I. Cell. 1985 Sep;42(2):449–456. doi: 10.1016/0092-8674(85)90102-3. [DOI] [PubMed] [Google Scholar]
- Geiger B. Membrane-cytoskeleton interaction. Biochim Biophys Acta. 1983 Aug 11;737(3-4):305–341. doi: 10.1016/0304-4157(83)90005-9. [DOI] [PubMed] [Google Scholar]
- Gorbsky G., Steinberg M. S. Isolation of the intercellular glycoproteins of desmosomes. J Cell Biol. 1981 Jul;90(1):243–248. doi: 10.1083/jcb.90.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Johnson G., Johnson R., Miller M., Borysenko J., Revel J. P. Do cellular slime molds form intercellular junctions? Science. 1977 Sep 23;197(4310):1300–1300. doi: 10.1126/science.561442. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luna E. J., Fowler V. M., Swanson J., Branton D., Taylor D. L. A membrane cytoskeleton from Dictyostelium discoideum. I. Identification and partial characterization of an actin-binding activity. J Cell Biol. 1981 Feb;88(2):396–409. doi: 10.1083/jcb.88.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E. J., Goodloe-Holland C. M., Ingalls H. M. A membrane cytoskeleton from Dictyostelium discoideum. II. Integral proteins mediate the binding of plasma membranes to F-actin affinity beads. J Cell Biol. 1984 Jul;99(1 Pt 1):58–70. doi: 10.1083/jcb.99.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. A., Niman H. L., Loomis W. F. Monoclonal antibody recognizing gp80, a membrane glycoprotein implicated in intercellular adhesion of Dictyostelium discoideum. Mol Cell Biol. 1983 May;3(5):863–870. doi: 10.1128/mcb.3.5.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Müller K., Gerisch G., Fromme I., Mayer H., Tsugita A. A membrane glycoprotein of aggregating Dictyostelium cells with the properties of contact sites. Eur J Biochem. 1979 Sep;99(2):419–426. doi: 10.1111/j.1432-1033.1979.tb13271.x. [DOI] [PubMed] [Google Scholar]
- Parish R. W., Müller U. The isolation of plasma membranes from the cellular slime mold Dictyostelium discoideum using concanavalin A and Triton X-100. FEBS Lett. 1976 Mar 15;63(1):40–41. doi: 10.1016/0014-5793(76)80190-1. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Edwards H. H. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980 Jul;86(1):77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W. R., Cooper D. N., Barondes S. H. Discoidin I is implicated in cell-substratum attachment and ordered cell migration of Dictyostelium discoideum and resembles fibronectin. Cell. 1984 Dec;39(3 Pt 2):557–564. doi: 10.1016/0092-8674(84)90462-8. [DOI] [PubMed] [Google Scholar]
- Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. II. Purification, properties, and membrane association of actin from amoebae of Dictyostelium discoideum. J Biol Chem. 1974 Sep 25;249(18):6013–6020. [PubMed] [Google Scholar]
- Stadler J., Gerisch G., Bauer G., Suchanek C., Huttner W. B. In vivo sulfation of the contact site A glycoprotein of Dictyostelium discoideum. EMBO J. 1983;2(7):1137–1143. doi: 10.1002/j.1460-2075.1983.tb01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin L. A. Structure and function of intercellular junctions. Int Rev Cytol. 1974;39:191–283. doi: 10.1016/s0074-7696(08)60940-7. [DOI] [PubMed] [Google Scholar]
- Sussman M., Boschwitz C. Adhesive properties of cell ghosts derived from Dictyostelium discoideum. Dev Biol. 1975 Jun;44(2):362–368. doi: 10.1016/0012-1606(75)90406-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Hancock K., Simons A. R. Calibration of prestained protein molecular weight standards for use in the "Western" or enzyme-linked immunoelectrotransfer blot techniques. Anal Biochem. 1984 Dec;143(2):304–307. doi: 10.1016/0003-2697(84)90667-5. [DOI] [PubMed] [Google Scholar]
- Yumura S., Mori H., Fukui Y. Localization of actin and myosin for the study of ameboid movement in Dictyostelium using improved immunofluorescence. J Cell Biol. 1984 Sep;99(3):894–899. doi: 10.1083/jcb.99.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]